Prevalence of emotional symptoms in Chilean oncology patients before the start of chemotherapy: potential of the distress thermometer as an ultra-brief screening instrument
Jorge Calderón1, Cristóbal Campla1, Nicole D’Aguzan1, Soledad Barraza2, Oslando Padilla3, Cesar Sánchez2, Silvia Palma2 and Matías González1
1Psychiatry Department, Pontificia Universidad Católica de Chile, Santiago 8330024, Chile
2Cancer Center, Pontificia Universidad Católica de Chile, Santiago 8330024, Chile
3Department of Public Health, Pontificia Universidad Católica de Chile, Santiago 8330024, Chile
Correspondence to: Jorge Calderón. Email: jcalder@med.puc.cl
Abstract
Emotional distress (ED) is greater for oncology patients in comparison with the general population, and this has implications for the quality of life of the patient and his/her family, adherence to the treatment, and eventually, survivorship. In general, the detection of these symptoms is low, which explains the need for detection systems appropriate to the clinical reality of the oncology team. The objective of this study is to evaluate for the first time the usefulness of an ultra-brief screening instrument [distress thermometer (DT)], in a group of Chilean oncology patients. A total of 166 outpatients were evaluated at the Cancer Center of the Pontificia Universidad Católica de Chile, before starting chemotherapy. Two screening instruments were applied: Hospital Anxiety and Depression Scale (HADS) and DT. The application of HADS resulted in a prevalence of 32.7% of anxiety symptoms (HADS-A ≥ 8), 15.7% of depression symptoms (HADS-D ≥ 8), and 39.8% had a total score of HADS-T ≥ 11. The DT resulted in the prevalence of 32.5% of distress or ED (DT ≥ 5). The validity of the DT was evaluated as a screening tool in comparison with HADS, observing, in relation to the anxiety scale (HADS-A), a sensitivity of 88.9% and specificity of 78.4% (DT ≥ 4); depression (HADS-D), a sensitivity of 69.2% and specificity of 74.3% (DT ≥ 5); and in relation to the total scale (HADS-T), a sensitivity of 68.2% and specificity of 73.0% (DT ≥ 4). This study demonstrates the elevated prevalence of emotional symptoms in Chilean oncology patients, before the start of chemotherapy, and confirms the potential of the DT as a brief screening instrument with easy application. The DT will allow the clinician to increase the detection threshold in the Chilean oncology population, intervene in a timely manner, and contribute to the comprehensive handling of the oncology patient without affecting the time needed for assistance.
Keywords: cancer, emotional stress, depression, screening
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In 2012, around 14.1 million new cancer cases were diagnosed worldwide, reaching a mortality rate of around 8.2 million. The largest cancer incidence around the world was of lung cancer, with 1.8 million new cases, followed by breast cancer, with 1.67 million [1].
In Chile, cancer is the second cause of death after cardiovascular illnesses, causing in 2005 24.8% of total deaths and reaching, in 2008 a figure of 21,824 deaths, with a rate of 130 deaths for every 100,000 inhabitants [2].
The prevalence of emotional distress (ED) in oncology patients is of about 35% in the course of the illness [3, 4], and it is greater for young people, depending on the location of the tumour (greater risk for cerebral tumours) and the oncology illness of worst prognosis, with a greater risk for patients with a survival prognosis of less than one year [5].
Psychiatric co-morbidity in cancer patients increases the number of days of hospitalisation, the demand for medical attention, and the risk of suicide [6, 7]; it delays adaptation to the cancer diagnosis for at least a month, and it is associated with lower adherence to anti-neoplastic treatments [8]. Depression symptoms increase sensitivity to pain and affect the rank and intensity of the side effects of the treatment, with a negative impact on the physical well-being and the social functioning of the patient [9].
As for the neoplastic illness prognosis, a meta-analysis of prospective studies found a statistically significant, although small, connection between depression symptoms and mortality, with an unadjusted relative risk of 1.25 (IC 95% 1.12–1.40; p < 0.001) increasing to 1.39 when given a major depressive disorder diagnosis (IC 95% 1.10–1.89; p = 0.03) [10].
