Water pollution and childhood leukaemia risk: a systematic review
Juan Carlos Nuñez-Enriquez1,a Daniela Medina-León2,b, Diana Tinoco-Montejano3,c, Karen Jacuinde-Trejo4,d, Janet Flores-Lujano5,e, Lissette Gómez-Rivera6,f, Omar Chávez-Martínez7,g, Francisco J García-Alvarado8,h, Patricia Blanco-Padilla9,i and Rosana Pelayo10,11,j
1División de Investigación en Salud, Unidad Médica de Alta Especialidad Hospital de Pediatría ‘Dr. Silvestre Frenk Freund’, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City 06720, Mexico
2Facultad Mexicana de Medicina, Universidad La Salle, Mexico City 14000, Mexico
3Departamento de Medicina, Facultad de Estudios Superiores Zaragoza, Universidad Nacional Autónoma de México, Mexico City 09230, Mexico
4Escuela Nacional de Medicina y Homeopatía, Instituto Politécnico Nacional, Mexico City 07320, Mexico
5Unidad de Investigación Médica en Epidemiología Clínica, Unidad Médica de Alta Especialidad Hospital de Pediatría ‘Dr. Silvestre Frenk Freund’, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City 06720, Mexico
6Centro de Documentación en Salud, Dirección de Educación e Investigación en Salud, Unidad Médica de Alta Especialidad Hospital de Oncología, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City 06720, Mexico
7Coordinación de Investigación en Salud, Unidad de Educación e Investigación, Instituto Mexicano del Seguro Social, Mexico City 06720, Mexico
8Departamento de Investigación en Salud, Unidad Médica de Alta Especialidad No. 71, Instituto Mexicano del Seguro Social, Torreón 27000, Mexico
9Jefatura de Laboratorios de la División Ciencias de la Salud. Instituto de Estudios Superiores de Tamaulipas, IEST - ANÁHUAC, Tamaulipas 89605, Mexico
10Laboratorio de Citómica del Cáncer Infantil, Centro de Investigación Biomédica de Oriente, Instituto Mexicano del Seguro Social, Puebla 74360, Mexico
11Unidad de Educación e Investigación, Instituto Mexicano del Seguro Social, Mexico City 06720, Mexico
ahttps://orcid.org/0000-0002-8070-9727
bhttps://orcid.org/0009-0005-2922-6219
chttps://orcid.org/0009-0003-8688-2330
dhttps://orcid.org/0009-0005-2584-9443
ehttps://orcid.org/0000-0003-0727-2837
fhttps://orcid.org/0000-0002-3337-1575
ghttps://orcid.org/0000-0003-2633-1898
hhttps://orcid.org/0000-0002-3760-1876
ihttps://orcid.org/0009-0004-4836-6041
jhttps://orcid.org/0000-0002-8070-9727
Abstract
Water pollution represents a major global health concern, especially in low- and middle-income countries where toxic metals (TMs) and pesticides can contaminate drinking water through industrial, agricultural and urban activities. Children are particularly susceptible due to their developing physiology and higher water intake relative to body weight. This review aims to explore the association between exposure to TMs and pesticides in drinking water and the risk of childhood leukaemia (CL), highlighting the broader significance for environmental health and child safety. A structured search in PubMed, Scopus and Google Scholar (2001–2024) identified studies on acute lymphoblastic leukaemia or acute myeloid leukaemia in individuals under 20 years of age, assessing exposure to trace metals or pesticides via drinking water. Observational designs were included, excluding studies unrelated to water exposure or lacking paediatric data. Records were screened and reviewed independently by four authors. Findings were heterogeneous, with several studies suggesting potential links between specific contaminants like arsenic, hexavalent chromium, pentachlorophenol and certain pesticides and an increased risk of leukaemia in children, while others found no significant associations and noted methodological challenges such as small sample sizes and difficulties in exposure measurement. Although current evidence remains inconclusive regarding a direct causal relationship, this review underscores the need for rigorous, long-term research to clarify the role of waterborne pollutants in CL and to guide public health strategies.
Keywords: water pollution; toxic metals; pesticides; childhood leukaemia; systematic review
Correspondence to: Juan Carlos Nuñez-Enriquez
Email: jcarlos_nu@hotmail.com
Published: 20/02/2026
Received: 17/07/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Water pollution (WP) is a significant global public health issue linked to cancer development [1–5]. Toxic metals (TM) and pesticides, detected in water from industrial, agricultural and domestic sources [1, 2, 6, 7], pose risks to children due to their developing physiology and longer life expectancy [8–12]. Childhood leukaemia (CL), mainly acute lymphoblastic leukaemia (ALL) and less commonly acute myeloid leukaemia (AML), is one of the most frequent paediatric cancers [13]. Although some studies associate exposure to TM and pesticides in water—like arsenic (As), lead (Pb), cadmium (Cd) and certain pesticides—with increased leukaemia risk, evidence remains inconsistent [14–19]. In Latin America, WP is severe, with elevated levels of TM and banned pesticides, stemming from natural sources or human activities, but monitoring is often limited, creating significant data gaps [20–25]. Assessing whether waterborne contaminants contribute to CL is crucial, especially in low- and middle-income countries with weaker environmental controls and higher CL incidence [20–22, 26, 27]. This systematic review examines literature from 2001 to 2024 to evaluate links between TM and pesticide exposure in water and CL risk, aiming to synthesize evidence, identify gaps and inform future research and public health actions.
