Pre-operative systemic therapy in locally advanced breast cancer: a single institution experience
Correspondence to EM Ibrahim. Email: ezzibrahim@kfshrc.edu.sa
Abstract
Background: Locally advanced breast cancer (LABC) is common in developing countries and it frequently affects younger women. Patients do very poorly when treated by locoregional therapy alone; therefore, pre-operative systemic therapy (PST) is commonly used.
Materials and methods: Medical records of 64 Saudi patients with LABC treated with PST in a single institution were retrospectively reviewed.
Results: At diagnosis, most patients were young (median age 41 years), and had poor clinicopathological characteristics. Following surgery, complete pathologic response (pCR) in the breast was achieved in 13 patients (20%). Of 62 patients with known nodal status, 22 (34%) had negative axillary nodes. Presence of oestrogen receptor (ER) negative tumour was the only dependent variable that predicted pCR in the breast (p = 0.03). At a median follow-up of 42 months, the median progression-free survival (PFS) was 48 months (95% CI, 20–76 months) and the projected five-year overall survival (OS) was 68%. The recently published scoring system (Jeruss et al (2008) J Clin Oncol 26 2 246–52), was the only variable that independently influenced PFS, while ER negative tumours and presence of lymphovascular space invasion were the only factors that adversely affected OS.
Conclusions: despite the use of standard multi-modality approach in the management of patients with LABC, prognosis remains guarded.
Introduction
Pre-operative systemic therapy (PST), initially used only for locally advanced breast cancer (LABC), has become more common for patients with operable disease [1–3]. In patients with operable breast cancer, randomized trials have demonstrated that PST and post-operative chemotherapy (using the identical agents and treatment schedules) result in the same disease-free (DFS) and overall survival (OS) [4]. It has also been shown that long-term outcome significantly correlates with both clinical and pathologic tumour response rates [5,6].
Recently, researchers at MD Anderson Cancer Center have developed scoring systems based on combined clinical and pathologic variables to define outcome for breast cancer patients treated with PST [7]. The study population was composed of 932 patients that were predominantly post- or perimenopausal with a median age of 50 years. The series included patients with LABC as well as patients with small primary tumour. In brief, the scoring systems assigned risk scores to clinical stage, pathologic stage, oestrogen receptor (ER) negative tumour, and grade III disease. A combined prognostic score ranging from 0 to 6 was developed (the higher score the worse was the prognosis). The study concluded that the scoring systems facilitated separation of the study population into more refined subgroups by outcome than the current staging system.
In Saudi Arabia like other developing countries, LABC is not only common but it also affects women at a much younger age, and it carries a distinctively poor outcome [8–11].
The primary objective of the current study was to describe the clinicopathologic features and to determine the outcome of patients with LABC treated in a single institution with PST. We also intended to test the utility of MD Anderson Cancer Center scoring systems in our patient population.
Patients and methods
This retrospective study was approved by the Institutional Review Board of the King Faisal Specialist Hospital and Research Center. Data of newly diagnosed consecutive patients with locally advanced non-inflammatory invasive breast cancer (T2 > 4 cm, T3 or T4) confirmed on tru-cut biopsy and who received PST, were retrospectively reviewed. The database was locked on March 2009. Those with bilateral breast cancer, or documented evidence of metastatic disease, were excluded.
Staging procedures included complete history and physical examination, laboratory studies, bilateral mammography and mammary ultrasound, computerized tomography of the chest and abdomen, and radionuclide bone scan. ER and progesterone receptors (PR) were measured using standard immunehistochemistry (IHC), and the positive score was defined as greater than or equal to 10% of tumour cells demonstrating nuclear staining. HER-2 was graded as per the Dako HercepTest (Dako, Carpinteria, CA). For Her-2 +2 by IHC, HER-2 gene amplification was assessed by fluorescent in situ hybridization (FISH), using the Vysis method (Abbott Molecular, Inc., Des Plaines, IL). HER-2 positive tumours were those scoring 3+ by IHC or with > two copies of the HER-2 gene by FISH assay.
Clinical size of primary breast cancers and axillary nodes, if the latter were palpable, was determined separately before the administration of each cycle of PST and also before surgery. At each assessment, the product of the two greatest perpendicular diameters of the tumours in the breast and axilla was measured. Assessment of response was determined by clinical examination combined with mammography and ultrasonography. Women with dense breasts were also evaluated by magnetic resonance imaging.
