Evolving treatment strategies for EGFRex20ins-mutated NSCLC: a comprehensive review of Amivantamab’s role and future directions
Rafael Alvim Pereira1, Milena Tumelero2, Wallace Klein Schwengber3 and Gabriel Lenz4
1Department of Medicine, Hospital Santa Casa, São José dos Campos, SP 12210-110, Brazil
2Department of Medicine, UNOESC University, Joaçaba, SC 89600-000, Brazil
3Mayo Clinic Cancer Center, Phoenix, AZ 85050, USA
4Internal Medicine, AdventHealth, Orlando, FL 32804, USA
Abstract
Epidermal growth factor receptor exon 20 insertion mutations (EGFRex20ins) are a unique molecular subtype of non-small cell lung cancer (NSCLC) linked to resistance to EGFR tyrosine kinase inhibitors of the first and second generations. Until recently, these patients had few and frequently ineffective treatment options. Amivantamab, a bispecific antibody targeting both EGFR and mesenchymal–epithelial transition factor, has become a novel therapeutic strategy for this population. This review explores the mechanism of action of Amivantamab and its clinical efficacy and safety as demonstrated in clinical trials. Additionally, the clinical development of the subcutaneous formulation of amivantamab, real-world evidence and its regulatory status were evaluated. Lastly, we contextualise Amivantamab in the current treatment landscape by contrasting it with mobocertinib and highlighting current studies that aim to improve central nervous system activity and overcome resistance mechanisms. This review highlights the therapeutic benefit of Amivantamab in EGFRex20ins-mutated NSCLC and offers guidance for future research in this quickly developing area.
Keywords: non-small cell lung cancer, EGFR exon 20 insertion, EGFRex20ins, amivantamab, bispecific antibody, MET, mobocertinib, targeted therapy, central nervous system metastases, drug resistance
Correspondence to: Rafael Alvim Pereira
Email: raralvim5@gmail.com
Published: 17/11/2025
Received: 08/05/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Lung cancer is still one of the most prevalent cancers in the world and the US, and socioeconomic and geographic factors affect how often it occurs [1]. It remains a significant public health concern even though rates in the United States have been steadily falling, particularly among men since the early 1990s and among women more recently [1–3]. 85% of primary lung tumours are non-small cell lung cancer (NSCLC), and many patients receive a diagnosis at an advanced stage. Palliative systemic therapy is frequently the cornerstone of treatment for these patients [4]. Nevertheless, since 2004 for epidermal growth factor receptor (EGFR)-mutated disease and 2011 for anaplastic lymphoma kinase-rearranged disease, patients with these oncogenic drivers can receive targeted therapies that are less harmful and more effective than chemotherapy, thanks to advancements in molecular diagnostic techniques [4–6].
More than 600 distinct EGFR mutation variants have been found due to the heterogeneity of NSCLC. Up to 40% of NSCLC cases have driver mutations in EGFR exons 18 to 21. Of these, approximately 85% of EGFR-positive cases have point mutations in exons 18, 19 and 21, which are known to react favourably to EGFR-targeted tyrosine kinase inhibitors (TKIs). Exon 20 insertions (exon20ins) are the second most frequent EGFR alteration, occurring in about 12% of cases [7–9].
Because they usually do not respond well to standard chemotherapy and immunotherapy and are resistant to conventional EGFR TKIs, EGFR exon20ins in NSCLC present unique clinical challenges [10, 11]. Amivantamab has shown encouraging potential as treatment for these mutations by bindings to the extracellular domains of EGFR and mesenchymal–epithelial transition factor (MET), enabling it to overcome resistance mechanisms associated with conventional TKIs, which target the intracellular kinase domain [12, 13].
Molecular and therapeutic rationale for Amivantamab
Insertion mutations in exon 20 of the EaGFR gene are resistant to traditional TKIs due to an altered conformation at the kinase active site. Furthermore, the EGFR and MET genes are highly expressed in NSCLC but are rarely co-expressed in healthy cells, promoting oncogenic signaling and tumour microenvironment remodeling [14–17]. Alterations in these pathways are among the most common mechanisms of resistance to EGFR TKIs.
Amivantamab was first approved and used in the US on 21 May 2021, for the treatment of adult patients with locally advanced or metastatic NSCLC harboring EGFR exon 20 insertion mutations whose disease had progressed on or after platinum-based chemotherapy [18]. Amivantamab is a fully human monoclonal antibody of the IgG1 Fc-active type, with bispecific specificity for EGFR and MET, developed using Genmab’s DuoBody technology. The drug has two distinct arms: one binds to the extracellular domain of EGFR, blocking interaction with the epidermal growth factor (EGF), while the other prevents binding to the MET receptor [7, 9]. This dual inhibition simultaneously blocks EGFR and MET signaling and is effective against resistance mechanisms to EGFR-targeted therapy mediated through the MET pathway. This unique structural design enables Amivantamab to eliminate antigen-expressing tumour cells through antibody-dependent cellular cytotoxicity, as well as antibody-dependent cellular phagocytosis and cytokine release. This activity leads to endocytosis of the receptor-antibody complex and its removal via lysosomal trafficking [19]. By targeting both receptors, Amivantamab provides a novel therapeutic approach for overcoming resistant mechanisms in these cancer types [20].
