Prevalence and associated factors of herbal medicine use among breast cancer patients: a cross-sectional study in Morocco
Anass Baladi1, Mohammed El Fadli1, Hassan Abdelilah Tafenzi1,2, Kawtar El Bouaouidi1, Nada Benhima1, Leila Afani1, Ismail Essâdi3,2 and Rhizlane Belbaraka1,2
1Department of Medical Oncology, Mohammed VI University Hospital of Marrakech, Marrakesh 40000, Morocco
2Laboratory of Biosciences and Health, Faculty of Medicine and Pharmacy of Marrakech, Marrakech 40000, Morocco
3Department of Medical Oncology, Avicenna Military Hospital of Marrakech, Marrakesh 40000, Morocco
Abstract
Background: Despite advances in modern medicine, an increasing number of breast cancer (BC) patients are turning to complementary and alternative medicine, such as phytotherapy. Instead of being prescribed by breast medical oncologists, patients are often seeking out phytotherapy themselves. They typically resort to herbal medicine as an alternative treatment to alleviate symptoms and side effects and enhance their quality of life during cancer treatment. This study, conducted in Morocco, aimed to investigate the prevalence and associated factors of herbal medicine use among BC patients.
Methods: A cross-sectional study of 170 patients with BC was carried out from October 2021 to January 2022 at the Mohamed VI University Hospital in Marrakech. Participants were selected using convenience sampling based on specific criteria such as being over 18 years old, having a histological diagnosis of BC, and being in active treatment. Data were collected through a structured questionnaire administered by trained clinicians, and medical records were reviewed for clinical data. Statistical analysis was conducted using Microsoft Forms for data collection and SPSS version 26 for statistical analysis. Descriptive statistics summarised demographic and health-related characteristics. Associations between herbal medicine use and categorical variables were assessed using chi-square and Fisher exact tests. Logistic regression analyses were performed to identify predictors of herbal medicine use, with statistical significance set at p < 0.05.
Results: Among the 170 BC patients included in the study, 37% reported using phytotherapeutics. One of the significant findings of this study was that nearly half of the BC patients surveyed believed herbal remedies to be harmless. None of the patients received information about herbal medicine use from their attending physicians. The use of herbal medication was significantly associated with marital status adjusted odds ratio (AOR: NR, p = 0.019), residence (AOR: 2.291, 95% confidence interval (CI): 1.214–4.324, p = 0.019), education levels (AOR: NR, p = 0.04) and receipt of radiotherapy (AOR: 0.128, 95% CI: 0.016–1.007, p = 0.023). Widowed women had a four times higher probability of using medicinal herbs than single or divorced women (AOR: 4.95, 95% CI: 1.16–20.90, p = 0.03). Illiterate women (AOR: 0.18, 95% CI: 0.052–0.65, p = 0.009) or those who attended Koranic school (AOR: 0.04, 95% CI: 0.004–0.47, p = 0.01) were less likely to use herbal medicine. Urban women were about twice as likely to use herbal remedies as women from rural areas (AOR: 2.02, 95% CI: 1.002–4.09, p = 0.049).
Conclusion: This study highlights the need for healthcare professionals to be aware of their patients’ possible use of herbal medicine, be familiar with commonly used herbal treatments, and take proactive steps to explain any potential drug interaction and associated benefits. The findings of this study also provide insight into information on the sociodemographic and health-related factors associated with the use of herbal medicines among BC patients.
