Adoptive cell therapy and modulation of the tumour microenvironment: new insights from ASCO 2016
Leila Khoja1,2 and Bishal Gyawali3
1Royal Free Hospital, Pond Street, London NW3 2QG, UK.
2Astrazeneca PLC, Cambridgeshire SG8 6HB, UK
3Department of Haemato-Oncology, Nobel Hospital, Kathmandu 21034, Nepal
Correspondence to: Bishal Gyawali. Email: bg.bishalgyawali@gmail.com
Abstract
Immuno-oncology has changed the landscape of cancer treatment in recent years. Immune checkpoint inhibitors (ICI) have shown survival advantage with long term remissions in a variety of cancers. However, there is another approach to harnessing the power of the immune system in combating cancer: the adoptive cell therapy (ACT) strategy. Although ACT is restricted to small specialized centres and has yet to deliver as much success as ICI, some important results were presented at this year’s ASCO meeting. Important lessons have been learned from these studies, including the prospects and challenges ahead. In this editorial, we summarize the important studies on ACT presented at the ASCO 2016 meeting and discuss the way forward.
Keywords: immunotherapy, adoptive cell therapy, immuno-oncology, chimeric antigen receptor-modified T cells, tumour infiltrating lymphocytes
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 01/09/2016; Received: 19/07/2016
Because of the remarkable success achieved with immune checkpoint inhibitors (ICI), most of the current immune-oncology research is focused on finding the optimal treatment schedules, combination partners and managing toxicities of these agents. However, another important facet of immuno-oncology with huge potential for clinical benefit is the strategy of Adoptive Cell Therapy (ACT). Although both these strategies act via enhancing the body’s immunity against cancer cells, they differ in one important aspect. While the ICI basically unleashes the already present immune response by blocking the immune-inhibitory molecules or their receptors, ACT may be able to make a tumour more immune responsive by harvesting T cells from the patient, expanding them in-vitro and reinfusing them into the host.
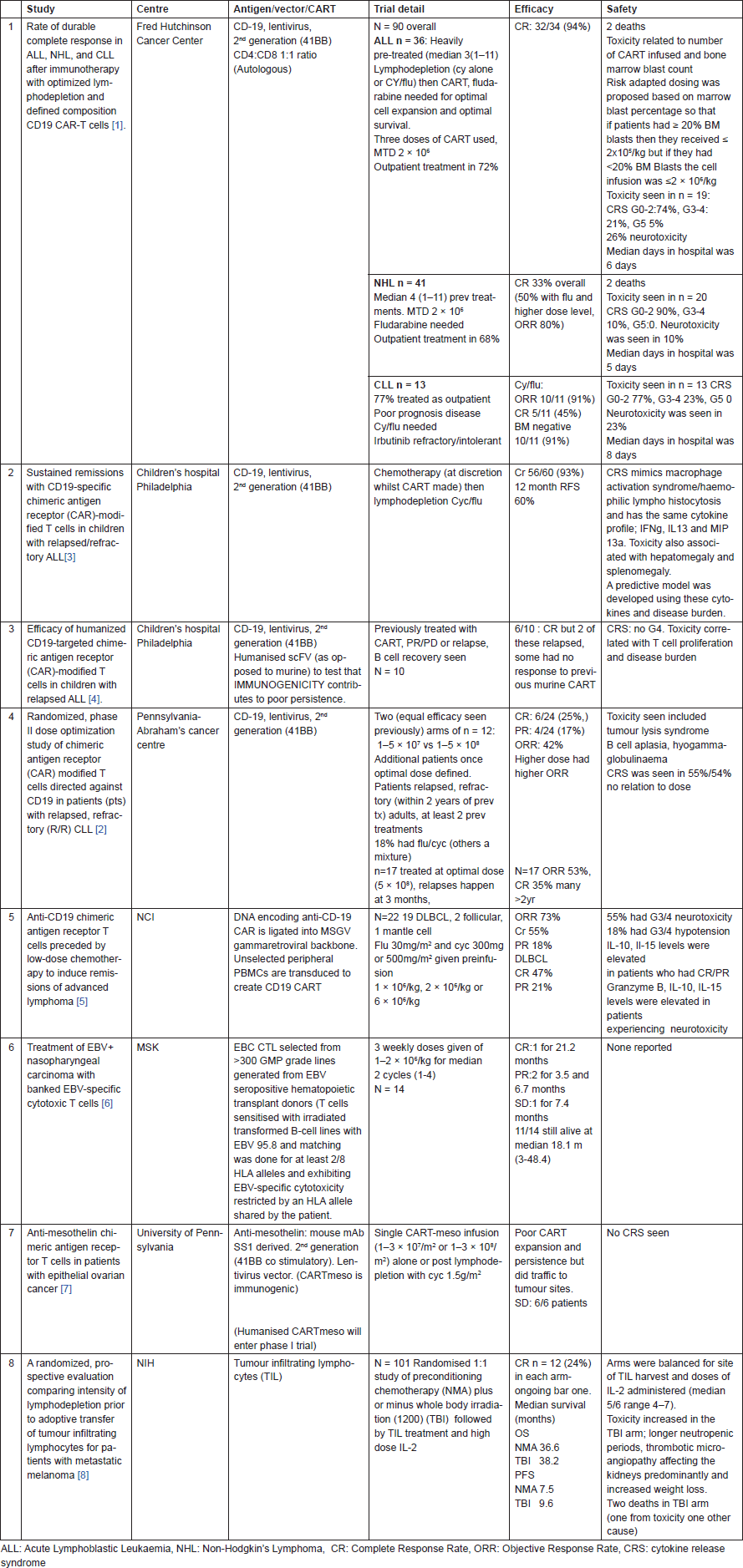
In accordance with the current trend, immunooncology remained the major attraction at the annual meeting of the American Society of Clinical Oncology (ASCO) 2016. This year though, in addition to the numerous abstracts, oral discussions and presentations on ICI in various tumours and in various combinations with small molecules and other antibodies, there were also a number of presentations on ACT. There were 8 oral presentations during the congress, 5 on chimeric antigen receptor-modified T cells (CART) in haematological cancers [1–5], 2 in solid tumours [6–7] and 1 randomized study using tumour-infiltrating lymphocytes (TIL) therapy in melanoma [8] (Table 1).
