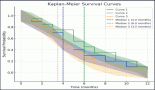
The international, phase III, multi-centre AZA-001 trial demonstrated azacitidine (AZA) is the first treatment to significantly extend overall survival (OS) in higher risk myelodysplastic syndromes (MDS) patients (Fenaux (2007) Blood 110 817). The current treatment paradigm, which is based on a relationship between complete remission (CR) and survival, is increasingly being questioned (Cheson (2006) Blood 108 419). Results of AZA-001 show CR is sufficient but not necessary to prolong OS (List (2008) Clin Oncol 26 7006). Indeed, the AZA CR rate in AZA-001 was modest (17%), while partial remission (PR, 12%) and haematological improvement (HI, 49%) were also predictive of prolonged survival. This analysis was conducted to assess the median number of AZA treatment cycles associated with achievement of first response, as measured by IWG 2000-defined CR, PR or HI (major + minor). The number of treatment cycles from first response to best response was also measured.