In preclinical studies, it was demonstrated that Amivantamab binds to the extracellular domains of the EGFR and MET receptors with binding affinities of 1.43 and 0.04 nM, respectively [20]. EGFR exon20ins mutations are a heterogeneous group of alterations that occur within the C-terminal portion of the tyrosine kinase domain, most commonly between codons 762 and 774. These insertions can be broadly classified into near-loop (within or adjacent to the C-helix, e.g., A763_Y764insFQEA) and post-alpha-helix (in the loop following the C-helix, e.g., D770_N771insNPG or H773_V774insNPH) subtypes [21]. In Ba/F3 cell lines containing mutations in EGFR exon20ins, Amivantamab reduced the expression of EGFR and MET and inhibited cell viability in a dose-dependent manner in five exon20ins variants (V769_D770insASV, D770delinsGY, H773_V774insH, Y764_V765insHH and D770_N771insSVD) [12]. The mechanism of action involved the inhibition of proliferative signaling pathways (pERK, pAkt and p-S6) and the induction of apoptosis via regulation of pro-apoptotic proteins such as Bcl-2–like protein 11 and cleaved caspase-3 [19]. Amivantamab was selected from a panel of bispecific anti-EGFR and anti-MET molecules with low fucose levels in the Fc region, which increases its affinity for immune effector cells, thereby enhancing immune-mediated antitumour activity. This combination of properties gives Amivantamab the potential to increase both the depth and duration of the therapeutic response in patients with EGFR mutations, particularly those associated with resistance to TKIs [22]. In addition to its favourable mechanism of action, pharmacokinetic data from vivo trials demonstrated that Amivantamab exposure increased proportionally at doses ranging from 350 to 1,750 mg. The drug exhibited a half-life of 11.3 (± 4.53) days and a median volume of distribution of 5.13 L. Furthermore, no clinically significant differences in exposure were observed across variables such as age, sex, race, creatinine clearance or hepatic impairment [19, 20]. Figure 1 below summarises the mechanism of action of Amivantamab.
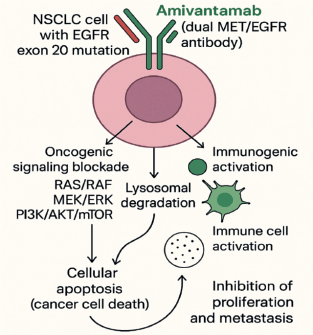
Figure 1. Amivantamab’s mechanism of action. Original figure.
Clinical evidence: efficacy and safety in EGFR exon20ins NSCLC
CHRYSALIS
In the first-in-human, open-label, phase 1 CHRYSALIS trial (NCT02609776), patients with advanced NSCLC who had EGFR exon20ins mutations—a subgroup known to respond poorly to conventional EGFR inhibitors—were assessed using Amivantamab. This non-randomised, multicenter study enrolled 114 patients in the EGFR exon20ins cohort who had progressed on platinum-based chemotherapy. Amivantamab was given intravenously to patients at a recommended phase 2 dose of 1,050 mg (or 1,400 mg for patients weighing more than 80 kg) once a week for the first 4 weeks and then every 2 weeks after that. The trial found that the objective response rate (ORR) among 81 patients who had progressed following platinum-based chemotherapy was 40% (95% CI, 29–51), with a median duration of response of 11.1 months (95% CI, 6.9–not reached) and a median progression-free survival (PFS) of 8.3 months (95% CI, 6.5–10.9). Responses were seen across various exon20ins variants, and the disease control rate (DCR) was 74%. In terms of safety, the most frequent adverse events associated with treatment were rash (86%), infusion-related reactions (IRRs) (66%), paronychia (45%) and stomatitis (21%). Most of these events were grades 1 or 2 and easily controlled. Notably, premedication and split-dosing techniques successfully managed IRRs, which mostly happened with the first dose [12]. These results demonstrate that Amivantamab is a promising treatment option with long-lasting clinical benefits for a patient population with limited options for targeted therapy, and they led to its expedited Food and Drug Administration (FDA) approval in 2021 [18].
PAPILLON trial
A phase III randomised study called PAPILLON trial (NCT04538664) compared 308 patients 1:1 to chemotherapy alone (155) versus Amivantamab plus carboplatin and pemetrexed chemotherapy (153) in patients with advanced NSCLC who had EGFR exon20ins mutations. The addition of Amivantamab resulted in a significant improvement in PFS: 11.4 months compared to 6.7 months in the group receiving chemotherapy alone (hazard ratio 0.40; 95% CI, 0.30–0.53; p < 0.001). The study also found that the combination arm had a significantly higher ORR than chemotherapy alone [23].
Table 1. Comparison of key clinical trials in EGFR exon20ins–mutant NSCLC [12, 23].

Table 2. Adverse effects across trials [12, 23].