Methods
A structured search strategy and selection criteria were applied to ensure the inclusion of relevant and methodologically sound studies.
Studies were included if they reported cases of ALL or AML in populations under 20 years of age, regardless of whether adult populations were also included in the study. That is, studies involving mixed-age populations were eligible, provided that data on individuals under 20 years were clearly analysed.
Eligible studies assessed exposure to trace metals (e.g., As, Cd and Pb) or pesticides via drinking water or directly related environmental pathways. We considered observational designs such as case-control, systematic reviews, ecological or geospatial analyses.
Exclusion criteria included studies focusing exclusively on chronic leukaemias or on populations over 20 years of age without clear inclusion of paediatric or adolescent subgroups. Studies were also excluded if the exposure pathway was unclear, unrelated to water or if they lacked sufficient information on the contaminants or exposure mechanisms.
A targeted literature search was conducted in PubMed, Scopus and Google Scholar. The most recent search was performed on May 15, 2025. The search strategy was structured around three core concepts: leukaemia, water contamination and paediatric population. Controlled vocabulary (MeSH, DeCS) and free-text terms were combined using Boolean operators and adapted to the syntax of each database.
Key search terms included
- Leukaemia: ‘Leukaemia’[Mesh], ‘Leukaemia, Lymphoid’[Mesh], ‘Leukaemia, Myeloid’[Mesh], leukaemia
- Water contamination: ‘Water Pollutants’[Mesh], ‘Drinking Water’[Mesh], water contamination, toxic water
- Population: ‘Child’[Mesh], ‘Adolescent’[Mesh], ‘Infant’[Mesh], child, adolescent, teen, paediatric
Custom searches were also conducted for specific contaminants (e.g., As, benzene and nitrates) and gray literature was retrieved through flexible queries in Google Scholar (e.g., ‘water contamination’ AND leukaemia AND children), applying manual filters for language (English/Spanish) and publication years (2001–2024).
Study selection and data summary
All records were independently reviewed by four authors (JFL, DML, DTM, KJT) in two phases: title/abstract screening followed by full-text review. Studies were selected based on relevance and methodological clarity. Disagreements were resolved by consensus (Figure 1).
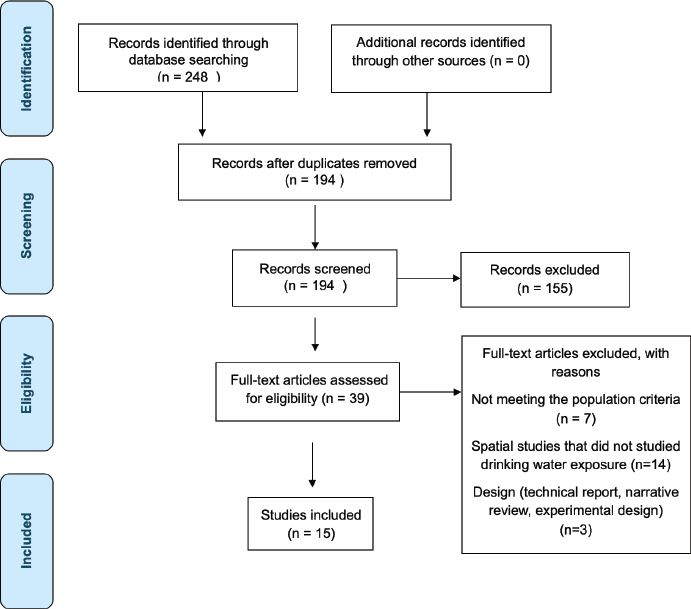
Figure 1. PRISMA flow diagram.
Results
A total of 15 studies were included in this systematic review, covering a range of geographical regions including North America, Europe, Asia and Africa. These studies were conducted between 1980 and 2023 and employed various study designs, including case-control, ecological and archival research, to investigate the potential association between WP by TM and pesticides and the risk of CL. The sample sizes ranged from small case-control studies (less than 100 participants) to larger ecological studies involving tens of thousands of individuals.
The studied populations varied, with most studies focusing on children or individuals under 20 years of age, though a few also included adults. The primary outcome assessed across these studies was CL, with some studies also examining other hematological malignancies such as lymphoma and myeloma. The cancers investigated were predominantly childhood ALL and other forms of leukaemia, though some studies also considered broader cancer types such as non-Hodgkin lymphoma, multiple myeloma and chronic lymphocytic leukaemia.
The studies primarily focused on exposure to specific substances in DW, such as As, chromium (hexavalent), radon, volatile organic compounds and various pesticides, including herbicides like atrazine, simazine and alachlor. Exposure mechanisms were primarily linked to DW, with a few studies also considering oral ingestion through food, geophagia and residential proximity to contaminated areas [28–32]. The exposure assessment methods varied, with some studies measuring contaminant concentrations directly in water samples using techniques such as liquid scintillation, atomic absorption spectrometry or gas chromatography, while others used geographic models or databases to estimate exposure levels [33, 34].
The primary aim of most studies was to evaluate whether there was an increased risk of CL or other cancers in populations exposed to these contaminants [28, 31, 32, 35–37]. Some studies sought to identify specific environmental factors or contaminants responsible for observed cancer clusters in certain regions, while others aimed to investigate the general health risks associated with long-term exposure to polluted water sources [29, 30, 33, 34, 38, 39].