Therapy
Patients received various PST regimens at the discretion of the treating physician. Following the PST regimen, response was assessed both clinically and by repeating relevant imaging. Patients with minimal or no response after two to three cycles were considered for alternative chemotherapy regimens. After the last cycle, patients were scheduled to undergo conservative surgery or modified radical mastectomy upon discretion of the surgeon and according to patient preference guided by the clinical response. Axillary lymph node dissection to levels I and II, aiming for excision of at least ten lymph nodes, was to be performed.
While most patients received post-surgery radiotherapy to the chest wall or the conserved breast and the axilla, post-operative adjuvant chemotherapy or hormonal therapy was given according to the discretion of the oncologist guided by the pathological response and the relevant clinicopathological characteristics.
Data source
A computerized database was created to capture hardcopy and electronic patient data. The following information was retrieved: patients' demographic and clinical data, laboratory and radiological studies, disease characteristics and PST details including clinical response and toxicity. The database also included surgery details, axillary lymph node dissection and pathologic response. Also, captured were post-surgery further chemotherapy, hormonal therapy, radiation therapy, recurrence and survival.
Definitions
Staging was defined according to the criteria determined by the International Union Against Cancer (UICC) [12], with group clinical and pathological staging according to the American Joint Committee on Cancer [13]. We adopted the criteria for LABC reported by Haagensen and Stout [14]. Best clinical response was assessed by physical and radiological examination and defined as complete response (cCR), partial response (cPR), stable disease (cSD), and progressive disease (cPD) according to response evaluation criteria in solid tumours [15]. This classification was also used to record the response of an axillary tumour to the PST regimen in patients who had clinically positive nodes at diagnosis. The development of a clinically suspicious ipsilateral axillary tumour during chemotherapy was considered as evidence of cPD in patients whose axilla was clinically negative when the first cycle of the PST was administered.
Histopathology
A median of 15 sections of the mastectomy or lumpectomy specimen was assessed; these included sections from each quadrant, from the nipple–areola complex (if appropriate), from areas of suspicious or prior tumour involvement and from the axillary contents (median of seven sections).
Pathologic response was assessed in surgical specimens of mammary tissue and lymph nodes using Sataloff et al [16] and Chevallier et al [17] criteria. Complete pathological response (pCR) in the breast, was defined as disappearance of invasive disease in the breast by pathologic examination, while residual invasive disease of < 1 cm and ≥ 1 cm was considered for descriptive purpose only as micro- and macro-residual disease, respectively. pCR in axilla was defined as absence of positive lymph nodes by haematoxylin and eosin staining.
Statistical methods
A two-sided Wilcoxon-Pratt test was used to compare tumour sizes before and after chemotherapy. To identify variables that predict pCR in the primary tumour, regression analysis was performed [18]. Overall survival (OS) was estimated from the date of starting PST to the date of last follow-up or death from any cause. Progression-free survival (PFS) was calculated from the date of definitive surgery until last contact; recurrence 'local, regional or distant'; occurrence of contralateral breast cancer; occurrence of second primary cancer other than in the contralateral breast or death. Survival was estimated by applying the method of Kaplan and Meier [19], while the statistical procedure of Brookmeyer-Crowley was used to estimate the 95% confidence interval (CI) of median survival [20]. The log-rank test was used to assess the significance of unadjusted differences in survival [21]. Exploring variables for their independent prognostic effect on OS or PFS was carried out using the multivariate stepwise regression model of Cox and Oakes to compute hazards ratio (HR) [22]. Being one of the objectives of our study, we specifically examined the prognostic significance of the scoring system as reported by Jeruss et al and alluded to in the introduction [7]. In the survival analysis, we grouped patients according to their assigned risk score. Variables with p value ≤ 0.1 in the univariate analysis were tested for the multivariate model. In this process, the predictor with the highest level of statistical significance was used to introduce the model; other variables were then evaluated for further predictive information and added in turn, beginning with the variables with the highest level of statistical significance (i.e. the lowest p values) and continuing until the p value for the variable added exceeded 0.05. Continuous prognostic variables were also considered for inclusion in the model as dichotomous variables using various cut-off points only if they attained a p value of ≤ 0.1 in the univariate analysis. We also compared the survival functions for variables after stratifying for baseline differences in additional variables [23]. All tests of significance were two sided, and differences were considered statistically significant when P <0.05. We performed all data analyses using the SPSS Statistical Software Package (SPSS software v. 17.0; SPSS Inc., Chicago, IL, USA).