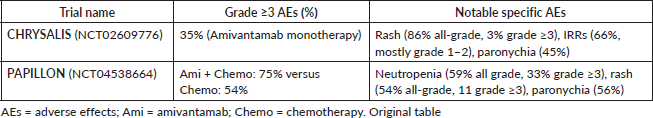
PAPILLON offered strong evidence for the drug’s use in the first-line setting, helping to establish a new standard of care for patients with EGFR exon20ins-mutant NSCLC, while CHRYSALIS demonstrated the drug’s effectiveness as a second-line option [12, 23, 24]. Table 1 below summarises the Key Clinical Trials in EGFR exon20ins-Mutant NSCLC.
Safety profile and IRRs
The safety profile of Amivantamab has been well documented in the CHRYSALIS and PAPILLON trials, particularly concerning IRRs, which occurred in about 66% of patients in the CHRYSALIS trial—most commonly during the first infusion. Chills, shortness of breath, flushing, nausea, chest pain and vomiting were typical symptoms of these reactions, which were typically mild to moderate in severity (grades 1 or 2). Antihistamines and antipyretics were usually prescribed to patients in advance to treat these occurrences and infusion rates were changed as needed [12, 18, 25].
The PAPILLON trial assessed Amivantamab in combination with chemotherapy and reported comparable IRR. These IRRs were primarily associated with the initial dose and decreased with subsequent infusions [10].
IRRs are among the most common side effects of Amivantamab, but with the right premedication and infusion procedures, they are usually controllable. Although these side effects rarely disrupt continued therapy, they emphasise how crucial close observation is during the initial dosage to guarantee patient safety and treatment continuity [18, 25]. Table 2 below summarises the adverse effects across trials.
The subcutaneous (SC) shift: clinical and operational impact
PALOMA-3 trial
A SC form of Amivantamab was created to preserve comparable pharmacokinetics and provide a more practical option for intravenous (IV) administration. The international, randomised, open-label Phase III PALOMA-3 trial (NCT05388669) aimed to determine whether SC versus IV Amivantamab administration, both in combination with lazertinib, was non-inferior in patients with advanced NSCLC that displayed EGFR
exon 19 deletions or L858R mutations and had advanced following previous treatment with osimertinib and platinum-based chemotherapy. A total of 418 patients were randomly assigned in a 1:1 ratio to receive Amivantamab intravenously (n = 212) or subcutaneously (n = 206), each in addition to daily oral lazertinib at a dose of 240 mg. The study achieved its primary goal by proving the SC formulation’s pharmacokinetic non-inferiority. The median PFS was 6.1 months for SC and 4.3 months for IV, while the ORR was similar for both arms (30% for SC and 33% for IV). Crucially, SC administration led to a five-fold decrease in venous thromboembolism incidence (9% versus 14%) and IRR (13% versus 66%). The SC group benefited from significantly shorter administration times (median 4.8 minutes versus 5 hours), but the treatment duration was comparable (median 7.2 versus 7.0 months). According to these results, SC amivantamab is safer, more practical and more efficient than IV delivery [26].
Amivantamab’s SC formulation preserves pharmacokinetic equivalency, improves patient convenience and satisfaction and offers significant advantages in operational efficiency and possible cost savings [27].
FDA rejection (2024)
Despite all its potential benefits, the SC form of Amivantamab (Rybrevant), which was being evaluated for patients with EGFR-mutated NSCLC, was rejected by the FDA in October 2024. Problems discovered during a routine manufacturing facility inspection served as the basis for the decision. Crucially, no additional clinical trials were needed, and the FDA expressed no concerns regarding the drug’s safety, efficacy or formulation [28].
EMA approval (2025)
The SC formulation of Amivantamab (Rybrevant) and lazertinib (Lazcluze) was approved by the European Commission Agency in April 2025 for use as a first-line treatment for adults with advanced NSCLC that has either exon 21 L858R mutations or EGFR exon 19 deletions. For patients with advanced NSCLC who have EGFR exon20ins mutations and have advanced following platinum-based chemotherapy, it was also authorised as a monotherapy [29].
Equity and access: challenges in low-resource systems
Some things are important to be considered depending on the healthcare setting. The SC route offers different advantages: first, it diminishes costs and time of administration, which could impact patient satisfaction. This can lead to a reduced financial burden on healthcare systems. These factors are very important to consider because some places, such as busy clinics or rural settings, have high demand and time is crucial. Although SC route offers great advantages, IV administration may be needed when the patient must be closely monitored, such as when early treatment of other IV drugs is being administrated [30].
Access and equity remain significant obstacles, particularly in low-resource environments where advanced therapies may be difficult to deliver due to supply chain problems, high treatment costs and inadequate healthcare infrastructure. Amivantamab and other SC formulations may lessen the logistical load, but safe administration requires trained personnel and appropriate storage. Since the high cost of targeted treatments also raises important questions about equity and access globally, stronger international health regulations and support networks are needed to make precision oncology more accessible to everyone, regardless of where they live [30].
Integration into clinical practice: NCCN guidelines
In the NCCN Guidelines for NSCLC, Amivantamab (Rybrevant) has taken center stage. Several Category 1 and 2A recommendations are specific to EGFR mutations and treatment contexts. Amivantamab combined with carboplatin and pemetrexed is now a Category 1 preferred first-line treatment for patients with newly diagnosed advanced or metastatic nonsquamous NSCLC that has EGFR exon20ins mutations.