In terms of study databases, several studies utilised national or regional environmental health databases, including public water system data, government environmental records and cancer registries [29, 30, 32, 40]. Some studies also used data from more specific sources, such as municipal water records or specialised cancer registries, to assess exposure and cancer incidence [28, 31, 39]. The variety in methodologies and data sources across the studies reflects the complexity of accurately assessing the link between WP and CL risk. Despite the heterogeneity in study designs, a consistent pattern emerged suggesting a potential association between exposure to TM and pesticides in DW and increased leukaemia risk in children.
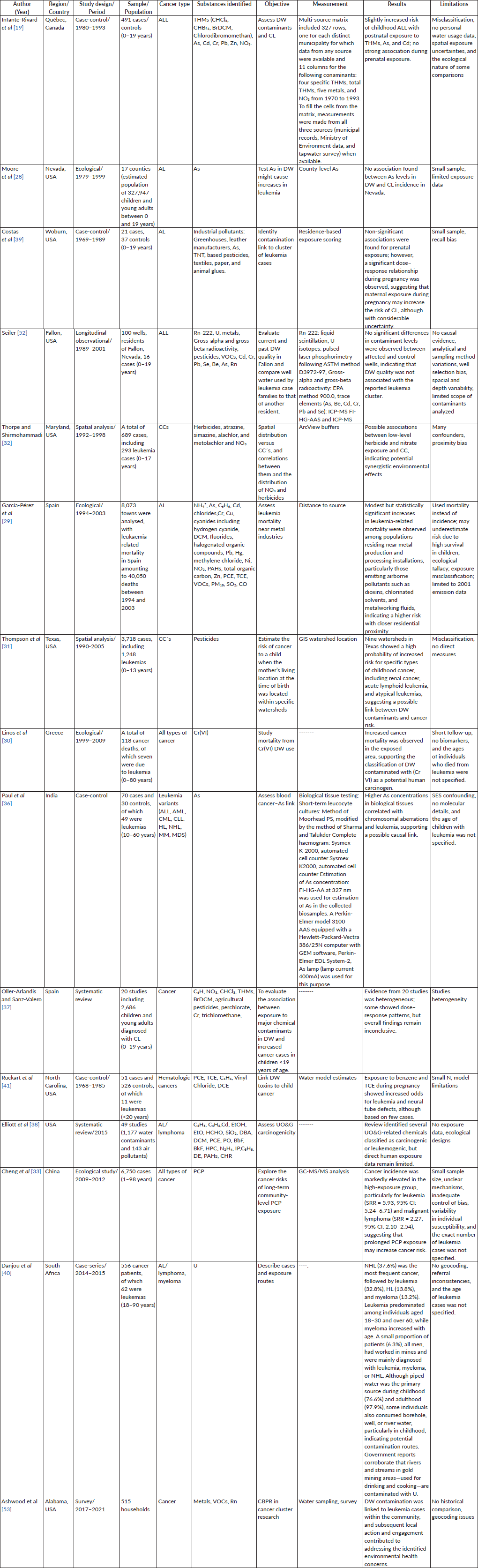
To provide a concise overview of the studies included in this review, Table 1 summarises the main characteristics of the 15 analysed articles. It describes the study design, population, type of leukaemia, exposure source, key findings and a brief quality commentary, allowing a comparative assessment of the methodological strengths and limitations of each investigation (Table 1).
Abbreviations
AL – Acute Leukaemias, ALL – Acute Lymphoblastic Leukaemia, AML – Acute Myeloid Leukaemia, As – Arsenic, ASR – Age-Standardised Rate, BbF – Benzo[b]fluoranthene, BkF – Benzo[k]fluoranthene, Be – beryllium (BrDCM) Bromodichloromethane, CHBr₃ – Bromoform (C₄H₆) 1,3-Butadiene, C₆H₆ – Benzene, C₈H₈ – Styren, CBPR – Community-Based Participatory Research, Cd – Cadmium, CHR – Chrysene, CC´s – childhood cancer(s), CI – Confidence Interval, CL – Childhood Leukaemia, CHCl₃ – chloroform, CO – Carbon Monoxide, Cr – Chromium, CrVI) – Hexavalent Chromium, DCE – trans-1,2-Dichloroethylene, DBA – Dibenz[a,h]anthracene, DE – Diesel Exhaust, DW – Drinking Water, EtOH – Ethanol, EtO – Ethylene Oxide, FI-HG-AAS – Flow Injection-Hydride Generation-Atomic Absorption Spectrometry, GC-MS/MS – Gas Chromatography–Mass Spectrometry, GIS – Geographic Information Systems, HCHO – Formaldehyde, Hg – Mercury, HL – Hodgkin Lymphoma, HM – Haematological Malignancy, HPC – Heptachlor, IARC – International Agency for Research on Cancer, ICP-MS – Inductively Coupled Plasma-Mass Spectrometry, IP – Indeno[1,2,3-cd]pyrene, MDS/MPS – Myelodysplastic/Myeloproliferative Syndrome, MM – Multiple Myeloma, N₂H₄ – Hydrazine, NHL – Non-Hodgkin Lymphoma, NH₄⁺ – Ammonia, NO₂ – Nitrogen Dioxide, NO₃ – Nitrates, NTDs – Neural Tube Defects, NTP – National Toxicology Program, OR – Odds Ratio, P – P Value, PAHs – Polycyclic Aromatic Hydrocarbons, PCP – Pentachlorophenol, Pb – Lead, PCE – Tetrachloroethylene, PM₁₀ – Particulate Matter 10, PO – 1,2-Propylene Oxide, Rn – Radon, RRs – Relative Risks, Se – selenium, SDs – standard deviations, SEER – Surveillance Epidemiology and End Results Program, SiO₂ – Quartz, SIRS – Standardised incidence ratios, SMRs – Standardised Mortality Ratios, SO₂ – Sulfur Dioxide, SRRs – Standardised Rate Ratios, TCE – Trichloroethylene, THMs – Trihalomethanes, TNT – Trinitrotoluene, U – Uranium, UO&G – Unconventional Oil and Gas, USA – United States of America, VOCs – Volatile Organic Compounds, y – years, Zn – Zinc
Table 1. Epidemiological evidence of childhood leukaemia and exposure to DW contaminants: summary table.