CART therapy responses in refractory haematological malignancies have been remarkable [9]. All the haematological studies presented at this ASCO meeting also showed impressive complete response (CR) rates in refractory disease. These were early, by day 28, deep and durable with minimal residual disease (negative bone marrows) following treatment. However, relapses could occur post CR and if they did, they seemed to occur within 6 months of treatment. There were other common themes to the studies: preconditioning chemotherapy especially fludarabine was important in getting the T cells to expand and engraft and toxicities could be very severe with treatment-related deaths occurring in at least 2 studies. The most common toxicities were cytokine release syndrome (thought to be the cause of the treatment related deaths) and neurotoxicity (confusion, delirium, aphasia, seizures, all of which completely resolved). The number of T cells infused and cytokine profiles were associated with toxicity as was the disease burden. Toxicities may be mitigated by altering the dose of infused cells depending on tumour burden.
TIL therapy has been trialed primarily in melanoma with some success largely before ICI [10–11]. The TIL therapy trial from the National Institute of Health (NIH) was interesting in that it was a randomized trial between two protocols that NIH has evaluated in several single arm studies [8]. Preconditioning chemotherapy plus or minus total body irradiation (TBI) was given to 101 patients, with 50 and 51 patients in each arm. Responses and outcomes were similar but toxicity increased in the TBI arm with an idiosyncratic side effect of thrombotic microangiopathy leading to the only death in the trial. Overall the NIH has now treated 196 melanoma patients with TIL, 44 CR (23%) have been seen and 42 of these are ongoing 14–140 months post treatment (there is a 62% partial response rate). Single digit numbers of patients had had ICI previously and they seemed to do worse but it is anecdotal.
In solid tumours the outcomes were much less impressive but one approach in particular was interesting. An EBV sensitized cytotoxic T cell was selected from a panel of over 300 GMP grade lines generated when EBV seropositive haematopoietic donor T cells were sensitized with irradiated transformed B-cell lines with EBV 95.8 and matching was done for at least 2/8 HLA alleles and exhibiting EBV-specific cytotoxicity restricted by an HLA allele shared by the patient [6]. To date, ACT has always been autologous making it labor intensive and expensive. Creating an off- the-shelf product would significantly simplify the process and possibly increase the number of eligible patients.