According to the MARIPOSA-2 trial (NCT04988295), Amivantamab plus chemotherapy, with or without lazertinib, is also a Category 1 subsequent therapy for patients with EGFR exon 19 deletions or exon 21 L858R mutations who do not progress on Osimertinib [31]. Moreover, amivantamab and lazertinib are now Category 1-recommended for these prevalent EGFR mutations in the first-line setting. Amivantamab can also be used on its own (monotherapy) as a Category 2A option for patients with EGFR exon20ins mutations whose cancer has progressed after platinum-based chemotherapy. These updates demonstrate the growing significance of Amivantamab as a treatment for EGFR-mutated NSCLC, providing new options for various therapy stages and mutation types [32].
Comparative landscape and positioning of Amivantamab
Mobocertinib
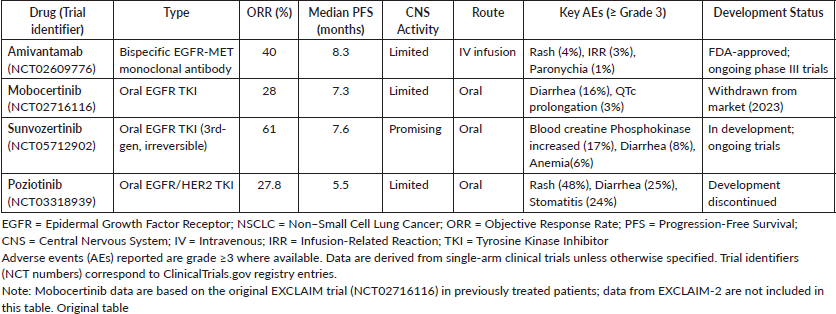
Mobocertinib (Exkivity) is an oral TKI designed to target EGFR exon20ins mutations in NSCLC, a subset known to be resistant to earlier-generation EGFR inhibitors. In 2021, the FDA gave accelerated approval for patients with metastatic or advanced NSCLC who had progressed while receiving platinum-based chemotherapy [18]. However, after the EXCLAIM-2 trial (NCT04129502) failed to demonstrate a definite advantage over conventional chemotherapy in the first-line setting, Takeda voluntarily removed the medication from the market in 2023 [33–35]. Although there was a lot of interest in mobocertinib at first, especially given how challenging EGFR exon20ins mutations are to treat—its modest effectiveness and side effects like diarrhea and QTc prolongation ultimately kept it from becoming a widely used option.
Sunvozertinib
An oral, third-generation, irreversible EGFR TKI called sunvozertinib is presently being developed to treat NSCLC with mutations in the EGFR exon20ins. It has demonstrated promising outcomes in patients who had previously received platinum-based chemotherapy. According to data from the pivotal WU-KONG6 trial (NCT05712902), 97 patients had a confirmed ORR of 59.8%, with 48.4% having baseline brain metastases [36]. Early-phase studies indicate a median PFS of approximately 8–9 months, and DCRs have been reported to be between 80% and 90% [37, 38]. In addition, sunvozertinib has demonstrated positive brain activity. Compared to amivantamab, it is a more convenient option because it is taken orally once daily. With common side effects like rash, diarrhea and paronychia, the safety profile is generally good [39]. Although both medications are equally effective (amivantamab has an ORR of about 40%), amivantamab must be administered intravenously and has limited central nervous system (CNS) penetration and infusion-related side effects. Sunvozertinib is a viable and practical therapeutic choice that is still being assessed in ongoing clinical trials [32].
Poziotinib
Poziotinib is an oral irreversible TKI that targets EGFR and human epidermal growth factor receptor 2 (HER2) exon20ins mutations in NSCLC. In the ZENITH20 trial (NCT03066206), the ORR ranged from 27% to 32%, with DCRs of nearly 70% in previously treated patients. Early-phase studies also revealed positive indications of activity [40, 41]. However, the drug’s limited effectiveness and frequent side effects ultimately led to its development being shut down. Many patients had unpleasant side effects like rash, diarrhea and mouth sores, which frequently required changing the dosage or discontinuing treatment completely [41]. Despite the benefit of being taken orally, poziotinib was withdrawn from FDA review and further development because it could not provide a robust enough risk-benefit profile.
In conclusion, amivantamab presently provides a clinically proven, validated and beneficial treatment option, particularly when used in conjunction with chemotherapy. However, the changing landscape—especially with drugs like sunvozertinib—may reshape treatment norms by providing improved CNS activity, oral administration convenience and equal or better efficacy. Table 3 below the efficacy, safety and development status of targeted therapies for ECGFR exon20ins-Mutant NSCLC.
Table 3. Efficacy, safety and development status of targeted therapies for EGFR exon20ins–mutant NSCLC [12, 36, 41, 42].
Real-world outcome data (2024–2025 studies)
The efficacy of Amivantamab in treating NSCLC with EGFR exon20ins mutations has been further supported by real-world data from 2024 to 2025. The 2025 American Society of Clinical Oncology Living Guideline emphasised the potential of combination therapies, pointing out that in patients with high-risk characteristics, like TP53 co-mutations, Amivantamab plus lazertinib enhanced PFS in comparison to osimertinib alone [43].