Discussion
The environmental factors influencing CL have been widely debated, with varying findings across different regions and exposure types. This systematic review examined the relationship between environmental contaminants, particularly in DW and CL. The findings from the included studies are diverse, with both significant associations and inconclusive results, pointing to the complexity of linking environmental exposure to leukaemia risk.
Water contaminants and CL risk
Several studies have highlighted the role of DW contaminants in the development of CL. Infante-Rivard et al [35] found an increased risk of ALL associated with exposure to trihalomethanes and metals like Cd and As in DW in Quebec, Canada. However, they acknowledged the challenges posed by measurement variability and exposure misclassification. Similarly, Moore et al [28] in Nevada found no significant association between As levels in DW and CL, suggesting that the relationship might not be as straightforward or might be influenced by other factors [28]. Costas et al [39] investigated potential associations in Woburn, USA, and found a non-significant link between contaminated water exposure during pregnancy and leukaemia diagnosis, although a significant dose-response relationship was observed for maternal exposure during pregnancy.
These findings underscore the complexities of exposure assessment in water contamination studies. The potential for misclassification and the difficulty in accurately measuring long-term exposure levels pose substantial challenges. Further, the studies often relied on ecological or case-control designs, which have limitations such as recall bias and limited power to detect weak associations.
Chemical exposure and childhood cancer
In addition to waterborne contaminants, industrial chemicals have been a focus of research. García-Pérez et al [29] conducted an ecological study in Spain and found an increased mortality risk from leukaemia in populations near metal industries that released pollutants into the air. This result aligns with earlier studies by Thorpe and Shirmohammadi [32], who reported potential associations between childhood cancers and the presence of pesticides, particularly herbicides like atrazine and simazine, in groundwater. The study highlighted that childhood cancers, particularly leukaemia, may be influenced by a combination of chemical exposures, but also noted that genetic factors and other environmental exposures, such as household pesticide use, could confound the results [32].
Geographic and temporal variability
Several studies have examined the spatial and temporal dimensions of environmental exposure to understand how localised pollution may contribute to CL. Thompson et al [31] found that certain watersheds in Texas were associated with higher risks of specific childhood cancers, including leukaemia, while García-Pérez et al [29] noted a dose-response relationship with proximity to industrial installations. This geographic variability suggests that localised environmental factors, such as the release of toxic substances by industries, may be a key contributor to increased cancer risks (Figure 2).
Furthermore, studies like Ruckart et al [41] and Elliott et al [38] have examined the role of specific chemical contaminants, such as tetrachloroethylene and benzene, in CL. While the evidence is still inconclusive, they emphasize the need for more comprehensive, longitudinal studies that include better exposure assessment methods and consider additional factors such as genetic predisposition and lifestyle.
Molecular interactions
In recent years, exposure to water contaminated with different TM and pesticides, especially organophosphates and organochlorines, has been increasing [42]. However, the consequences derived from their consumption and their possible relationship with the development of CL
have not been explored in depth. Among what has been described, there have been a few environmental factors reported to be linked with the development of different types of cancer. Some examples of these established risk factors are radiation exposure, genetic syndromes, chemotherapy and pesticides (e.g., benzene) (Figure 3). On the other hand, it has been suspected that TM and other types of pesticides interact directly with hematopoietic stem cells, altering their capacity to proliferate and differentiate [43].
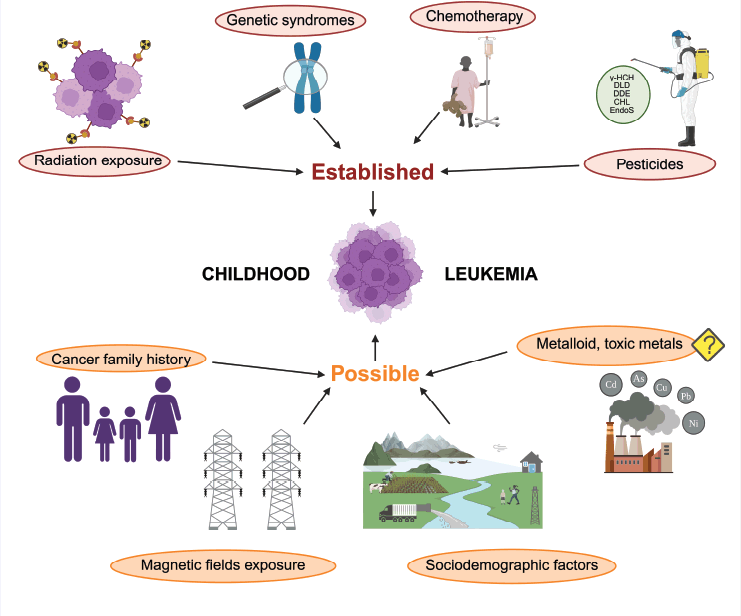
Figure 2. Established and possible risk factors for the development of leukaemia [47–49].
