Assessment of treatment plans from high-dose-rate-brachytherapy of prostate cancer in Nigeria: findings from pioneer centre
Bidemi I Akinlade1, Iyobosa B Uwadiae2, Abbas A Abdus-Salam1, Atara I Ntekim1, Ayorinde M Folasire1, Mutiu A Jimoh1, Afolabi A Oladeji1, Foluke O Sarimiye1 and Adeniyi A Adenipekun1
1Department of Radiation Oncology, Faculty of Clinical Sciences, College of Medicine, University College Hospital, University of Ibadan, Ibadan 200212, Oyo State, Nigeria
2Department of Radiation Oncology, University College Hospital, Ibadan 200212, Oyo State, Nigeria
Abstract
Introduction: High dose rate (HDR) brachytherapy is a promising therapeutic approach for localised prostate cancer. Optimised treatment plans have been shown to improve disease control and reduce toxicity on the organs-at-risk (OAR).
Aim: To report findings from the treatment plan parameters (TPPs) obtained from two different HDR brachytherapy treatment regimens at the University College Hospital, Ibadan Nigeria.
Methods: The treatment plans of 90 patients, who had HDR brachytherapy to the prostate gland between August 2020 and October 2023 were considered. All were treated with Bebig Saginova machine, housing Cobalt-60 source, the first multi-channel unit in the country then. 44% of patients received dose of 18 Gy in 2 fractions (category A), while the remaining received 27 Gy in 2 fractions (category B). Treatment plans were generated on the Sagiplan 2.0 treatment planning system from Eckert & Ziegler, BEBIG and relevant TPP were extracted and analysed using IBM SPSS 27.
Results: The mean age(years) of patients in Categories A and B were 65.3 ± 6.59 and 66.5 ± 5.32, respectively; their gleason score and prostate-specific-antigen were (7 ± 1; and 7 ± 1) and (12.83 ± 16.32; and 12 ± 17 ng/mL), respectively. The mean volume (cm3) of prostate volume (PVol.) for both categories were 46 ± 21 and 31 ± 8, respectively. The paired t-test performed on TPP from patients in both categories was statistically significant (p < 0.005), except their age (p < 0.873) and dose homogeneity index (p < 0.639). Also, the regression analysis showed that V100 is statistically dependent (p < 0.05) on Total Reference Air Kerma, conformal index, Rectum D10 and PVol. in both categories.
Conclusion: Although, some level of optimal dose coverage around the prostate gland was achieved for some of the patients, especially those in Category B, there is still room for improvement to minimise the dose to OAR.
Keywords: high dose rate brachytherapy, prostate cancer, temporary prostate implant, treatment plan quality, dose optimisation, organs at risk, dose volume parameters
Correspondence to: Bidemi I Akinlade
Email: bidylade@gmail.com and biakinlade@com.ui.edu.ng
Published: 15/05/2025
Received: 04/11/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
High dose rate (HDR) brachytherapy is a form of radiotherapy where radioactive sources are directly implanted into the target to achieve a highly conformal therapy. It is an effective method of treating localised prostate cancer which is the most common cancer among men of age 50 years and above. Low dose rate (LDR) permanent seed brachytherapy is most commonly used for low-risk cancer, while HDR is usually combined with external beam radiotherapy (EBRT) to treat higher risk disease [1]. The HDR of radiation dose deliverable and the possibility of the large fractional size of radiation employed in HDR techniques have been found to be a radiobiological advantage, especially for tumours with high sensitivity to radiation fractional size and can be administered as a monotherapy or as a boost with adjuvant EBRT [2, 3].
Effective treatment of prostate cancer requires a good radiation dose distribution around the target volume and minimal dose to the surrounding healthy tissues [4]. In HDR, treatment is usually delivered using a high-strength stepping source with treatment planning and dwell time optimisation performed after treatment applicators (plastic or steel needles) have been implanted [5]. This assures dosimetry that is more consistent when compared with LDR brachytherapy permanent seed implants which usually faces challenges of seed misplacement, seed displacement and swelling of the prostate. All of these may result in variations between the planned and delivered doses [6]. The quality of treatment plan parameters for prostate cancer depends largely on the operator’s skills, the prostate gland anatomy, the prostate volume (PVol) and the surrounding tissues (organs-at-risk (OAR)) among others [7]. As HDR continues to evolve, a strong focus on treatment plan quality (TPQ) is expected to further enhance its effectiveness in managing localised prostate cancer.