In one study, Wang et al [44] found that patients from various cancer centers had a DCR of 64.3% and a clinical response rate of 35.7%. Even when Amivantamab was used in conjunction with radiation therapy or osimertinib, the safety profile stayed the same and no new toxicities were observed [44].
The Lung Cancer Genomic Screening Project for Individualized Medicine in Japan-Asia study also provided additional information by demonstrating that the location of exon20ins may impact treatment outcomes. When Amivantamab was administered to patients instead of docetaxel, immune checkpoint inhibitors or classical TKIs, the patient’s overall survival (OS) and PFS improved [45].
These results demonstrate that amivantamab is a potent treatment option for patients with EGFR exon20ins mutations because it is practical and well-tolerated, particularly when combined with other targeted therapies or customised to the patient’s risk factors.
Future directions and unanswered questions
Resistance mechanisms to amivantamab
Although the exact mechanism of amivantamab resistance in NSCLC with EGFR exon20ins mutations is still unknown, information from comparable EGFR-targeted therapies provides some insight. By attaching to the extracellular domains of EGFR and MET, the bispecific antibody amivantamab can help get around some of the resistance that TKIs cause [9, 12]. However, there are a few ways that resistance can arise. One potential mechanism involves secondary mutations in EGFR that alter the receptor’s shape and decrease the drug’s binding ability.
Another possible pathway is MET amplification, which permits cancer cells to depend more on MET signaling even when EGFR is inhibited [46]. To avoid EGFR and MET, tumours may activate other survival pathways, such as those involving HER2 or HER3 [47]. Finally, histologic transformation—where the tumour changes type, for instance, becoming small cell lung cancer—is a known resistance mechanism with other EGFR therapies and may also occur here, even though it has not been documented with amivantamab specifically yet. As we seek methods to get past resistance and enhance amivantamab results, it is critical to comprehend these potentialities.
The MET pathway acts as a compensatory mechanism to EGFR signaling, with MET amplification being a well-established mechanism of resistance to EGF receptor TKIs. However, it remains unclear whether this resistance mechanism also applies to TKIs specifically targeting EGFR exon20ins. Amivantamab, a bispecific EGFR/MET antibody, stands out for its dual mechanism of action and has shown efficacy in patients with EGFR mutations who have progressed after osimertinib therapy [48, 49].
The exploration of anti-MET antibody therapies represents a promising approach in the treatment of NSCLC, particularly in the context of resistance to EGFR inhibitors. Dysregulation of the MET pathway, through overexpression, exon 14 mutation or gene amplification, plays a critical role in oncogenesis and therapeutic resistance. While the METex14 mutation is the most validated biomarker, MET amplification, particularly assessed by the MET/CEP7 ratio using FISH, demonstrates superior predictive potential [50].
Another point is amivantamab’s possible activity outside of this subgroup of exon 20. G719X, S768I, L861Q and specific exon 18 insertions are examples of uncommon EGFR mutations that are typically less sensitive to early-generation TKIs but may still be partially sensitive to third-generation agents. Preclinical research has demonstrated that amivantamab’s bispecific EGFR/MET binding is effective against some rare variants, possibly circumventing resistance resulting from changed kinase conformations [51].
Furthermore, complex resistance patterns and distinct biological behaviour can be conferred by compound EGFR mutations, which are defined as two or more EGFR alterations in the same tumour. Early clinical experience and retrospective reports indicate that amivantamab—alone or paired with lazertinib—may help some patients with compound EGFR mutations, particularly when one of the alterations is an exon20ins. However, because these genotypes are highly diverse and treatment responses can vary, prospective studies are needed to better define which patients are most likely to benefit [44].
Importantly, strategies to overcome or delay resistance are under investigation. For instance, in patients with EGFR exon20ins–mutated NSCLC, amivantamab added to carboplatin–pemetrexed chemotherapy in the first-line setting significantly extended PFS when compared to chemotherapy alone (PAPILLON trial) [23]. Furthermore, real-time early detection of new resistance mechanisms is made possible by liquid biopsy, which includes circulating tumour DNA analysis [52]. This allows for prompt therapeutic adaptation and may extend clinical benefit.
The future of NSCLC treatment will depend on the precise integration of biomarkers, optimisation of the safety of combination therapies and further understanding of MET pathway biology. Ongoing clinical trials and translational studies will be crucial in consolidating these therapies into clinical practice [49].