Finally other engineered cells are also being evaluated. A phase Ib/II trial of expanded and activated autologous natural killer (NK) cells with trastuzumab in refractory HER2 metastatic breast cancer (MBC) [12] poster presentation described a trial designed to increase the antibody-dependent cell-mediated cytotoxicity effect of trastuzumab. Cells were harvested from patients and incubated with irradiated k562-mb15-41BBl cells for 10 days. Eleven patients were treated with trastuzumab (3 weekly up to 8 cycles). Cycle 1 was followed by a NK cell infusion and IL2 1MU/m2 x 3 weekly for 6 doses. No responses were seen but 7 of 10 patients had stable disease and 3 patients had a second infusion at Cycle 6 and 2 patients had a third infusion at Cycle 8. NK cells did expand with increased ADCC but these expansions were modest.
Immunophenotyping of cells in tumours may ultimately help in identifying patients that can benefit from immunotherapies. Immunoscore in colorectal cancer [13] has been validated now and there were numerous presentations on biomarkers of response to ICI. Conversely there is also a need for biomarkers of resistance to identify patients who simply do not respond to immunotherapy. The recently proposed ‘Cancer Immunogram’ could serve as a framework to think of effective immunotherapy on an individualized basis [14]. One requirement in this immunogram is immune (T-cell) infiltration. It could therefore be proposed that so called immunologically cold tumours are suitable for ACT.
Table 1. Important studies of ACT presented at ASCO 2016.
This was a topic for discussion in an educational session on CART in sarcoma by Seth Pollack of the Fred Hutchinson Centre. Synovial and myxoid round cell sarcomas are homogenous for NYESO positivity (a cancer testes antigen) but apart from some early data on TIL in synovial sarcoma from NIH, immunotherapy (including ICI) has failed to impress. Biopsies were done in patients at baseline and during treatment. These tumours are described as immunologically cold tumours: very few T cells are seen to infiltrate the tumour and these have low PD1 staining in addition to low PDL1 staining in the tumour. Large numbers of CD168 (M2 macrophages) are seen but MHC I and II expression is low. A trial was started of IFNg and preconditioning chemotherapy followed by an engineered T cell to NYESO infusion and low dose Il2. There was 1 partial response but also 1 death from myositis thought to be cyclophosphamide related and the study has halted. The concept of modulating the microenvironment to be more immunogenic is also being tested with the use of the gp100 TCR construct produced by immunocore [15]. The antibody to IMCgp100 is coupled to a TCR thus attracting the T cells into the tumour once they bind to tumour cells. A three arm study is underway; alone, in combination with durvulumab (PDL1) or in combination with combination durvulumab and tremelimumab (NCT02535078).
So where does ACT fit in the current landscape of cancer treatment? In haematological disease it looks as if it has a place particularly in refractory disease and especially if the toxicity can be better mitigated. It is conceivable that if toxicity is improved upon it may find a place earlier in treatment paradigms. In solid tumours the responses are poor so that combinations possibly with ICI might be an area to explore. Alternately engineered T cells constructed so as to release stimulatory cytokines such as IL-12 or not express PD-1 could have greater efficacy. TIL therapy at least in melanoma looks more promising and will be a continued area for research possibly in combination with other agents like anti-angiogenics or ICI to increase efficacy further. One drawback of all these ACT approaches, toxicity aside, is the autologous nature of the constructs. T-cells must be harvested and then reinfused post expansion. Treatment with ACT only takes place in specialized centres although the number of such centres worldwide is increasing. Commercialization of this therapy will require an off-the-shelf product that can be used in a wide population of patients. New molecular techniques such as CRISPR/Cas 9 should facilitate this and indeed the first trial in humans is planned for the end of the year [16].
The way forward
Single agent ICIs, especially anit-PD-1 antibodies, carry relatively low toxicity profiles when compared to chemotherapy. The desire to build upon single agent activity has seen a multitude of different combination strategies explored in clinical trials. However, even with combination immunotherapies, nearly half of the patients do not respond. Without validated predictive biomarkers to select patients for either single agent or a specific combination it will be challenging to determine which patients respond to particular therapeutic strategies or indeed if all patients will respond to immunotherapy given the right combination. Furthermore, the combinatorial approach with at least 2 agents will increase toxicity compromising the applicability to wide patient populations.