Our centre is the first radiotherapy centre in Nigeria to acquire a full-fledged multi-channel afterloading HDR facility which is required to treat patients living with prostate cancer using a temporary implant. This study presents our initial findings with respect to dosimetry procedures: dose volume parameters (DVPs) and TPQ, obtained from the treatment planning system of 90 patients managed between August 2020 and October 2023.
Materials and methods
Ninety patients living with prostate cancer and managed in the Department of Radiation Oncology, University College Hospital (UCH), the pioneer centre to offer HDR temporary prostate brachytherapy in Nigeria, between August 2020 and October 2023 were considered in this study. Forty patients received a prescription boost dose of 18 Gy in 2 fractions (9 Gy per day) to complement EBRT of 45–50 Gy, while 50 patients received a monotherapy dose of 27 Gy in 2 fractions (13.5 Gy per day). All fractions were completed within 1 week of each patient’s admission to the ward.
The patients were carefully selected to ensure that they were in the early stages of the disease limited to the prostate. Most of the patients (64%) were in TNM stages 1 and 2 and more than 70% had prostate-specific antigen (PSA) below 20 ng/mL. Only 12% had TNM stage 4 diseases while 24.6% had PSA above 20 ng/mL. None of the patients had distant metastases to the lungs, liver, bones or the nervous system at the time of treatment.
All patients were treated with the Bebig multi-channel remote afterloading HDR brachytherapy unit, model SagiNova. This machine was manufactured by Eckert & Ziegler Bebig GmbH, Berlin Germany. It was acquired and installed between 2018 and 2019 through a public–private partnership initiative between UCH and a private investor. The HDR unit uses a radioactive Cobalt-60 source with a half-life of 5.26 years. The source strength as at the time of installation (16 October 2018) was 23.43 mGym2/h. It has the capacity to deliver a nominal dose rate of 12 Gy/hr or higher at the prescription point. This type of radioactive source greatly relieved the burden of quarterly source replacements, which are usually associated with challenges of foreign exchange, customs clearance, shipping and transportation among others.
The image sequences used for treatment planning of all the patients were obtained from an Ultrasound machine, Flex Focus 400 from bK Medical, Germany, after the insertion of appropriate number of needles, first at the peripheral and then at the internal, while observing the spacing distance of at least 5 mm between needles. These images are then used by the treatment planning software from Bebig, SagiPlan 2.0, for planning the treatment. The treatment planning procedures include calibration or matching of the physical prostate template with the electronic template, contouring of the treatment volume (Prostate gland) and OAR (Urethra and Rectum). Contouring of the bladder is not required when using Ultrasound imaging technique.
All patients underwent standard HDR brachytherapy treatment procedures/protocols which were adopted from the GEC/ESTRO recommendations [8]. The patients’ selections and other procedures before actual treatment were as earlier reported by Abdus-Salam et al [9] in our first publication on prostate HDR brachytherapy experience.
Treatments were delivered using a high-strength stepping source (Cobalt-60) with planning and dwell time optimisation (inverse planning) performed after the needles had been properly implanted under ultrasound guidance and carefully identified on the images in the planning software [10, 11]. The planning software have the capability to calculate dose distribution per volume of the organ, based on the parameters set as constraints (dose volume histogram) and determine the overall plan quality. These are usually arrived at after several mathematical iterations, dose optimisation/adjustment of the isodose curves around the contoured organs and then display for assessment and approval. The patients are usually treated after these treatment plans are approved by the Radiation Oncologists. For the purpose of this study, the following was extracted from the approved treatment plans: conformal index (COIN), dose homogeneity index (DHI), Total Reference Air Kerma (TRAK) and total treatment time (min) used to deliver the prescribed fractional dose. TRAK is the total radiation exposure when the source is outside of its shielded position. While DHI is the tool used to analyse the uniformity of dose distributions in the target volume, COIN represents the relationship between isodose distributions and the target volume coverage. Other parameters documented for this study are the patient’s age, Gleason score (GS), PSA (GS and PSA are values before the HDR brachytherapy procedure), number of inserted needles (peripheral and internal), DVP of the contoured organs’ of interest namely: Prostate (Dose received by its minimum volume (Dmin), dose received by 90% of its volume (D90), the volume that received 100% of the prescribed dose (V100) and the volume that received 150% of the prescribed dose (V150)); Urethra (Dose received by 10% of its volume (D10) and the maximum dose received (Dmax)); Rectum (Dose received by 10% of its volume (D10) and the maximum dose received (Dmax)). Bladder is not quantified among OAR for ultrasound-planned dosimetry [12].