Conclusion
The treatment of NSCLC with EGFR exon20ins has been entirely transformed by the discovery of amivantamab, a more effective alternative to traditional chemotherapy. As research into its use in different combinations and environments continues, its role in treating this troublesome form of lung cancer is becoming increasingly apparent. More recently, the development of an SC formulation has increased the appeal of treatment by making it quicker and simpler to administer. Compared to IV infusion, SC delivery improves the overall quality of treatment, takes only a few minutes and minimises side effects associated with infusion. This change relieves the burden on infusion facilities and employees while promoting more adaptable care models, such as outpatient and possibly even home-based options. Because SC delivery eliminates the need for specialised infrastructure, it may also increase access in rural or resource-constrained areas. This strategy aligns nicely with the increasing emphasis on patient-centered, value-based cancer care. As SC amivantamab is used more frequently, it may improve the treatment of EGFR-mutated NSCLC and influence the way targeted therapies are administered in oncology in the future. Nonetheless, resistance mechanisms remain a vital frontier, with ongoing studies exploring optimal sequencing, novel combinations and activity across broader
EGFR mutation subtypes. Amivantamab’s therapeutic footprint is anticipated to grow as empirical data mount and biomarker-driven methods improve patient selection, impacting not only the treatment of EGFR-mutated NSCLC but also the delivery models of targeted therapies in oncology going forward.
Disclosure
Rafael Alvim Pereira has no disclosures. Milena Tumelero has no disclosures. Wallace Klein Schwengber has no disclosures. Gabriel Lenz has no disclosures.
Conflicts of interest
The authors Rafael Alvim Pereira, Milena Tumelero, Wallace Klein Schwengber and Gabriel Lenz declare that they have no conflicts of interest related to this work.
Funding
No funding was received for the publication of this article.
References
1. Zhang Y, Vaccarella S, and Morgan E, et al (2023) Global variations in lung cancer incidence by histological subtype in 2020: a population-based study Lancet Oncol 24(11) 1206–1218 https://doi.org/10.1016/S1470-2045(23)00444-8 PMID: 37837979
2. Smith RA, Andrews KS, and Brooks D, et al (2019) Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening CA Cancer J Clin 69(3) 184–210 PMID: 30875085
3. Huang J, Deng Y, and Tin MS, et al (2022) Distribution, risk factors, and temporal trends for lung cancer incidence and mortality Chest 161(4) 1101–1111 https://doi.org/10.1016/j.chest.2021.12.655 PMID: 35026300
4. Gridelli C, Peters S, and Sgambato A, et al (2014) ALK inhibitors in the treatment of advanced NSCLC Cancer Treat Rev 40(2) 300–306 https://doi.org/10.1016/j.ctrv.2013.07.002
5. Guo L, Zhu C, and Cai L, et al (2024) Global burden of lung cancer in 2022 and projected burden in 2050 Chin Med J (Engl) 137(21) 2577–2582 https://doi.org/10.1097/CM9.0000000000003268 PMID: 39313774 PMCID: 11557091
6. Yang X, Yang K, and Kuang K (2014) The efficacy and safety of EGFR inhibitor monotherapy in non-small cell lung cancer: a systematic review Curr Oncol Rep 16(6) 390 https://doi.org/10.1007/s11912-014-0390-4 PMID: 24807015
7. Vijayaraghavan S, Lipfert L, and Chevalier K, et al (2020) Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis Mol Cancer Ther 19(10) 2044–2056 https://doi.org/10.1158/1535-7163.MCT-20-0071 PMID: 32747419
8. Passaro A, Mok T, and Peters S, et al (2021) Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations J Thoracic Oncol 16(5) 764–773 https://doi.org/10.1016/j.jtho.2020.12.002
9. Yun J, Lee SH, and Kim SY, et al (2020) Antitumor activity of amivantamab (JNJ-61186372), an EGFR–MET bispecific antibody, in diverse models of EGFR exon 20 insertion–driven NSCLC Cancer Discov 10(8) 1194–1209 https://doi.org/10.1158/2159-8290.CD-20-0116 PMID: 32414908
10. Riely GJ, Wood DE, and Ettinger DS, et al (2024) Non–small cell lung cancer, version 4.2024 J Nat Comprehensive Cancer Netw 22(4) 249–274 https://doi.org/10.6004/jnccn.2204.0023
11. Russell MC, Garelli AM, and Reeves DJ (2023) Targeting EGFR Exon 20 insertion mutation in non–small cell lung cancer: amivantamab and mobocertinib Ann Pharmacotherapy 57(2) 198–206 https://doi.org/10.1177/10600280221098398
12. Park K, Haura EB, and Leighl NB, et al (2021) Amivantamab in EGFR exon 20 insertion–mutated non–small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study J Clin Oncol 39(30) 3391–3402 https://doi.org/10.1200/JCO.21.00662 PMID: 34339292 PMCID: 8791812