Results
This retrospective analysis included the records of 64 patients, and they were all evaluable for efficacy and toxicity analysis. The median age was 41 years (range, 25–75). Patient and disease characteristics are depicted in Table 1. The median largest tumour diameter at diagnosis was 7.5 cm (range, 2–15 cm). Four (6%) and 60 (94%) patients had invasive lobular and invasive ductal cancer, respectively.
Table 1: Patient and disease characteristics (64 patients)
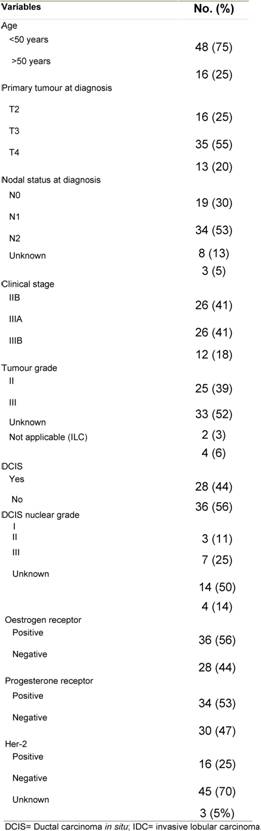
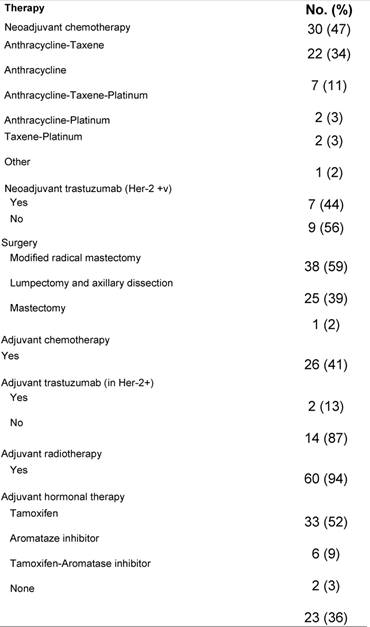
Table 2 shows the PST regimens and the adjuvant treatment given. Due to minimal or no response to initial anthracycline-based therapy, four patients required substitution of PST by taxane-platinum-based regimens. The median time from diagnosis to the initiation of PST was 24 days (95% CI, 32–69 days), and the median number of PST cycles was four (range, 2–8). Patients with HER-2 over-expression (seven patients) received neoadjuvant trastuzumab concomitantly with chemotherapy according to the MD Anderson protocol [7].
Table 2: Neoadjuvant and adjuvant therapy
All patients were evaluable for the best clinical response (BCR). According to the pre-defined criteria, 18 (28%), 31 (48%), nine (14%) and six (10%) of the patients achieved cCR, cPR, cSD and cPD, respectively. The median time to the best clinical response was 2.7 months (95% CI, 2.6–4.6 months).
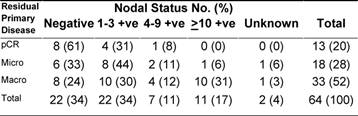
Following PST, patients underwent surgery (Table 2). The median time from starting therapy to surgery was 4.2 months (95% CI, 4–5.8 months). Despite a combined cCR and cPR rate of 76%, only 26 patients (41%) had conservative surgery. The pathologic response in the primary tumour was considered complete (pCR) in 13 (20%) of patients. On the other hand, residual invasive disease was microscopic (< 1 cm in diameter) and macroscopic (≥ 1 cm in diameter), in 18 (28%) and 33 (52%) patients, respectively. The median largest residual tumour diameter was 1.8 cm (range, 0–8). Compared with baseline assessment that downstaging was statistically significant (Table 3 depicts the pathologic data for the primary tumour and axillary lymph nodes. Of all 64 patients, eight (13%) achieved pCR in the primary tumour and in the axilla. None of the eight patients had residual non-invasive disease. The main clinicopathologic features of those eight patients were as follows: six patients (75%) were ≤ 50 years; four patients (50%) each had clinical stage IIB and IIIA, respectively; tumour grade II/III in 2/6 patients; lymphovascular space invasion -ve/unknown in 6/2 patients; ER+/ER- in 3/5 patients; PR+/PR- in 1/7 patients; and HER-2+/HER-2- in 4/4 patients. ER negative tumour was the only variable that independently predicted pCR in the breast (p = 0.03).