In Chile, there is no specific record of psychiatric morbidity in oncology patients, and standardised instruments to study these disorders in said population have not been evaluated. The use of the Hospital Anxiety and Depression Scale (HADS) in a study of patients with chronic pathologies in an advanced stage (of which 77.6% were oncology patients) reported anxiety and depression symptoms in 51.1% and 27.9% of the patients, respectively (cut-off point ≥ 8). Considering only the ‘clinically relevant’ cases (cut-off point ≥ 11) in both sub-scales, the frequency was of 30.2% and 11.6%, respectively [11].
There are several instruments used to evaluate the psychosocial health of people with cancer, and although these instruments have been used mainly in research, there is a growing interest in incorporating them into clinical practice as part of the standardised evaluation. Amongst these are highlighted the HADS, Psychological Distress Inventory, Brief Symptom Inventory, and others. Although many patients who complain of emotional problems do not meet the criteria for major depressive disorder according to the DSM-IV, the presence of ED affects the experience of the patient and his/her family. This has led to the development of the concept of distress (hereafter, ED), which has even been suggested to be the ‘sixth vital sign’ and which has allowed for the formulation of ultra-brief screening tools such as the distress thermometer (DT) [9].
The aim of this study is to measure the prevalence of depression and anxiety symptoms in an outpatient group of oncology patients in a university clinical hospital in Chile, before the start of chemotherapy, and to show the usefulness and diagnostic precision of the DT in comparison with the HADS, as an ultra-brief screening tool to detect the ED in this sample. This will allow for the establishment of the psychometric properties of the DT in a group of Chilean patients and the comparison with the properties in other populations.
Materials and methods
The study included a total of 166 consecutive patients from the Cancer Center of the Pontificia Universidad Católica de Chile. The evaluation took place immediately after the introduction talk, prior to the start of chemotherapy. The study included patients with different cancer types who will undergo chemotherapy.
The HADS was initially designed to evaluate the psychological status of patients who are physically ill, and it was used to determine the presence of depression and anxiety symptoms [12]. It has been widely accepted as an effective tool in the study of anxiety and depression symptoms in oncology patients [13–15], and it is validated in Spanish [16]. It consists of 14 questions, divided into seven questions that identify anxiety symptoms (HADS-A) and seven questions that identify depression symptoms (HADS-D). Each answer gets between 0 and 3 points, for a total of 21 points for each sub-scale and 42 for the whole scale. The greater the score, the greater the degree of anxiety or depression. In the original report, the cut-off point was 8 for suspicious cases and 11 for definitive cases, both for anxiety (HADS-A) and depression (HADS-D) scales. Our study used a value of ≥ 8 as a cut-off point in the depression and anxiety sub-scales and a value of ≥ 11 in the total scale.
The second tool used was the DT. To study ED in oncology patients in a quick, simple, and non-stigmatising manner, Roth et al. [17] designed the DT. Similar to the analogue visual pain evaluation scale, the patient is asked questions related to his/her ED grade in the last week on a scale of 0–10. This scale has been incorporated in the clinical practice guides for ED management of the National Comprehensive Cancer Network (NCCN) [18]. The NCCN later developed the ‘list of problems’ (LP), consisting of 34 problems, commonly experimented by oncology patients, grouped in five categories: practical, physical, family related, emotional, and spiritual. Initially, the NCCN recommended a cut-off point of 5 in the DT to determine a significant ED, which requires referral to the appropriate service. The DT together with the LP have been proven to be effective screening tools for detecting ED in patients with different types of cancer [19–21].
Statistical analysis
Measurements for age and the different scales were calculated. The prevalence of ED amongst men and women or according to the type of cancer diagnosis was compared using the chi-square test, or the Fisher exact probability test. Receiver operating characteristic (ROC) curve analysis was performed. The level of statistical significance was set at 0.05. The analyses were made using the program SPSS Statistics for Windows, Version 17.0 (Chicago, 2008, SPSS Inc.).
Results
A total of 166 patients were included: 109 women (65.6%) and 57 men (34.5%) between 16 and 79 years of age. The most frequent diagnoses were breast cancer (27.9%) and colon and rectum cancer (19.9%). Of the total, 78 (47.3%) patients presented curable neoplasms, 67 (40.6%) incurable, and in 21 cases (12.7%), the prognosis was uncertain (Table 1).