13. Chouaid C, Bosquet L, and Girard N, et al (2023) An adjusted treatment comparison comparing amivantamab versus real-world clinical practice in Europe and the United States for patients with advanced non-small cell lung cancer with activating epidermal growth factor receptor exon 20 insertion mutations Adv Ther 40(3) 1187–1203 https://doi.org/10.1007/s12325-022-02408-7 PMID: 36652175 PMCID: 9988783
14. Kumar A, Petri ET, and Halmos B, et al (2008) Structure and clinical relevance of the epidermal growth factor receptor in human cancer J Clin Oncol 26(10) 1742–1751 https://doi.org/10.1200/JCO.2007.12.1178 PMID: 18375904 PMCID: 3799959
15. Eck MJ and Yun CH (2010) Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer Biochim Biophys Acta 1804(3) 559–566 https://doi.org/10.1016/j.bbapap.2009.12.010 PMCID: 2859716
16. Travis WD, Brambilla E, and Nicholson AG, et al (2015) The 2015 World Health Organization classification of lung tumors J Thoracic Oncol 10(9) 1243–1260 https://doi.org/10.1097/JTO.0000000000000630
17. Zhang YL, Yuan JQ, and Wang KF, et al (2016) The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis Oncotarget 29(48) 78985–78993 https://doi.org/10.18632/oncotarget.12587
18. Chon K, Larkins E, and Chatterjee S, et al (2023) FDA approval summary: amivantamab for the treatment of patients with non–small cell lung cancer with EGFR exon 20 insertion mutations Clin Cancer Res 29(17) 3262–3266 https://doi.org/10.1158/1078-0432.CCR-22-3713 PMID: 37022784 PMCID: 10523842
19. Brazel D, Smith J, and Ou SHI, et al (2025) The user’s guide to amivantamab Target Oncol 20(2) 235–245 https://doi.org/10.1007/s11523-025-01128-6 PMID: 39903428 PMCID: 11933153
20. Brazel D and Nagasaka M (2023) The development of amivantamab for the treatment of non-small cell lung cancer Respir Res 24(1) 256 https://doi.org/10.1186/s12931-023-02558-4 PMID: 37880647 PMCID: 10601226
21. Riess JW, Gandara DR, and Frampton GM, et al (2018) Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC J Thoracic Oncol 13(10) 1560–2158 https://doi.org/10.1016/j.jtho.2018.06.019
22. Grugan KD, Dorn K, and Jarantow SW, et al (2017) Fc-mediated activity of EGFR x c-Met bispecific antibody JNJ-61186372 enhanced killing of lung cancer cells MAbs 9(1) 114–126 https://doi.org/10.1080/19420862.2016.1249079 PMCID: 5240640
23. Zhou C, Tang KJ, and Cho BC, et al (2023) Amivantamab plus chemotherapy in NSCLC with EGFR Exon 20 Insertions New England J Med 389(22) 2039–2051 https://doi.org/10.1056/NEJMoa2306441
24. Cho BC, Lu S, and Felip E, et al (2024) Amivantamab plus lazertinib in previously untreated EGFR-mutated advanced NSCLC New England J Med 391(16) 1486–1498 https://doi.org/10.1056/NEJMoa2403614
25. Park K, Sabari JK, and Haura EB, et al (2023) Management of infusion-related reactions (IRRs) in patients receiving amivantamab in the CHRYSALIS study Lung Cancer 178 166–171 https://doi.org/10.1016/j.lungcan.2023.02.008 PMID: 36868177
26. Leighl NB, Akamatsu H, and Lim SM, et al (2024) Subcutaneous versus intravenous amivantamab, both in combination with lazertinib, in refractory epidermal growth factor receptor–mutated non–small cell lung cancer: primary results from the phase III PALOMA-3 study J Clin Oncol 42(30) 3593–3605 https://doi.org/10.1200/JCO.24.01001 PMID: 38857463 PMCID: 11469630
27. Bazhenova L, Ismaila N, and Abu Rous F, et al (2024) Therapy for stage IV non–small cell lung cancer with driver alterations: ASCO living guideline, version 2024.2 J Clin Oncol 42(36) https://doi.org/10.1200/JCO-24-02133 PMID: 39531596
28. Update on U.S. Regulatory Review of Subcutaneous Amivantamab [Internet]. 2024 [https://www.jnj.com/media-center/press-releases/update-on-u-s-regulatory-review-of-subcutaneous-amivantamab]Date accessed: 14/04/2025
29. Ryan C (2025) OncLive. Subcutaneous Amivantamab Wins EU Approval in Multiple EGFR+ Advanced NSCLC Indications [https://www.onclive.com/view/subcutaneous-amivantamab-wins-eu-approval-in-multiple-egfr-advanced-nsclc-indications?utm_source=chatgpt.com] Date accessed: 14/04/2025
30. Aguiar-Ibáñez R, Fotheringham I, and Mittal L, et al (2024) Differences between intravenous and subcutaneous modes of administration in oncology from the patient, healthcare provider, and healthcare system perspectives: a systematic review Adv Ther 41(12) 4396–4417 https://doi.org/10.1007/s12325-024-02985-9 PMID: 39425890
31. Passaro A, Wang J, and Wang Y, et al (2024) Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study Ann Oncol 35(1)
32. Kristina Gregory N, Lisa Hang M, and Aisner DL, , et al (2025) NCCN Guidelines Version 3.2025 Non-Small Cell Lung Cancer Continue NCCN Guidelines Panel Disclosures [Internet] [https://www.nccn.org/home/member]