Table 3: Relationship between pathologic responses of the primary tumour versus pathologic nodal status
Seven patients who over-expressed HER-2 received neoadjuvant trastuzumab. Three patients achieved pCR in the primary tumour and the axilla. Two patients attained pCR in the primary tumour but with one positive lymph node in each patient. Of the remaining two patients, one had micro-residual disease and one had macro-residual disease. Neither of the latter two patients had positive axillary disease.
Survival analysis
At a median follow-up of 42 months (95% CI, 34–49 months), 13 patients (20%) were dead, 15 (24%) were alive with evidence of disease and the remaining 35 (56%) were still alive with no apparent evidence of disease. All mortality events were attributed to progressive breast cancer or its related complications.
Disease recurrence was documented in 29 patients (45%). The first documented recurrence was local and/or regional, contralateral breast, contralateral lymph nodes, distant, distant and locoregional, in five, three, two, 18 and one patients, respectively. Of the eight patients, who attained pCR in the breast and axilla, three patients (38%) experienced relapse as compared with 26 of the 56 patients (46%) with less than pCR. The same three patients were the only patients who experienced relapse among all 13 patients with pCR in the breast. Of the seven patients, who received neoadjuvant trastuzumab, only one developed regional and distant relapse after 27 months. The median PFS for the series was 48 months (95% CI, 20–76 months) and the five-year PFS rate (±SE) was 48% (±7%).
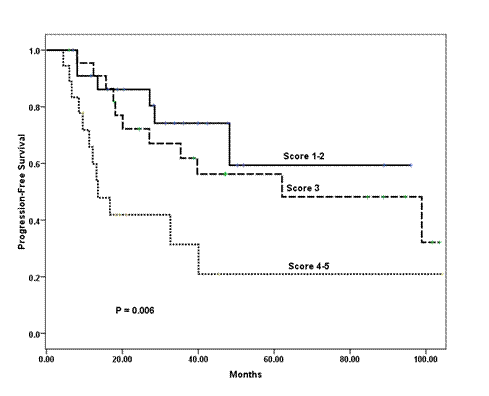
In a univariate analysis, the following variables prognosticated poor PFS and were subsequently tested in a multivariate model: age ≤ 35 years (p = 0.08); ER negative tumour (p = 0.07); tumour grade III (p = 0.001); pathologically positive lymph node (p = 0.008); extra-nodal extension (p = 0.07); and higher scoring system (p = 0.006) according to that proposed by Jeruss et al [7]. For the latter variable, we defined three distinctive risk groups (low-risk = 1–2 score, intermediate-risk = 3 score, and high-risk = 4–5 score). The Cox proportional hazards model identified higher score in the scoring system as the only variable that independently influenced PFS (p = 0.006) (Table 4 and Figure 1).
Table 4: Multivariate analysis of prognostic variables for progression-free (PFS) and overall survival (OS)
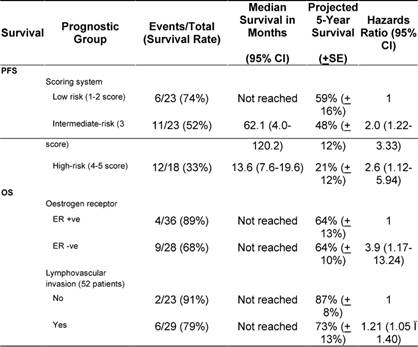
Figure 1: Kaplan-Meier estimates of progression-free survival of patients stratified into three risk groups according to the combined scoring system
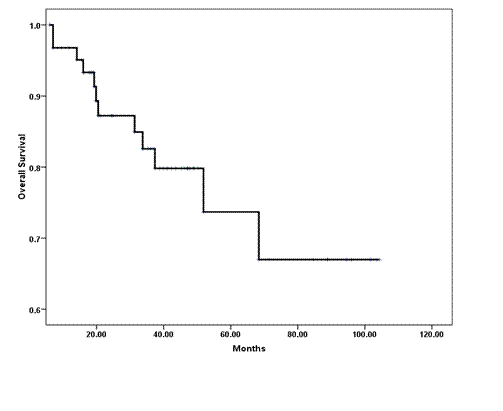
The median OS has not been reached (Figure 2); however, the estimated five-year OS (±SE) was 68% (±9%). Disease-related mortality occurred in two of the eight patients who attained pCR in the breast and axilla. However, none of those who received neoadjuvant trastuzumab have died. In a univariate analysis, the following variables predicted poor OS and were subsequently tested in a multivariate model: tumour grade III (p = 0.02); ER- (p = 0.014); PR- (p = 0.03); lymphovascular invasion (p = 0.07); less than pCR (p = 0.06) and extra-nodal extension (p = 0.01). The scoring system showed no prognostic significance, and only ER negative tumours and the presence of lymphovascular space invasion were associated with an independent adverse effect on OS (Table 4).