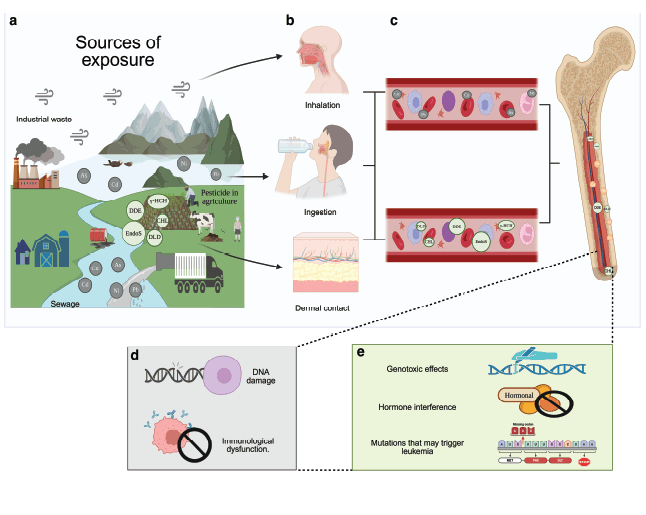
Figure 3. Main contact pathways of contaminated water by TM and pesticides related with CL development. (a) WP with TM and pesticides by sewage, industrial waste and pesticide use in agriculture. (b) The contaminants may enter into the human body through different ways: inhalation, ingestion and/or dermal contact. (c) Circulation of the toxic agent in the vascular system up to the bone marrow and their absorption. (d) Cellular impact of TM in hematopoietic cells potentially involved to the development of CL. (e) Described possible effects of pesticides exposure in the hematopoietic cells related to the development of CL [50, 51].
Also, both pesticides and TM have been related to the alteration of the hematopoietic microenvironment (Figure 4) [44–46]. This alteration is generated by the hyperproduction of reactive oxygen species, producing oxidative stress. This causes damage to cellular DNA, including double-strand breaks and mutations in genes critical for hematopoiesis [44–46].
This microenvironment favours the formation of free radicals, which modify or hinder the function of various enzymes and isomerases involved in DNA replication or DNA repair enzymes, such as PARP-1 and OGG1. In consequence, the accumulation of mutations triggers chromosomal aberrations, among the most common, translocations, resulting in genomic instability [45, 47, 48].
Similarly, epigenetic alterations occur, affecting the cell cycle. First, by modulating DNA methylation and histone acetylation, resulting in a decrease in the expression of tumour suppressor genes and activating oncogenes. Second, by altering cell signaling pathways such as or NF-κB, which directly alter the proliferation and differentiation of hematopoietic precursors or by altering genes such as BRCA1 and TP53, key in tumour suppression [49, 50].
All these mechanisms chronically generate an alarming immunosuppression, since the levels of natural killer and CD8+ T lymphocytes are reduced, thus allowing the proliferation of leukaemic cells [51] (Figure 4).
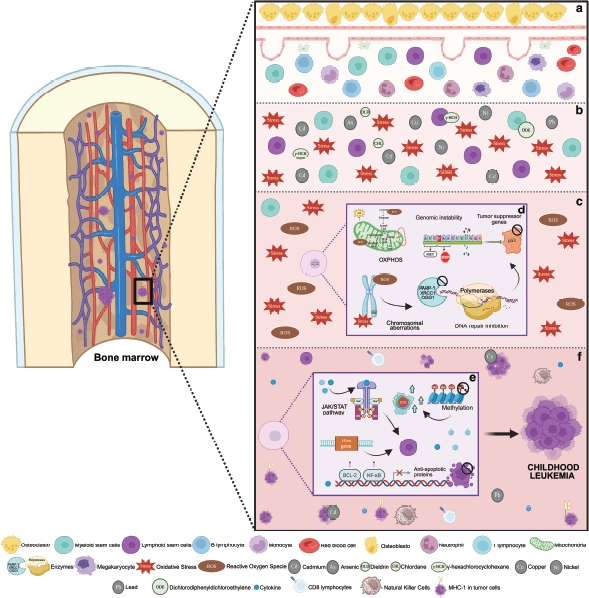
Figure 4. Molecular pathways disrupted by TM and pesticide exposure potentially linked to leukaemia. (a) Normal hematopoiesis. (b) Bone marrow contamination with TM and pesticides generating cellular stress. (c) Alteration of the microenvironment by oxidative stress with the generation of free radicals and reactive oxygen species. (d) DNA damage generating mutations in critical genes (TP53), chromosomal aberrations such as translocations and inhibition of enzymes involved in DNA repair (PARP-1, OGG1, XRCC1 and polymerases). (e) Modification of hematopoietic precursors by alteration of signaling pathways such as JAK/SAT and alteration in HOX gene expression. Interruption of cell apopotosis due to activation of BCL-2 and NF-KB. Global DNA hypomethylation activating oncogenes such as RAS. (f) Bone marrow with leukaemic clones secondary to immunosuppression and evasion of the immune response due to reduced expression of MHC-I in tumour cells [37–39].
Challenges and limitations in environmental exposure research
Despite the vast body of literature on environmental contaminants and CL, many studies, including those by Oller-Arlandis and Sanz-Valero [37] and Cheng et al [33], face significant methodological limitations. These include the reliance on ecological data, small sample sizes and lack of direct exposure measurements. Most studies rely on residential proximity to contamination sources or indirect exposure estimates, which can lead to exposure misclassification. Furthermore, the limited availability of data on individual-level factors like diet, occupation and personal habits makes it difficult to establish clear causal links.