Keywords: complementary and alternative medicine, integrative medicine, phytotherapy, breast cancer
Correspondence to: Anass Baladi
Email: anassbaladi2020@gmail.com
Published: 09/10/2024
Received: 02/06/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Breast cancer (BC) is one of the most substantial global health issues [1–4]. In women globally, it is the leading cancer by incidence with more cases diagnosed than lung cancer worldwide as of 2020 [1]. The disease makes up a huge proportion of cancer cases and deaths all over the world; almost 2.3 million new cases were reported last year along with 685,000 lives lost to it [2]. BC is still on the rise everywhere around the world which means that we are all affected differently based on where we live more advanced regions see higher survival rates while less developed ones struggle due to delays in diagnosis and ineffective treatment access [3]. The use of herbal medicine is considered very effective in combating the secondary effects that can result from conventional cancer treatment. According to research, herbal drugs such as licorice, astragalus, turmeric and others can prevent or weaken adverse reactions produced by chemotherapy and radiation therapy which helps improve the effectiveness of anti-cancer treatment as a whole [2, 5]. In a recent survey, 70% of cancer patients reported using complementary or alternative medicine (CAM) as part of their treatment plan [6]. In Spain, the use of complementary medicine (CM) among cancer patients is notably high, with studies indicating that approximately 81% of the general population has utilised some form of complementary therapy at least once [7]. Saudi studies found that the use of CAMs by cancer patients ranged between 25% and 80%, with natural products topping the list as the most commonly chosen type [8]. The prevalence of phytotherapy use among BC patients in Africa is notable, with traditional healers and practitioners incorporating medicinal plants in the treatment of BC due to factors like the high cost of conventional treatments and limited access to healthcare services [9–11]. The Moroccan population frequently practices phytotherapy. Studies have shown that, depending on the region, 55% to 90% of patients use plants to treat chronic diseases such as diabetes, hypertension and urinary infections [12].
Phytotherapy involves using plant-based medicines and supplements to treat illnesses and BC patients often use it to alleviate symptoms and the side effects of conventional cancer treatments. Studies have shown that phytotherapy, which involves the use of medicinal plants, is increasingly recognised for its potential in BC treatment, either alone or in combination with traditional therapies [9]. However, there is a substantial variation in prevalence, patterns, reasons for use and user characteristics across different geographic regions [13]. Many complementary therapies, particularly herbal remedies, lack controlled clinical trials to test their effects and some may interact with standard treatments and cause adverse effects. This poses a significant challenge for oncologists [14]. This study aimed to investigate the prevalence and associated factors of herbal medicine use among BC patients on standard treatment regimens in Morocco.
Methods
Study design and setting
We conducted a cross-sectional study between October 2021 and January 2022 in the medical oncology Department of Mohamed VI University Hospital in Marrakech, Morocco.
Sample size and sampling technique
The sample size was not determined using a specific estimation formula, as we included all available and willing patients during the data collection period. Since we worked with all eligible patients who consented to participate in the study, this was not a random sample or sample size drawn, but rather a convenience sample based on the inclusion criteria and patient availability. There were two groups of patients: Phyto users and non-Phyto users.
Inclusion and exclusion criteria
Participants had to be over 18 years old with a histological diagnosis of BC. Exclusion criteria included patients currently in the diagnosis process, those who refused the questionnaire or those in palliative care who were too weary to participate. Patients younger than 18 years were excluded from the study for several reasons. First, BC is sporadic among adolescents, meaning their inclusion could skew results and statistical analyses. Second, the ethical considerations and parental consent required to include minors in research studies can be complex and may limit the ability to collect reliable and consistent data.
Data collection technique
We used a structured questionnaire to collect data on the use of herbal medicines among BC patients. However, we acknowledge that the questionnaire was not subjected to a formal local validation process specific to the Moroccan context. Instead, the questionnaire was adapted from previous studies with similar objectives and was reviewed by oncologists, to ensure cultural relevance and comprehensibility for the study population. Illiterate patients were assisted in completing the questionnaire to ensure accurate data collection. Information collected included sociodemographic variables, use of medicinal plants, reasons for their use, sources of information and patient perspectives on herbal versus synthetic medicines. Clinical parameters and follow-up data were obtained from medical records. In this study, patients were recruited from a diverse range of medical situations within the oncology department. These included patients undergoing curative treatment regimens as well as those with metastatic disease. The distinction between these groups was based on clinical assessments and staging according to the eighth edition of the American Joint Committee on Cancer TNM staging system.
Operational definitions
Phyto users: Patients who reported using herbal medicines.
Non-Phyto users: Patients who did not report using herbal medicines.
Herbal medicines: Plant-based substances used for medicinal purposes.