Figure 2: Kaplan-Meier estimate of overall survival
PST was well tolerated with the expected treatment-related toxicity. There was no incidence of therapy-related mortality and no patient developed secondary malignancy other than contralateral breast cancer in three patients.
Discussion
Patients with LABC do very poorly when treated by locoregional therapy alone. Such therapy favourably affects locoregional control, but most relapses are due to the development of distant metastases [24,25]. However, while PST regimens have been shown to have a favourable effect on the outcome of patients with LABC, the survival advantage of primary chemotherapy is yet to be shown [26–28].
In this series, there was significant prevalence of poor clinicopathologic features among patients. Nevertheless, PST achieved a significant downstaging of the primary tumour size and the clinical nodal status resulting in a high-clinical response rate of 76%, of which 28% was complete. However, only 20% and 28% of patients demonstrated pCR and microscopic residual invasive tumour, respectively. Moreover, of the 62 patients with known nodal status, 22 (35%) had negative axillary lymph nodes and, of those, eight patients (13%) had pCR in the breast and the axilla.
The regression model showed that ER negativity was the only variable that predicted pCR in the primary tumour. That finding is consistent with other published series that have shown that ER negative tumours tend to have a higher pathologic response rate to chemotherapy than ER positive disease [29–32].
Despite only seven patients with HER-2+ tumours receiving trastuzumab, three patients (45%) attained pCR in the primary tumour and the axilla. The achieved results are consistent with published studies. Incorporating trastuzumab with chemotherapy in the neoadjuvant setting in HER-2 over-expressing disease has uniformly demonstrated impressive pCR rate ranging from 39% to 67% [33–35].
At a median follow-up of 42 months (95% CI, 34–49 months), disease recurrence was documented in 45% of patients. The five-year PFS was 48%, and the median PFS was 48 months. Three of the seven patients who attained pCR in breast and axilla had relapsed. Reported series have shown a five-year recurrence rate in patients with a pCR ranging from 13% to 35% [3,36,37]. The five-year OS was projected as 68%, which is somewhat inferior to that reported in other series [38,39]; however, the attained OS was expected considering the poor clinicopathologic features of patients.
Interestingly, while the combined scoring system was developed on older patients with less advanced locoregional disease [7]; in our patients, the system was the only variable that distinctly classified patients into three risk groups with significantly different PFS rates. The scoring system, however, did not prognosticate OS. The lack of a prognostic effect of the scoring system on OS may be related to the few number of events. On the other hand, the Cox model identified ER tumour and the presence of lymphovascular space invasion as independent adverse prognostic variables for OS. In a large, recently published series, lymphovascular space invasion was a strong independent variable that predicted higher locoregional failure among patients with LABC [40].
The current series has several limitations. First, the series was relatively small; however, the series included a group of patients that largely had poor clinicopathologic characteristics. Second, various PST regimens have been used; however, all patients were offered the most active PST agents and received a uniform management strategy at a single institution.
In conclusion, this study described the clinical and pathological features, management strategy and outcome of young patients with LABC in a developing country. The study also identified predictor and prognostic variables that could influence outcome in a similar patient population. The prognosis of LABC remains guarded. The aggressive nature of LABC and the ability to measure drug effect in vivo indicate that more clinical trials of combining existing and newer biological agents with chemotherapy are needed [41,42].