Table 1. Patient characteristics, type of cancer, and prognosis.

Prevalence of anxiety, depression, and ED symptoms in oncology patients
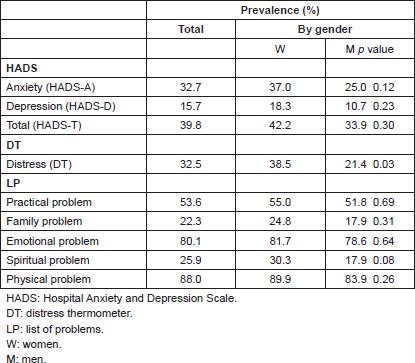
There was a prevalence of anxiety symptoms (anxiety, hereafter) of 32.7% (HADS-A ≥ 8), of 15.7% for symptoms of depression (depression, hereafter; HADS-D ≥ 8) and 39.8% for the total score (HADS-T ≥ 11). The average score for HADS-A was 6.42, with a standard deviation of 3.87: in HADS-D, it was 3.69, with a standard deviation of 3.34, and in HADS-T, it was 10.34, with a standard deviation 6.57. The DT resulted in a prevalence of ED of 32.5% (DT ≥ 5), with an average score of 3.52 and a standard deviation of 2.57. The LP identified 53.6% of patients who admit having practical problems, 22.3% family problems, 80.1% emotional problems, 25.9% spiritual problems, and 88% physical problems (Table 2).
With respect to gender differences, these were not significant for anxiety (p = 0.12), depression (p = 0.23), and HADS-T (p = 0.30; Table 2). Neither were there significant gender differences in the problems reported, where only the spiritual problem report showed a tendency to statistical significance, with a greater amount reported in women (p = 0.08).
With respect to the ED measured by the DT, women showed a prevalence of 38.5% (DT ≥ 5), considerably greater than the 21.4% of men (p = 0.03; Table 2).
With respect to cancer type, no significant differences were found between breast cancer and the rest of the neoplasms, both in anxiety prevalence (p = 0.31), depression (p = 0.84), HADS-T (p = 0.83), and ED (p = 0.95). The same analyses took place for colorectal cancer, where considerable differences were also not found in comparison with the rest of the neoplasms both for anxiety (p = 0.20), depression (p = 0.63), HADS total (p = 0.34), and ED (p = 0.15). The analysis of these diagnoses was favoured, as they had the most prevalence. There were no significant differences in these measurements between patients with curable and incurable cancer.
Table 2. Prevalence of anxiety, depression, and ED symptoms and problems.
Determination of cut-off points, sensitivity, and specificity of the DT with relation to HADS
The validity of the DT was evaluated as a screening tool in comparison with HADS, observing, in relation to the anxiety scale (HADS-A), a sensitivity of 88.9% and specificity of 78.4%; area under the curve (AUC) 0.89 (DT ≥ 4); depression (HADS-D), a sensitivity of 69.2% and specificity of 74.3% AUC 0.76 (DT ≥ 5); and in relation to the HADS-T, a sensitivity of 68.2% and specificity of 73.0% AUC 0.77 (DT ≥ 4) (Table 3; Figures 1–3)
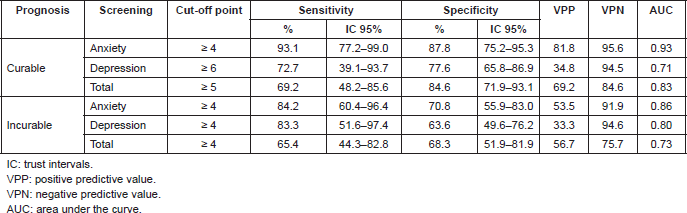
When evaluating the converging validity of the DT as a screening tool, the sensitivity and specificity of different cut-off points of the DT in relation to the HADS through the AUC curve analysis for two categories depending to the prognosis (curable or incurable) were considered. The AUC was used to calculate the precision of the cut-off points, with a range of 1 (perfect precision) to 0.5 (poor precision).