A notable example of these challenges is the study by Ruckart et al [41], which found associations between contaminated DW and childhood cancers but acknowledged the difficulty in controlling for all potential confounding variables, including the lack of detailed information on household water usage and occupational exposures.
Future directions
Future research should focus on more robust study designs, including cohort studies that can better assess individual exposure and account for confounding variables. The use of biomarkers and more precise exposure assessments, including the measurement of specific contaminants over time, could significantly enhance the accuracy of findings. Additionally, the role of genetic factors in modulating the effects of environmental exposures should be explored more thoroughly, as genetic susceptibility may play a crucial role in determining the risks of leukaemia from environmental pollutants.
Longer-term, multi-center studies with diverse populations, particularly in low- and middle-income countries, are needed to strengthen the evidence and address the geographic and temporal variability in exposure patterns. Moreover, integrating environmental monitoring data with clinical cancer registries would provide a more comprehensive understanding of the relationship between environmental exposures and CL.
In conclusion, while evidence of a link between environmental contaminants in DW and CL is accumulating, further studies are needed to clarify the strength of these associations and to overcome the methodological challenges of exposure assessment and confounding factors. The complexity of these relationships requires ongoing investigation and a multidisciplinary approach to understand how environmental factors contribute to childhood cancer risk.
List of abbreviations
ALL – acute lymphoblastic leukaemia, AML – acute myeloid leukaemia, As – arsenic, Cd – cadmium, CL – childhood leukaemia, DeCS – Descriptores en Ciencias de la Salud, IMSS – Instituto Mexicano del Seguro Social, MeSH – Medical Subject Headings, Pb – lead, PRISMA – Preferred Reporting Items for Systematic Reviews and Meta-Analyses, TM – toxic metals, UMAE – Unidad Médica de Alta Especialidad, USA – United States of America, WP – water pollution.
Acknowledgments
Invaluable contributions and dedication of the PRONAII Childhood Leukaemia fieldwork team. Each member plays a vital role in advancing a project. We are grateful to Fundacion IMSS, A.C. for exceptional administrative management of the Leukaemia National Project.
Conflicts of interest
The author(s) declare that they have no conflicts of interest.
Funding
The author(s) declare that financial support was received for the research, authorship and/or publication of this article. This work was supported by grants from the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI), previously known as the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT) FORDECYT-PRONACES 302994 to RP and FORDECYT-PRONACES 303019 to JN-E.
References
1. Morris RD (1995) Drinking water and cancer Environ Health Perspect 103(Suppl 8) 225–231. https://doi.org/10.1289/ehp.95103s8225
2. Lin L, Yang H, and Xu X (2022) Effects of water pollution on human health and disease heterogeneity: a review Front Environ Sci 10 1–16 https://doi.org/10.3389/fenvs.2022.880246
3. Zhao J, Han W, and Guo XB, et al (2024) Spatial association of surface water quality and cancer in the Huaihe river basin Zhongguo Yi Xue Ke Xue Yuan Xue Bao 46 849–861
4. Wang Z, Gu W, and Guo X, et al (2023) Spatial association of surface water quality and human cancer in China NPJ Clean Water 6 1–9 https://doi.org/10.1038/s41545-023-00267-5
5. Picetti R, Deeney M, and Pastorino S, et al (2022) Nitrate and nitrite contamination in drinking water and cancer risk: a systematic review with meta-analysis Environ Res 210 1–22 https://doi.org/10.1016/j.envres.2022.112988 PMID: 35217009
6. Reyes D, Ganesan N, and Boffetta P, et al (2023) Arsenic-contaminated drinking water and cholangiocarcinoma Eur J Cancer Prevention 32 10–17 https://doi.org/10.1097/CEJ.0000000000000740
7. Garcia D and Matthews T (2024) Contaminated drinking water and its effect on cancer ACS EST Water 4 3340–3347 https://doi.org/10.1021/acsestwater.4c00210
8. Brown M (2024) Examples of Different Environmental Hazards and How They Affect Children 2 Climate-Related Hazards Malaria [Internet] [https://ceh.unicef.org/sites/default/files/2025-02/250210_Fragile_Beginnings_Childhood.pdf] Date accessed: 16 May 2025
9. Carroquino MJ, Posada M, and Landrigan PJ (2013) Environmental toxicology: children at risk Environmental Toxicology ed RA Meyers (New York: Springer Science+Business Media) p 239 https://doi.org/10.1007/978-1-4614-5764-0_11
10. Postma J, Butterfield PW, and Odom-Maryon T, et al (2011) Rural children’s exposure to well water contaminants: implications in light of the American Academy of Pediatrics’ recent policy statement J Am Acad Nurse Pract 23 258–265 https://doi.org/10.1111/j.1745-7599.2011.00609.x PMID: 21518074 PMCID: 3190600