Data analysis procedure
Data collection was done using Microsoft Forms, and statistical analysis was performed using SPSS version 26. Descriptive statistics, including frequency counts, percentages and mean standard deviations for categorical data, were calculated. The relationship between herbal remedy usage and other demographic and health-related factors was assessed using chi-square and Fisher exact tests. We used univariate and multivariate logistic regression analyses to control for confounders in this study. These analyses allowed us to evaluate the impact of each independent variable on the use of herbal medicine while adjusting for other variables that could confound the results. Variables that showed a statistically significant correlation in the univariate analysis were included in the multivariate model to determine their independent effect on medicinal plant use. Additionally, patients’ sociodemographic and clinical data were carefully collected and considered to minimise potential bias. Statistically significant differences were defined as p-values less than 0.05.
Results
Sociodemographic and health-related characteristics
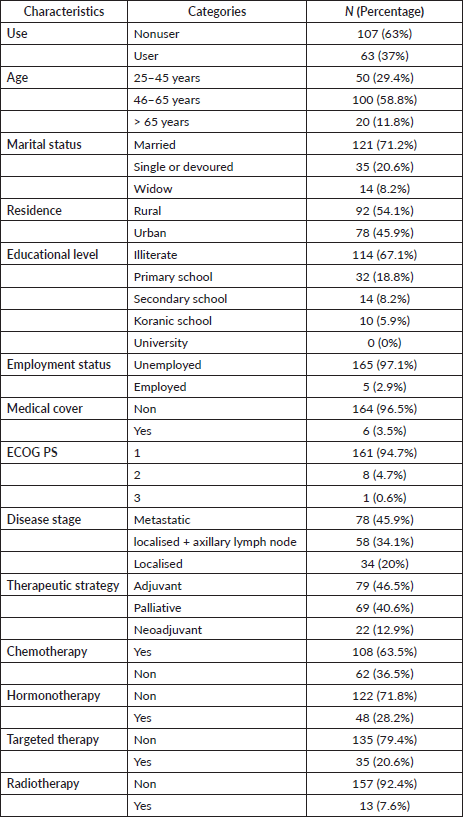
A total of 170 participants were selected in the medical oncology department, out of which 63 patients (37%) were identified as herbal medicine users compared to 107 patients (62.94%) who were not. Thirty patients (47.6%) had metastatic-stage cancer. All patients included in the study were female, with a mean age of 51.3 years (range: 25–75 years). The majority of patients fell between the ages of 40 and 70, with a standard deviation of 10.9 and a median of 52.5 (43.0; 59.0). All patients received treatments, including chemotherapy (63.5%, n = 108), targeted therapy (20.6%, n = 35), hormonal therapy (28.2%, n = 48) and radiotherapy (7.6%, n = 13), either alone or in combination.
Of the total respondents, 121 patients (71.17%) were married and the majority of respondents, 114 patients (67.1%), were illiterate. Ninety-two patients (54.1%) were from rural areas, and only six had medical insurance (Table 1).
Table 1. Sociodemographic and health-related characteristics of BC patients at Mohammed VI Oncological Center, Marrakech (October 2021–January 2022).
Association between herbal medicine use and sociodemographic and health-related factors
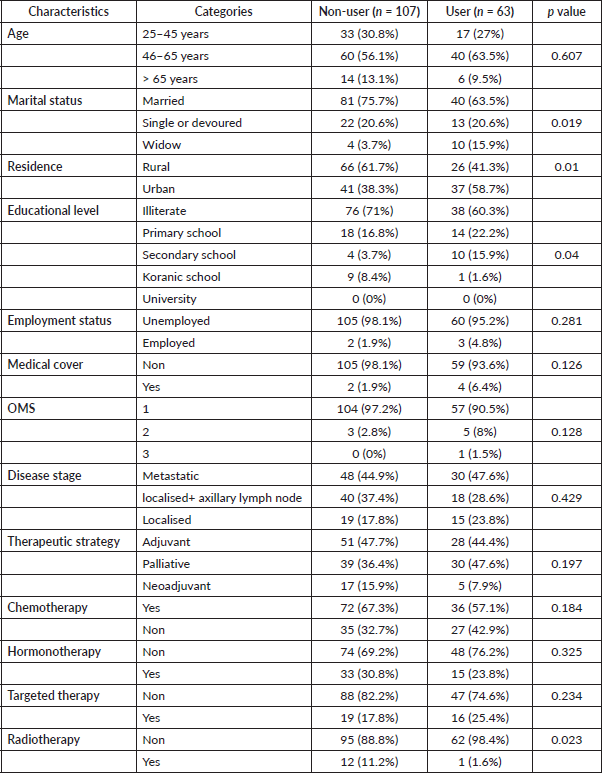
Table 2 displays the statistically significant associations between the usage of herbal medication and marital status adjusted odds ratio (AOR: NR, p = 0.019), residence (AOR: 2.291, 95% confidence interval (CI): 1.214–4.324, p = 0.019), education levels (AOR: NR, p = 0.04) and receipt of radiotherapy (AOR: 0.128, 95% CI: 0.016–1.007, p = 0.023).