References
1. Hortobagyi GN (1990) Comprehensive management of locally advanced breast cancer Cancer 66 1387–91 PMID: 2205369 DOI: 10.1002/1097-0142(19900915)66:14+3.0.CO;2-I
2. Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, Fellin FM and McFarlane J (1994) Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast Cancer 73 362–9 PMID: 8293401 DOI: 10.1002/1097-0142(19940115)73:23.0.CO;2-L
3. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer J Clin Oncol 16 2672–85 PMID: 9704717
4. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27 J Clin Oncol 26 778–85 PMID: 18258986 DOI: 10.1200/JCO.2007.15.0235
5. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B et al (2006) Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27 J Clin Oncol 24 2019–27 PMID: 16606972 DOI: 10.1200/JCO.2005.04.1665
6. Machiavelli MR, Romero AO, Perez JE, Lacava JA, Dominguez ME, Rodriguez R et al (1998) Prognostic significance of pathological response of primary tumour and metastatic axillary lymph nodes after neoadjuvant chemotherapy for locally advanced breast carcinoma Cancer J Sci Am 4 125–31 PMID: 554929
7. Jeruss JS, Mittendorf EA, Tucker SL, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, Cormier JN, Buzdar AU, Hortobagyi GN and Hunt KK (2008) Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy J Clin Oncol 26 246–52 PMID: 18056680 DOI: 10.1200/JCO.2007.11.5352
8. Ibrahim EM, al-Mulhim FA, al-Amri A, al-Muhanna FA, Ezzat AA, Stuart RK and Ajarim D (1998) Breast cancer in the eastern province of Saudi Arabia Med Oncol 15 241–7 PMID: 9951687 DOI: 10.1007/BF02787207
9. Ezzat AA, Ibrahim EM, Ajarim DS, Rahal MM, Raja MA, Stuart RK, Tulbah AM, Kandil A, Al-Malik OA and Bazarbashi SM (2000) High complete pathological response in locally advanced breast cancer using paclitaxel and cisplatin Breast Cancer Res Treat 62 237–44 PMID: 11072788 DOI: 10.1023/A:1006434406989
10. Ezzat AA, Ibrahim EM, Raja MA, Al-Sobhi S, Rostom A and Stuart RK (1999) Locally advanced breast cancer in Saudi Arabia: high frequency of stage III in a young population Med Oncol 16 95–103 PMID: 10456657 DOI: 10.1007/BF02785842
11. Parkin DM, Bray F, Ferlay J and Pisani P (2005) Global cancer statistics, 2002 CA Cancer J Clin 55 74–108 PMID: 15761078 DOI: 10.3322/canjclin.55.2.74
12. Hermanek P, Hutter RV, Sobin LH and Wittekind C (1999) International Union Against Cancer. Classification of isolated tumour cells and micrometastasis Cancer 86 2668–73 PMID: 10594862 DOI: 10.1002/(SICI)1097-0142(19991215)86:123.0.CO;2-R
13. Greene FL (2002) The American Joint Committee on Cancer: updating the strategies in cancer staging Bull Am Coll Surg 87 13–5 PMID: 17387902
14. Haagensen CD and Stout AP (1943) Carcinoma of the Breast. II-Criteria of Operability Ann Surg 118 1032–51 PMID: 17858324 DOI: 10.1097/00000658-194312000-00010
15. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumours. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst 92 205–16 PMID: 10655437 DOI: 10.1093/jnci/92.3.205
16. Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP and Baloch Z (1995) Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome J Am Coll Surg 180 297–306 PMID: 7874340
17. Chevallier B, Roche H, Olivier JP, Chollet P and Hurteloup P (1993) Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate Am J Clin Oncol 16 223–8 PMID: 8338056 DOI: 10.1097/00000421-199306000-00006
18. Abbott RD (1985) Logistic regression in survival analysis Am J Epidemiol 121 465–71 PMID: 4014135
19. Kaplan EL and Meier P (1958) Nonparametric estimation from incomplete observations Amer Statist Ass 53 457–81 DOI: 10.2307/2281868
20. Brookmeyer R (1983) Prediction intervals for survival data Stat Med 4 485–95 PMID 6672946
21. Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration Cancer Chemother Rep 50 163–70 PMID: 5910392
22. Cox DR and Oakes D (1972) Regression models and life tables (with discussion) J Roy Statist Soc B34 187–20
23. Kalbfleisch JD and Prentice RL (1990) The Statistical Analysis of Failure Time Data (New York: Wiley)