In patients with a curable prognosis, a cut-off point of DT ≥ 4 was identified for anxiety risk, a value that presented an AUC of 0.93 (IC 95% 0.86–0.98, p < 0.0001), sensitivity of 93.1% (IC 95% 77.2–99.0), and specificity of 87.8% (IC 95% 75.2–95.3), with a positive predictive value (VPP) of 81.8 and a negative predictive value (VPN) of 95.6 (Table 4). In patients with an incurable prognosis, the cut-off point of DT for anxiety risk was also ≥ 4, with AUC of 0.86 (IC 95% 0.8–0.9, p < 0.0001), sensitivity of 84.2% (IC 95% 60.4–96.4) and specificity of 70.8% (IC 95% 55.9–83.0), VPP of 53.5, and VPN of 91.9.
When evaluating the risk of depression in patients with a curable prognosis, the cut-off point of the DT was ≥ 6, with AUC of 0.71 (IC 95% 0.6–0.8, p < 0.0262), sensitivity of 72.7% (IC 95% 39.1–93.7) and specificity of 77.6% (IC 95% 65.8–86.9), VPP of 34.8, and VPN of 94.5 (Table 4). In patients with an incurable prognosis, the cut-off point was of ≥ 4, with AUC of 0.8 (IC 95% 0.69–0.89, p < 0.0001), sensitivity of 83.3% (IC 95% 51.6–97.4) and specificity of 63.6% (IC 95% 49.6–72.7), VPP of 33.3, and VPN of 94.6.
In relation to the total scale (TADS-T), the cut-off point established for the DT in patients with a curable prognosis was > 4, with AUC of 0.83 (IC 95% 0.7–0.9, p < 0.0001), sensitivity of 69.2% (IC 95% 48.2–85.6) and specificity of 84.6% (IC 95% 71.9–93.1), VPP of 69.2, and VPN of 84.6. In patients with an incurable prognosis, the cut-off point was of ≥ 4, with AUC of 0.73 (IC 95% 0.6–0.8), sensitivity of 65.4% (IC 95% 44.3–82.8) and specificity of 68.3% (IC 95% 51.9–81.9), VPP of 56.7, and VPN of 75.7.
Table 3. Sensitivity and specificity of the DT with relation to HADS.
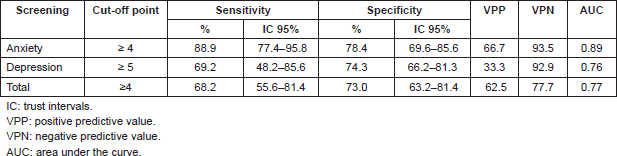
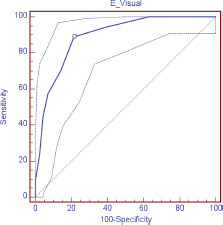
Figure 1. ROC curve visual scale (DT) for anxiety.
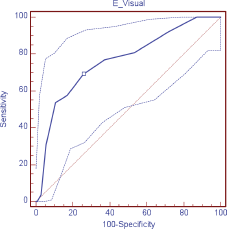
Figure 2. ROC curve visual scale (DT) for depression.
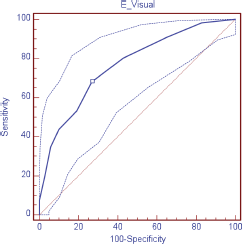
Figure 3. ROC curve visual scale (DT) for total HADS.
Table 4. Sensitivity and specificity of the DT with relation to HADS, according to the prognosis.
Discussion
Ultra-brief screening instruments such as DT, although they do not have the ability on their own to diagnose depression or anxiety disorders, are useful to detect, at an early stage, the patients who are more prone to developing these disorders [22]. In Chile, there are no studies that evaluate screening methods for ED, which is why this study contributes to an advancement on this objective.
The DT has been used in several countries and languages, showing adequate psychometric properties [23, 24]. The results of our study are similar to those of the reported studies. In a recent systematic review of screening instruments for ED [25], the cut-off point to identify ED in a clinically relevant manner was of 4 or 5, depending on the validation measures used. The sensitivity and specificity were lower than 80% in half and the two-thirds of the validation studies, respectively. The higher levels of sensitivity contrasted with the moderate or low levels of specificity. Complementary studies suggest that modifications to the DT, such as the mood DT and the impact DT, may represent an advancement in relation to the original scale.