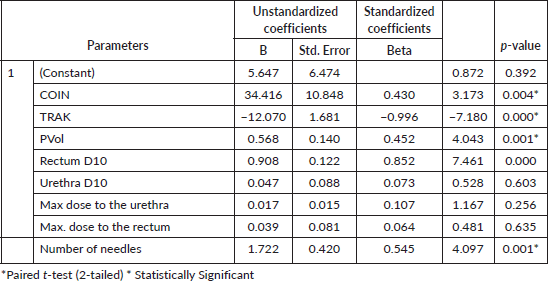
These data were analysed using IBM SPSS statistics 27, and the results are presented in Tables 1–4. Part of the analysis was to compare the mean values of all the variables obtained for patients in category A with those in category B using a paired samples T-test and to determine the variables that have statistical significance (p < 0.05) on the volume of the prostate that will receive 100% of the prescribed dose (V100). To achieve the latter, V100 was set as the dependent variable, while the Rectum Dmax, Rectum D10, Urethra Dmax, Urethra D10, PVol contoured, COIN and TRAK were set as predictor variables.
Results
Out of the 90 patients managed with the remote afterloading HDR unit for interstitial temporary prostate implants, 44% received a total dose of 18 Gy in 2 fractions (category A), while the remaining 56% received a total dose of 27 Gy in 2 fractions (category B). All patients had one fraction per day and completed their treatment within a week. The patients’ prognostic factors such as GS and PSA values before the treatment and the age of the patients are presented in Table 1 for those in categories A and B. The mean age of patients in category A (65.3 ± 6.59) and category B (66.5 ± 5.32) were almost the same (p < 0.873) and so were their GS and PSA values (p = 0.000). Also presented in Table 1 is the average volume (cm3) of the prostate gland treated for patients in category A which was significantly larger (46 ± 21) than those in category B (31 ± 8) (p < 0.001). The number of implanted needles used for treatment of patients in category A were smaller (13 ± 2) than those in category B (16 ± 3), (p < 0.001). Also presented in Table 1 for patients in categories A and B are DVP in percentages ((median (range)) of the prescribed dose and volumes for the prostate and OAR (urethra and rectum).
The summary of the regression analysis performed on the DVP and the TPQ to determine their relative dependence or statistical significance on V100 at the 95% confidence interval is presented in Tables 2–4.
Table 1. Comparison between DVP and plan quality for patients in category A and B.
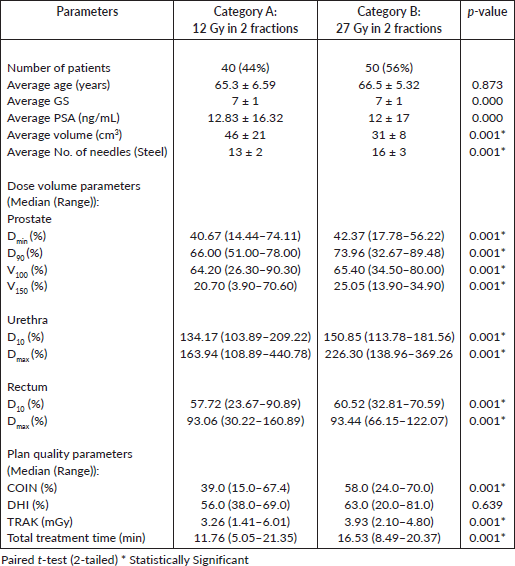
Table 2. Regression analysis between the DVP, plan quality parameters (Predictors) and V100 (Dependent variable).

Table 3. Significance of the predictors (combined) on the dependent variable.
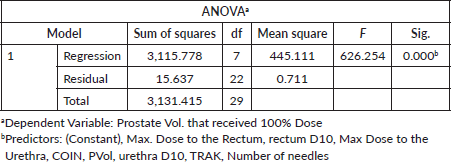
Table 4. Significance of individual predictor on the dependent variable.
Discussion
As the pioneer Oncology centre for interstitial temporary prostate implant with HDR in Nigeria, the TPQ and DVP obtained from the treatment planning of 90 patients living with prostate cancer have been assessed and analysed. The age of the patients considered in this study ranged from 51 to 78 years. This is consistent with the age at which the incidence of prostate cancer is high (1 in 52 males) and hence the recommendation that screening should take place at age 50 years for men who are at an average risk of prostate cancer [13].