11. Pronczuk-Garbino J (2005) Children’s Health and the Environment : A Global Perspective : A Resource Manual for the Health Sector (Geneva: World Health Organization) 367 p
12. Early Childhood Scientific Council on Equity and the Environmen. A Cascade of Impacts: The Many ways water affects child development [Internet]. 2024 [https://developingchild.harvard.edu/wp-content/uploads/2024/06/HCDC-ECSCEE-Water-Apr-25.pdf] Date accessed: 16 May 2025
13. Steliarova-Foucher E, Colombet M, and Ries LAG, et al (2025) International incidence of childhood cancer, volume III (electronic version). IARC Scientific Publication No. 170 (Lyon, France: International Agency for Research on Cancer) [https://iicc.iarc.who.int/results] Date accessed: 02/02/2026
14. Mejía-Aranguré JM (2016) Etiology of acute leukemias in children (Springer International Publishing) p 320
15. Schmidt JA, Hornhardt S, and Erdmann F, et al (2021) risk factors for childhood leukemia: radiation and beyond Front Public Health 9 1–13 https://doi.org/10.3389/fpubh.2021.805757
16. Metayer C, Dahl G, and Wiemels J, et al (2016) Childhood leukemia: a preventable disease Pediatrics 138(Suppl 1) S45–S55 https://doi.org/10.1542/peds.2015-4268H PMID: 27940977 PMCID: 5080868
17. Wiemels J (2012) Perspectives on the causes of childhood leukemia [Internet] Chem Biol Interact Internet 196(3) 59–67 https://doi.org/10.1016/j.cbi.2012.01.007
18. Fagliano J, Berry M, and Bove F, et al (1990) Drinking water contamination and the incidence of leukemia: an ecologic study Am J Public Health 80 1209–1212 https://doi.org/10.2105/AJPH.80.10.1209 PMID: 2400032 PMCID: 1404802
19. Infante-Rivard C, Olson E, and Jacques L, et al (2001) Drinking water contaminants and childhood leukemia Epidemiology 12 13–19 https://doi.org/10.1097/00001648-200101000-00004 PMID: 11138808
20. Souza MCO, Rocha BA, and Adeyemi JA, et al (2022) Legacy and emerging pollutants in Latin America: a critical review of occurrence and levels in environmental and food samples Sci Total Environ 848 1–29 https://doi.org/10.1016/j.scitotenv.2022.157774 PMID: 35932867
21. Galvão L, Câmara V, and Buss D (2020) Environmental health in Latin American countries Oxford Research Encyclopedia of Global Public Health (Oxford: Oxford University Press) https://doi.org/10.1093/acrefore/9780190632366.013.31
22. Costa FCR, Moreira VR, and Guimarães RN, et al (2024) Arsenic in natural waters of Latin-American countries: occurrence, risk assessment, low-cost methods, and technologies for remediation Process Saf Environ Prot 184 116–128 https://doi.org/10.1016/j.psep.2023.11.075
23. Su CC, Lu JL, and Tsai KY, et al (2011) Reduction in arsenic intake from water has different impacts on lung cancer and bladder cancer in an arseniasis endemic area in Taiwan Cancer Causes Control 22 101–108
24. Comba P, Iavarone I, Kogevinas M and Cohen AJ (2020) Contamination of air, water, soil, and food: The challenge is to characterize specific risks World Cancer Report: Cancer Research For Cancer Prevention eds CP Wild, E Weiderpass, and BW Stewart {International Agency for Research on Cancer) pp 115–126
25. Working Group on the Evaluation of Carcinogenic Risks to Humans I (2004) Some drinking-water disinfectants and contaminants, including arsenic IARC Monogr Eval Carcinog Risks Hum 84 1–477
26. Núñez-Enríquez JC, Mejía-Aranguré JM, and Cruz-Muñoz ME, et al (2024) Editorial: childhood leukemias in Latin America: epidemiology, causality, novel predictive profiles and therapeutic strategies Front Oncol 14 1509943 https://doi.org/10.3389/fonc.2024.1509943 PMID: 39624629 PMCID: 11609072
27. Fuller R, Landrigan PJ, and Balakrishnan K, et al (2022) Pollution and health: a progress update Lancet Planet Health 6 e535–e547 https://doi.org/10.1016/S2542-5196(22)00090-0 PMID: 35594895 PMCID: 11995256
28. Moore LE, Lu M, and Smith AH (2002) Childhood cancer incidence and arsenic exposure in drinking water in Nevada [Internet] Arch Environ Health 57(3) 201–206 [https://pubmed.ncbi.nlm.nih.gov/12507173/] https://doi.org/10.1080/00039890209602937
29. García-Pérez J, López-Cima MF, and Boldo E, et al (2010) Leukemia-related mortality in towns lying in the vicinity of metal production and processing installations Environ Int 36(7) 746–753 https://doi.org/10.1016/j.envint.2010.05.010 PMID: 20576291
30. Linos A, Petralias A, and Christophi CA, et al (2011) Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece - An ecological study [Internet] Environ Health Internet 10(1) 50 [https://click.endnote.com/viewer?doi=10.1186%2F1476-069x-10-50&token=WzQwMjAzNTEsIjEwLjExODYvMTQ3Ni0wNjl4LTEwLTUwIl0.0wVV5GUNXXeMKNT8hpz76do8Q7o]
31. Thompson JA, Carozza SE, and Bissett WT, et al (2010) Risks of childhood cancer among Texas watersheds, based on mothers’ living locations at the time of birth [Internet] J Water Health 2025 139–146 [http://iwaponline.com/jwh/article-pdf/8/1/139/397337/139.pdf] https://doi.org/10.2166/wh.2009.018