24. Zucali R, Uslenghi C, Kenda R and Bonadonna G (1976) Natural history and survival of inoperable breast cancer treated with radiotherapy and radiotherapy followed by radical mastectomy Cancer 37 1422–31 PMID: 816452 DOI: 10.1002/1097-0142(197603)37:33.0.CO;2-Y
25. Bruckman JE, Harris JR, Levene MB, Chaffey JT and Hellman S (1979) Results of treating stage III carcinoma of the breast by primary radiation therapy Cancer 43 985–93 PMID: 106955 DOI: 10.1002/1097-0142(197903)43:33.0.CO;2-1
26. Hortobagyi GN, Ames FC, Buzdar AU, Kau SW, McNeese MD, Paulus D et al (1988) Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy Cancer 62 2507–16 PMID: 3056604 DOI: 10.1002/1097-0142(19881215)62:123.0.CO;2-D
27. Valagussa P, Zambetti M, Bonadonna G, Zucali R, Mezzanotte G and Veronesi U (1990) Prognostic factors in locally advanced noninflammatory breast cancer. Long-term results following primary chemotherapy Breast Cancer Res Treat 15 137–47 PMID: 2372569 DOI: 10.1007/BF01806351
28. D Mauri, N Pavlidis and JP Ioannidis (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis J Natl Cancer Inst 97 188–94 PMID: 15687361
29. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B et al (2003) The effect on tumour response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27 J Clin Oncol 21 4165–74 PMID: 14559892 DOI: 10.1200/JCO.2003.12.005
30. Ring AE, Smith IE, Ashley S, Fulford LG and Lakhani SR (2004) Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer Br J Cancer 91 2012–7 PMID: 15558072 DOI: 10.1038/sj.bjc.6602235
31. Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V et al (2006) Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors J Clin Oncol 24 1037–44 PMID: 16505422 DOI: 10.1200/JCO.2005.02.6914
32. Ellis P, Smith I, Ashley S, Walsh G, Ebbs S, Baum M, Sacks N and McKinna J (1998) Clinical prognostic and predictive factors for primary chemotherapy in operable breast cancer J Clin Oncol 16 107–14 PMID: 9440730
33. Coudert BP, Arnould L, Moreau L, Chollet P, Weber B, Vanlemmens L et al (2006) Pre-operative systemic (neo-adjuvant) therapy with trastuzumab and docetaxel for HER2-overexpressing stage II or III breast cancer:results of a multicenter phase II trial Ann Oncol 17 409–14 PMID: 16332965 DOI: 10.1093/annonc/mdj096
34. Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL et al (2007) Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen Clin Cancer Res 13 228–33 PMID: 17200359 DOI: 10.1158/1078-0432.CCR-06-1345
35. Andre F, Mazouni C, Liedtke C, Kau SW, Frye D, Green M, Gonzalez-Angulo AM, Symmans WF, Hortobagyi GN and Pusztai L (2008) HER2 expression and efficacy of preoperative paclitaxel/FAC chemotherapy in breast cancer Breast Cancer Res Treat 108 183–90 PMID: 17468948 DOI: 10.1007/s10549-007-9594-8
36. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K et al (1999) Clinical course of breast cancer patients with complete pathologic primary tumour and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy J Clin Oncol 17 460–9 PMID: 10080586
37. Chollet P, Amat S, Cure H, de Latour M, Le Bouedec G, Mouret-Reynier MA, Ferriere JP, Achard JL, Dauplat J and Penault-Llorca F (2002) Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer Br J Cancer 86 1041–6 PMID: 11953845 DOI: 10.1038/sj.bjc.6600210
38. Mathew J, Asgeirsson KS, Cheung KL, Chan S, Dahda A and Robertson JF (2009) Neoadjuvant chemotherapy for locally advanced breast cancer: a review of the literature and future directions Eur J Surg Oncol 35 113–22 PMID: 18502088
39. Barni S and Mandala M (2006) Locally advanced breast cancer Curr Opin Obstet Gynecol 18 47–52 PMID: 16493260 DOI: 10.1097/01.gco.0000192998.04793.ba
40. Huang EH, Tucker SL, Strom EA, McNeese MD, Kuerer HM, Hortobagyi GN et al (2005) Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy Int J Radiat Oncol Biol Phys 62 351–7 PMID: 15890574
41. Salter JT and Miller KD (2007) Antiangiogenic agents in breast cancer Cancer Invest 25 518–26 PMID: 18027149 DOI: 10.1080/07357900701648516
42. Guix M, Granja Nde M, Meszoely I, Adkins TB, Wieman BM, Frierson KE et al (2008) Short preoperative treatment with erlotinib inhibits tumour cell proliferation in hormone receptor-positive breast cancers J Clin Oncol 26 897–906 PMID: 18180460 DOI: 10.1200/JCO.2007.13.5939