Our study reported an ED prevalence of 32.5% (DT ≥ 5), similar to what was detected in large sample studies [3, 4].
In spite of the prevalence of ED in oncology patients, there are few studies that have evaluated the research methods used by health-care professionals. Lawrie et al. [26] surveyed 134 physicians who worked in palliative care; 73% reported having routinely evaluated depression symptoms in their patients, of which only 50% used standardised instruments, 10% used one single question (‘are you depressed?’), and 27% used the HADS. Physicians show a tendency to underestimate depression symptoms when these are more severe and seem to be more influenced by symptoms such as crying and a depressive mood than by more specific symptoms such as anhedonia, suicidal thoughts, despair, and feelings of guilt [27].
In this respect, it shows the efficiency of the DT as an research tool for detecting ED in Chilean oncology patients who are about to begin chemotherapy, their best performance is in screening anxiety symptoms, with a cut-off point of ≥ 4, obtaining a sensitivity of 93.1% and a specificity of 87.8% in relation to HADS. The lower cut-off points for the DT do not provide any comparative advantages neither for anxiety nor for depression, decreasing considerably the specificity of the instrument.
The LP complements the results of the DT by determining which is the area of greater concern for the patient. In this study, the main problems noted by patients were of physical nature (88.0%), followed by emotional (80.1%) and practical (53.6%).
The advantage of this instrument lies in the possibility of a brief standardised evaluation that can be completed by the patient without support from the health personnel, providing a quick result that increases the detection threshold and facilitates timely referral without affecting the time needed for attention.
Our work has some limitations. The number of patients was relatively low, which is why the results are not easy to generalise. Different cancer types of oncology pathologies were considered, which does not allow for a more appropriate evaluation of the differences in ED related to the location or type of cancer. In our study, the most prevalent diagnoses, such as breast and colon cancer, did not show considerable differences with those of other locations, with regard to ED. The instruments were used the moment before the start of chemotherapy, which is why it is not possible to compare them with other moments during treatment. The prevalence of anxiety, depression, and ED was not considerably different in patients whose pathology is incurable versus curable. This is an interesting finding that will have to be analysed in greater detail in a future study. It is possible that the moment of measuring, before the start of chemotherapy, generates anxiety and fear at the same time, and provides the patient with the hope for partial or definitive improvement.
Conclusion
It is possible to offer oncology patients with considerable ED the option for a timely referral and effective treatment. This is reinforced by the increase in publications that refer to a considerable reduction in anxiety and depression symptoms for high-risk patients who are given psycho-oncological interventions aimed at maintaining their autonomy, providing defence mechanisms, strengthening hope and confidence, and ensuring good communication with the health-care team [28]. These results extended to even a year after the intervention and corroborate the importance of timely screening of ED and support in the initial stages.
For the first time, a study has been carried out that evaluated DT in the Chilean population. The instrument shows a behaviour similar to that of its application in other populations, and is established as an efficient initial screening tool for Chilean patients.