33. 2024-15371
34. Duke ES, Stapleford L, and Drezner N, et al (2023) FDA approval summary: mobocertinib for metastatic non-small cell lung cancer with EGFR Exon 20 insertion mutations Clin Cancer Res 29(3) 508–512 https://doi.org/10.1158/1078-0432.CCR-22-2072 PMCID: 9898076
35. Jänne PA, Wang BC, and Cho BC, et al (2025) First-line mobocertinib versus platinum-based chemotherapy in patients with EGFR exon 20 insertion–positive metastatic non–small cell lung cancer in the phase III EXCLAIM-2 trial J Clin Oncol https://doi.org/10.1200/JCO-24-01269
36. Wang M, Fan Y, and Sun M, et al (2024) Sunvozertinib for patients in China with platinum-pretreated locally advanced or metastatic non-small-cell lung cancer and EGFR exon 20 insertion mutation (WU-KONG6): single-arm, open-label, multicentre, phase 2 trial Lancet Respir Med 12(3) 217–224 https://doi.org/10.1016/S2213-2600(23)00379-X
37. Wang M, Yang JCH, and Mitchell PL, et al (2022) Sunvozertinib, a selective EGFR inhibitor for previously treated non–small cell lung cancer with EGFR exon 20 insertion mutations Cancer Discov 12(7) 1676–1689 https://doi.org/10.1158/2159-8290.CD-21-1615 PMID: 35404393 PMCID: 9262839
38. Liu H, Qin J, and Qian X (2024) Targeting EGFR exon 20 insertion mutations in non-small-cell lung cancer: changes in treatment strategies are coming Cancer Control 31 10732748241292782 https://doi.org/10.1177/10732748241292782 PMID: 39417568 PMCID: 11489933
39. Watanabe N, Horio Y, and Fujiwara Y (2022) Emerging therapies for non-small cell lung cancer harboring EGFR exon 20 insertion mutations: narrative review Ann Transl Med 10(23) 1283 https://doi.org/10.21037/atm-2022-56
40. Robichaux JP, Elamin YY, and Tan Z, et al (2018) Mechanisms and clinical activity of an EGFR and HER2 exon 20–selective kinase inhibitor in non–small cell lung cancer Nat Med 24(5) 638–646 https://doi.org/10.1038/s41591-018-0007-9 PMID: 29686424 PMCID: 5964608
41. Elamin YY, Robichaux JP, and Carter BW, et al (2022) Poziotinib for patients with HER2 exon 20 mutant non–small-cell lung cancer: results from a phase II trial J Clin Oncol 40(7) 702–709 https://doi.org/10.1200/JCO.21.01113 PMCID: 8887948
42. Zhou C, Ramalingam SS, and Kim TM, et al (2021) Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion–positive metastatic non–small cell lung cancer JAMA Oncol 7(12) 214761 https://doi.org/10.1001/jamaoncol.2021.4761
43. Owen DH, Ismaila N, and Ahluwalia A, et al (2025) Therapy for stage IV non–small cell lung cancer with driver alterations: ASCO living guideline, version 2024.3 J Clin Oncol 43(10) 43
44. Wang K, Du R, and Myall NJ, et al (2024) Real-world efficacy and safety of amivantamab for EGFR-mutant NSCLC J Thoracic Oncol 19(3) 500–506 https://doi.org/10.1016/j.jtho.2023.11.020
45. Okahisa M, Udagawa H, and Matsumoto S, et al (2024) Clinical outcomes in patients with non-small cell lung cancer harboring EGFR Exon20 in-frame insertions in the near-loop and far-loop: results from LC-SCRUM-Asia Lung Cancer 191 107798 https://doi.org/10.1016/j.lungcan.2024.107798 PMID: 38669727
46. Park S, Park S, and Kim TM, et al (2024) Resistance mechanisms of EGFR tyrosine kinase inhibitors, in EGFR exon 20 insertion-mutant lung cancer Eur J Cancer 208 114206 https://doi.org/10.1016/j.ejca.2024.114206 PMID: 38981315
47. Shah MP and Neal JW (2022) Targeting acquired and intrinsic resistance mechanisms in epidermal growth factor receptor mutant non-small-cell lung cancer Drugs 82(6) 649–662 https://doi.org/10.1007/s40265-022-01698-z PMID: 35412115
48. Vyse S and Huang PH (2022) Amivantamab for the treatment of EGFR exon 20 insertion mutant non-small cell lung cancer Expert Rev Anticancer Ther 22(1) 3–16 https://doi.org/10.1080/14737140.2022.2016397
49. Wang K and Hsu R (2024) Anti-MET antibody therapies in non-small-cell lung cancer: current progress and future directions Antibodies 13(4) 88 https://doi.org/10.3390/antib13040088 PMID: 39449330 PMCID: 11503282
50. Cho BC, Simi A, and Sabari J, et al (2023) Amivantamab, an epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) bispecific antibody, designed to enable multiple mechanisms of action and broad clinical applications Clin Lung Cancer 24(2) 89–97 https://doi.org/10.1016/j.cllc.2022.11.004
51. Oh SY, Park S, and Lee S, et al (2025) The potential of lazertinib and amivantamab combination therapy as a treatment strategy for uncommon EGFR-mutated NSCLC Cell Rep Med 6(2) 101929 https://doi.org/10.1016/j.xcrm.2025.101929 PMID: 39874964 PMCID: 11866483
52. Rolfo C, Mack PC, and Scagliotti GV, et al (2018) Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC J Thoracic Oncol 13(9) 1248–1268 https://doi.org/10.1016/j.jtho.2018.05.030