The patients were further divided into two categories: those treated at the commencement of the HDR clinical services (Category A) and those treated after modification of the treatment regimen (Category B). This modification followed some months of clinical observation of the treatment outcomes of the previous regimen. The patients in category A (44%) received a total dose of 18 Gy in 2 fractions as an adjuvant therapy to EBRT [14] while those in category B (56%) received 27 Gy in 2 fractions as monotherapy. These dose prescriptions were similar to those reported in literature, when HDR is given as a monotherapy [2, 15, 16]. It has also been reported that the use of HDR as monotherapy technique seems to be a promising treatment modality for low-risk prostate cancer [5]. The mean volumes of the prostate glands contoured for patients in categories A and B were within the recommended volume (≤ 60 cm3) of the prostate gland that should be implanted for HDR [4, 8, 16]. Also, the mean number of needles used for the prostate implants for patients in categories A and B were similar to the number of needles inserted for patients in the study reported by Kini et al [15]. In another study by Adam et al [7], they suggested that the minimum number of needles implanted for prostate cancer HDR should be greater than 9 and optimally greater than or equal to 13.
The DVP considered in this study (for prostate: Dmin, D90, V100 and V150; for urethra: D10 and Dmax; and for rectum: D10 and Dmax) for patients in both categories were among the parameters recommended for reporting for HDR prostate implants by GEC/ESTRO [8]. Their respective values obtained for patients in both categories were statistically significant (p < 0.001). The median values of DVP obtained were within the range of values reported in the literature [7] namely prostate D90 (65.9%–102.8%); urethra D10 (78.8%–152.9%); rectal D10 (37.4%–101.0%). The median volume of the prostate tissue covered by the 100% isodose surface (V100) in category A (64.20%) and category B (65.40%) were lower than the range of values (75%–99%) reported in the literature [14]. Likewise, the minimum doses to the target volume which was 40.67% of the prescribed dose in category A and 42.37% in category B were lower than the range of values reported (45%–97%) by the same authors. The median prostate D90’s obtained for category A (66.0%) and category B (73.96%) were lower than the value (110%) reported in literature [17]. However, the median urethra D10’s and rectum D10’s obtained in this study for category A (134.17%, 57.72%) and category B (150.85%, 60.52%), respectively, were higher than the values (114%, 55%) reported by the same authors.
Similarly, the Plan Quality Parameters (COIN, DHI, TRAK and total treatment time) obtained and compared between the two categories showed some level of significance (p < 0.001) in predicting V100 except for DHI (p < 0.639). The conformity of the isodose curve with the volume of the prostate gland (COIN) and homogenous distribution of dose (DHI) around the target volume obtained for patients in category A (39%, 56%) and category B (58%, 63%), respectively, were lower than the values (80%, 67%) reported by Major et al [17]. The TRAK, total treatment time, and the average number of needles obtained for patients in category A (3.26 mGy, 11.76 minutes and 13) were lower than those in category B (3.93 mGy, 16.53 minutes and 16), respectively. This variation may be due to the increases in the prescribed dose (18 Gy versus 27 Gy) and the number of treatment needles (13 versus 16) used for patients in category B. It is important to note that the greater number of implanted needles and increased PVol (PVol.) with improved D90 have been shown to produce better target coverage (V100), better dose distribution and decreased dose to the urethra (urethra D10) [7]. The regression analysis performed on some of the variables (COIN, TRAK, PVol., rectum D10, rectum Dmax, urethra D10, urethra Dmax) considered in this study was done to determine their statistical significance (p < 0.05) on V100 and showed (R2 = 0.995; direct relationship) that when all the variables are combined, they contributed significantly (p = 0.000) to produce better target coverage. However, on an individual basis, only COIN (p = 0.004), TRAK (p = 0.000), PVol. (p = 0.001), rectum D10 (p = 0.000) and number of needles (p = 0.001) have significance predicting V100.
Conclusion
It can be seen from this study that if all the variables mentioned in this study are carefully acquired during treatment planning procedures, the resultant effects will be better coverage of the prostate gland and minimal dose to the OAR. Further study to assess the overall treatment outcome of these patients is still ongoing.
Acknowledgment
We would like to acknowledge Dr Edward Brandner, for his technical advice and Prof Saiful Huq, for providing professional mentorship (Online) for some of the authors (Medical Physicists).
Conflicts of interest
The authors have no conflicts of interest and therefore nothing to disclose.
Funding
No funding was received.