References
1. WHO (2012). International Agency for Research on Cancer Home Available from: http://globocan.iarc.fr/Default.aspx
2. Ministerio de Salud. Gobierno de Chile. Dirección de Estadísticas e Información de Salud (DEIS) Available from: http://www.deis.cl/
3. Zabora J et al (2001) The prevalence of psychological distress by cancer site Psychooncology 10 19–28 PMID: 11180574
4. Carlson LE et al (2004) High levels of untreated distress and fatigue in cancer patients Br J Cancer 90 2297–304 PMID: 15162149 PMCID: 2410292
5. Hinz A et al (2010) Anxiety and depression in cancer patients compared with the general population Eur J Cancer Care (Engl) 19 522–9 DOI: 10.1111/j.1365-2354.2009.01088.x
6. Labisi O (2006) Assessing for suicide risk in depressed geriatric cancer patients J Psychosoc Oncol 24 43–50 DOI: 10.1300/J077v24n01_04 PMID: 16803751
7. Levenson JL, Hamer RM and Rossiter LF (1990) Relation of psychopathology in general medical inpatients to use and cost of services Am J Psychiatry 147 1498–503 PMID: 2121054
8. Colleoni M et al (2000) Depression and degree of acceptance of adjuvant cytotoxic drugs Lancet 356 1326–7 DOI: 10.1016/S0140-6736(00)02821-X PMID: 11073026
9. Holland JC and Bultz BD (2007) The NCCN guideline for distress management: a case for making distress the sixth vital sign J Natl Compr Canc Netw 5 3–7 PMID: 17323529
10. Satin JR, Linden W and Phillips MJ (2009) Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis Cancer 115 5349–61 DOI: 10.1002/cncr.24561 PMID: 19753617
11. Palma A et al (2008) Frequency and assessment of symptoms in hospitalized patient with advanced chronic diseases: is there concordance among patients and doctors? Rev Med Chil 136 561–9 PMID: 18769802
12. Zigmond A and Snaith R (1983) The hospital anxiety and depression scale Acta Psychiatr Scand 67 361–70 DOI: 10.1111/j.1600-0447.1983.tb09716.x PMID: 6880820
13. Razavi D et al (1990) Screening for adjustment disorders and major depressive disorders in cancer in-patients Br J Psychiatry 156 79–83 DOI: 10.1192/bjp.156.1.79 PMID: 2297623
14. Carroll BT et al (1993) Screening for depression and anxiety in cancer patients using the hospital anxiety and depression scale Gen Hosp Psychiatry 15 69–74 DOI: 10.1016/0163-8343(93)90099-A PMID: 8472942
15. Osborne RH et al (2004) The value of the hospital anxiety and depression scale (HADS) for comparing women with early onset breast cancer with population-based reference women Qual Life Res 13 191–206 DOI: 10.1023/B:QURE.0000015292.56268.e7 PMID: 15058800
16. Herrero MJ et al (2003) A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population Gen Hosp Psychiatry 25 277–83 DOI: 10.1016/S0163-8343(03)00043-4 PMID: 12850660
17. Roth AJ et al (1998) Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study Cancer 82 1904–8 PMID: 9587123
18. NCCN Clinical Practice Guidelines in Oncology Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
19. Trask PC et al (2002) Assessment of psychological distress in prospective bone marrow transplant patients Bone Marrow Transplant 29 917–25 DOI: 10.1038/sj.bmt.1703557 PMID: 12080358
20. Graves KD et al (2007) Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress Lung Cancer 55 215–24 DOI: 10.1016/j.lungcan.2006.10.001 PMCID: 1857305
21. Hegel MT et al (2006) Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer Cancer 107 2924–31 DOI: 10.1002/cncr.22335 PMID: 17103381
22. Mitchell AJ (2007) Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders J Clin Oncol 25 4670–81 DOI: 10.1200/JCO.2006.10.0438 PMID: 17846453
23. Martínez P et al (2013) The distress thermometer in Spanish cancer patients: convergent validity and diagnostic accuracy Support Care Cancer 21 3095–102 DOI: 10.1007/s00520-013-1883-7 PMID: 23832312
24. Bidstrup PE et al (2012) Accuracy of the Danish version of the “distress thermometer” Psychooncology 21 436–43 DOI: 10.1002/pon.1917
25. Vodermaier A, Linden W and Siu C (2009) Screening for emotional distress in cancer patients: a systematic review of assessment instruments J Natl Cancer Inst 101 1464–88 DOI: 10.1093/jnci/djp336 PMID: 19826136 PMCID: 3298956
26. Lawrie I, Lloyd-Williams M and Taylor F (2004) How do palliative medicine physicians assess and manage depression Palliat Med 18 234–8 DOI: 10.1191/0269216304pm865oa PMID: 15198136
27. Passik SD et al (1998) Oncologists’ recognition of depression in their patients with cancer J Clin Oncol 16 1594–600 PMID: 9552071
28. Goerling U et al (2011) The impact of short-term psycho-oncological interventions on the psychological outcome of cancer patients of a surgical-oncology department - a randomised controlled study Eur J Cancer 47 2009–14 DOI: 10.1016/j.ejca.2011.04.031 PMID: 21612912