ACT can cause life threatening toxicities but reported efficacies, particularly in refractive haematological malignancies, is impressive. Thus, reducing the toxicities of ACT should be the prime focus of future research. Without mitigating the associated toxicities, ACT cannot gain wider application notwithstanding survival results in phase 3 trials. In solid tumours, the lack of a predictive biomarker further hinders the wider use of this therapy although immunologically cold tumours may be particularly suited to the ACT approach and could be hypothesized to be resistant to ICI therapy. Response to ACT post ICI is unknown at the current time as the majority of available ACT data is from patients pre-ICI development.
Conclusion
Although significant challenges lie ahead especially in solid tumours, ACT might find its place in the treatment paradigms of a variety of tumours if and when the two major problems associated with ACT are mitigated: the logistics in administering the ACT therapy and the seriousness of the toxicities.
Conflicts of Interest
Dr Khoja works as a research physician for Astrazeneca but this work is independent of Astrazeneca. Dr Gyawali has no conflicts of interest to declare.
References
1. Turtle CJ et al (2016) Rate of durable complete response in ALL, NHL, and CLL after immunotherapy with optimized lymphodepletion and defined composition CD19 CAR-T cells ASCO Meeting Abstracts 34(15_suppl) 102
2. Porter DL et al (2016) Randomized, phase II dose optimization study of chimeric antigen receptor (CAR) modified T cells directed against CD19 in patients (pts) with relapsed, refractory (R/R) CLL ASCO Meeting Abstracts 34(15_suppl) 3009
3. Maude SL et al (2016) Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL ASCO Meeting Abstracts 34(15_suppl) 3011
4. Maude SL et al (2016) Efficacy of humanized CD19-targeted chimeric antigen receptor (CAR)-modified T cells in children with relapsed ALL ASCO Meeting Abstracts 34(15_suppl) 3007
5. Kochenderfer J et al (2016) Anti-CD19 chimeric antigen receptor T cells preceded by low-dose chemotherapy to induce remissions of advanced lymphoma ASCO Meeting Abstracts 34(15_suppl) LBA3010.
6. Prockop S et al (2016) Treatment of EBV nasopharyngeal carcinoma with banked EBV-specific cytotoxic T cells ASCO Meeting Abstracts 34(15_suppl) 3012
7. Tanyi JL et al (2016) Anti-mesothelin chimeric antigen receptor T cells in patients with epithelial ovarian cancer ASCO Meeting Abstracts 34(15_suppl) 5511
8. Goff SL et al (2016) A randomized, prospective evaluation comparing intensity of lymphodepletion prior to adoptive transfer of tumour infiltrating lymphocytes for patients with metastatic melanoma ASCO Meeting Abstracts 34(15 suppl) 3006
9. Dai HY et al (2016) Chimeric antigen receptors modified T-Cells for cancer therapy J Natl Cancer Inst 108(7)
10. Dudley ME et al (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens J Clin Oncol 26(32) 5233–9
11. Rosenberg SA et al (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy Clin Cancer Res 17(13) 4550–7
12. Lee SC et al (2016) Phase Ib/II trial of expanded and activated autologous natural killer (NK) cells with trastuzumab (Tras) in refractory HER2 metastatic breast cancer (MBC) ASCO Meeting Abstracts 34(15_suppl) 3045
13. Galon J et al (2016) Validation of the Immunoscore (IM) as a prognostic marker in stage I/II/III colon cancer: Results of a worldwide consortium-based analysis of 1,336 patients ASCO Meeting Abstracts 34(15_suppl) 3500
14. Blank CU et al (2016) CANCER IMMUNOLOGY. The “cancer immunogram” Science 352(6286) 658–60
15. Middleton MR et al (2016) Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific t cell redirector with solid tumour activity: results from the FIH study in melanoma ASCO Meeting Abstracts 34(15_suppl) 3016
16. https://www.statnews.com/2016/06/21/crispr-human-trials/ STAT reporting from the frontiers of health and medicine, 2016