References
1. Morton GC (2005) The emerging role of high-dose-rate brachytherapy for prostate cancer Oncology 17(4) 219–227
2. Harris AA, Martin B, and Stang K, et al (2018) Impact of prostate gland size ≥60 cc on Physician and patient-reported toxicity after high dose rate prostate brachytherapy Int J Radiat Oncol Biol Phys 102(3) E116 https://doi.org/10.1016/j.ijrobp.2018.07.315
3. Yoshiya Y, Leland R, and Jeffrey D, et al (2012) American Brachytherapy Society Consensus guidelines for high-dose-rate prostate brachytherapy Brachytherapy 11(1) 20–32 https://doi.org/10.1016/j.brachy.2011.09.008
4. White EC, Kamrava MR, and Demarco J, et al (2013) High-dose-rate prostate brachytherapy consistently results in high quality dosimetry Int J Radiat Oncol Biol Phys 85(2) 543–548 https://doi.org/10.1016/j.ijrobp.2012.03.035
5. Morton GC (2015) Prostate high-dose-rate brachytherapy. Transrectal ultrasound-based planning, a technical note Pract Radiat Oncol 5(4) 238–240 https://doi.org/10.1016/j.prro.2014.12.009 PMID: 25703529
6. Morton GC and Hoskin PJ (2013) Brachytherapy: current status and future strategies- can high dose rate replace low dose rate and external beam radiotherapy? Clin Oncol 25 474–482 https://doi.org/10.1016/j.clon.2013.04.009
7. Chichel A, Marek K, and Janusz S, et al (2019) Correlation between treatment plan parameters and particular prognostic factors in prostate cancer treated with high-dose-rate brachytherapy (HDR_BT) as a boost J Contemp Brachytherapy 1(1) 11–17
8. Hoskin PJ, Colombo A, and Henry A, et al (2013) GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: an update Radiot Oncol 107 325–332 https://doi.org/10.1016/j.radonc.2013.05.002
9. Abdus-salam AA, Takure AO, and Akinlade BI, et al (2023) Early experience of high-dose-rate interstitial brachytherapy for prostate cancer in Nigeria J Radiat Med Trop 4(1) 1–5 https://doi.org/10.4103/jrmt.jrmt_24_21
10. Nath R, Meli JA, and Olch AJ, et al (1997) Code of practice for brachytherapy physics: report of the AAPM Radiation Therapy Committee Task Group No. 56 Med Phys 24(10) 1557–1598 https://doi.org/10.1118/1.597966 PMID: 9350711
11. Kubo HD, Glasgow GP, and Pethel TD, et al (1998) High dose-rate brachytherapy treatment delivery: report of the AAPM Radiation Therapy Committee Task Group No. 59 Med Phys 25(4) 375–403 https://doi.org/10.1118/1.598232 PMID: 9571605
12. Krauss DJ (2017) High dose rate brachytherapy for prostate cancer: current techniques and applications to varying disease presentations Appl Radiat Oncol 2017 7–17
13. American Cancer Society (2023) The American Cancer Society, downloaded from American Cancer Society Recommendations for Prostate Cancer Early Detection Washington, DC: American Cancer Society
14. Martinez A, Gonzalez J, and Ye H, et al (2011) Dose escalation improves cancer-related events at 10 years for intermediate and high-risk prostate cancer patients treated with hypofractionated high-dose rate boost and external beam radiotherapy Int J Radiat Oncol Biol Phys 79 363–370 https://doi.org/10.1016/j.ijrobp.2009.10.035 PMID: 21195875
15. Solanki AA, Mysz ML, and Patel R, et al (2019) Transitioning from a low-dose-rate to a high-dose-rate prostate brachytherapy program: comparing initial dosimetry and improving workflow efficiency through targeted interventions Adv Radiat Oncol 4 105–111
16. Kini VR, Edmundson GK, and Vicini FA, et al (1999) Use of three-dimensional radiation therapy planning tools and intraoperative ultrasound to evaluate high dose rate prostate brachytherapy implants Int J Radiat Oncol Biol Phys 43(3) 571–578 https://doi.org/10.1016/S0360-3016(98)00420-9 PMID: 10078639
17. Major T, Polgar C, and Jargo K, et al (2017) Dosimetric comparison between treatment plans of patients treated with low-dose-rate vs. High-dose-rate interstitial prostate brachytherapy as monotherapy. Initial findings of a randomized clinical trial Brachytherapy 16(3) 608–615