32. Thorpe N and Shirmohammadi A (2005) Herbicides and nitrates in groundwater of Maryland and childhood cancers: a geographic information systems approach J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 23(2) 261–278 PMID: 16291529
33. Cheng P, Zhang Q, and Shan X, et al (2015) Cancer risks and long-term community-level exposure to pentachlorophenol in contaminated areas, China Environ Sci Pollut Res 22(2) 1309–2171 https://doi.org/10.1007/s11356-014-3469-4
34. Seller RL (2004) Temporal changes in water quality at a childhood leukemia cluster Ground Water 42 446–455 https://doi.org/10.1111/j.1745-6584.2004.tb02692.x
35. Infante-Rivard C, Olson E, Jacques L, Ayotte P. Drinking water contaminants and childhood leukemia. Environ Health Perspect. 2001;109(6):539–544. https://doi.org/10.1097/00001648-200101000-00004
36. Paul S, Chakraborty T, and Halder A, et al (2011) Association of genotoxic effects of arsenic with haematological malignancy in West Bengal Hum & Exp Toxicol 30(2) 165–170 https://doi.org/10.1177/0960327110368694
37. Oller Arlandis V and Javier SV (2012) OK Cáncer por contaminación química del agua de consumo humano en menores de 19 años: una revisión sistemática Rev Panam Salud Publica 32 435–443 https://doi.org/10.1590/S1020-49892012001400007
38. Elliott EG, Trinh P, and Ma X, et al (2017) Unconventional oil and gas development and risk of childhood leukemia: assessing the evidence Sci Total Environ 576 138–147 https://doi.org/10.1016/j.scitotenv.2016.10.072
39. Costas K, Knorr RS, and Condon SK (2002) A case–control study of childhood leukemia in Woburn, Massachusetts: the relationship between leukemia incidence and exposure to public drinking water Sci Total Environ 300(1-3) 23–35 https://doi.org/10.1016/S0048-9697(02)00169-9
40. Danjou A, Patel M, and Espina C, et al (2019) Prospective case-series analysis of haematological malignancies in goldmining areas in South Africa South Afr Med J 109(5) 340–346 https://doi.org/10.7196/SAMJ.2019.v109i5.13538
41. Ruckart PZ, Bove FJ, and Maslia M (2013) Evaluation of exposure to contaminated drinking water and specific birth defects and childhood cancers at Marine Corps Base Camp Lejeune, North Carolina: a case-control study [Internet] Environ Health 12(1) 1–10 https://doi.org/10.1186/1476-069X-12-104
42. Zhang P, Yang M, and Lan J, et al (2023) Water quality degradation due to heavy metal contamination: health impacts and eco-friendly approaches for heavy metal remediation Toxics 11 2023 https://doi.org/10.3390/toxics11100828
43. Scharf P, Broering MF, and Oliveira Da Rocha GH, et al (2020) Cellular and molecular mechanisms of environmental pollutants on hematopoiesis [Internet] Int J Mol Sci Internet 21(19) 6996 https://doi.org/10.3390/ijms21196996
44. Hernández A and Menéndez P (2016) Linking pesticide exposure with pediatric leukemia: potential underlying mechanisms [Internet] Int J Mol Sci 17(4) 1–16 [https://pubmed.ncbi.nlm.nih.gov/27043530/] https://doi.org/10.3390/ijms17040461
45. Wang H, Gan X, and Tang Y (2024) Mechanisms of heavy metal cadmium (Cd)-induced malignancy [Internet] Biol Trace Elem Res 203(2) 608–623 [https://pubmed.ncbi.nlm.nih.gov/38683269/]
46. Joseph P (2009) Mechanisms of cadmium carcinogenesis [Internet] Toxicol Appl Pharmacol Internet 238(3) 272–279 (Accessed May 2025) https://doi.org/10.1016/j.taap.2009.01.011
47. Filipič M (2012) Mechanisms of cadmium induced genomic instability [Internet] Mutation Res - Fundam Mol Mech Mutagenesis 733(1–2) 69–77 [https://pubmed.ncbi.nlm.nih.gov/21945723/]
48. Sebastian R and Raghavan SC (2016) Induction of DNA damage and erroneous repair can explain genomic instability caused by endosulfan [Internet] Carcinogenesis 37(10) 929–940 https://doi.org/10.1093/carcin/bgw081 PMID: 27492056
49. Xu D, Liang D, and Guo Y, et al (2018) Endosulfan causes the alterations of DNA damage response through ATM-p53 signaling pathway in human leukemia cells [Internet] Environ Pollut 238 1048–1055 [https://pubmed.ncbi.nlm.nih.gov/29705383/](Accessed 16 May 2025) https://doi.org/10.1016/j.envpol.2018.03.044 PMID: 29705383
50. Rafeeinia A, Asadikaram G, and Moazed V, et al (2023) Organochlorine pesticides may induce leukemia by methylation of CDKN2B and MGMT promoters and histone modifications [Internet] Gene Internet 851 851 [https://pubmed.ncbi.nlm.nih.gov/36261081/]
51. Zhang Y, Yu X, and Sun S, et al (2016) Cadmium modulates hematopoietic stem and progenitor cells and skews toward myelopoiesis in mice [Internet] Toxicol Appl Pharmacol 313 24–34 https://doi.org/10.1016/j.taap.2016.10.016 PMID: 27771405
52. Seiler RL (2004) Temporal changes in water quality at a childhood leukemia cluster Sci Total Environ 322(1-3) 185–200 https://doi.org/10.1111/j.1745-6584.2004.tb02692.x
53. Ashwood L, Vick K, and Hiett C, et al (2023) Rural and community-based cancer cluster research Environ Justice 16(4) 272–285 https://doi.org/10.1089/env.2021.0118