Table 2. Association between sociodemographic and health-related factors and herbal medicine use among BC patients at Mohammed VI Oncological Center, Marrakech (October 2021–January 2022).
Predictors of herbal medicine use
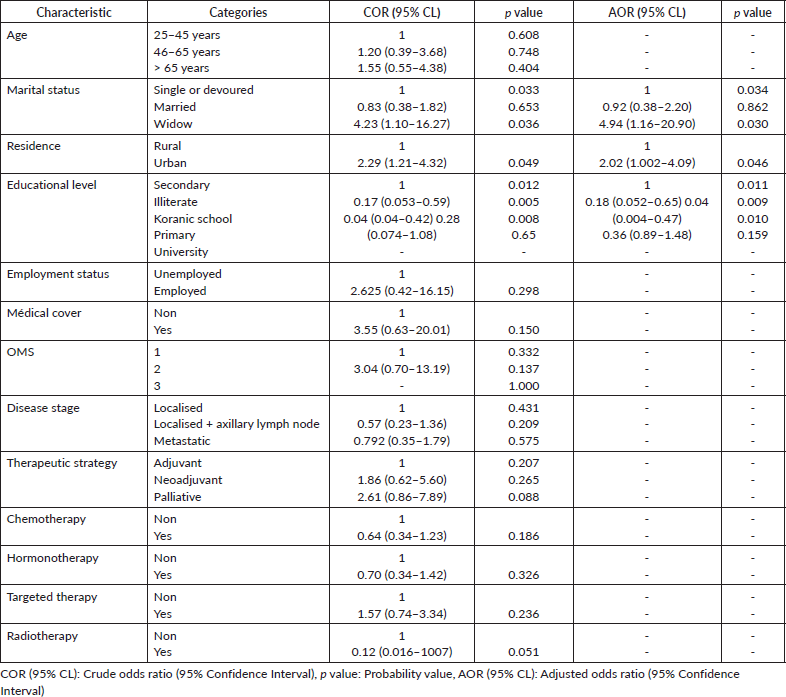
Table 3 displays the results of both univariate and multivariate logistic regression analyses concerning predictors of herbal medicine usage among BC patients after treatment. The analysis revealed that widowed women (AOR 4.95, 95% CI 1.16–20.90, p = 0.03) had a four times higher probability of using herbal remedies compared to single or divorced women. In addition, illiterate women (AOR: 0.18, 95% CI: 0.052–0.65, p = 0.009) and those who attended Koranic school (AOR: 0.04, 95% CI: 0.004–0.47, p = 0.01) were less likely to use herbal medicine. Moreover, women from urban areas were twice as likely to use herbal remedies as women from rural areas (AOR: 2.02, 95% CI: 1.002–4.09, p = 0.049).
Table 3. Predictors of herbal medicine use among BC patients at Mohammed VI Oncological Center, Marrakech (October 2021–January 2022).
Sources of information for herbal medicine
Table 4 shows the sources of information on the herbal medicines used among patients with BC. Almost half of the patients (63,49%, n = 40) obtained information from their direct entourage, while 38% (n = 29) obtained information through discussions with other patients. The use of medicinal plants was recommended by family or friends in 66.66% (n = 42) of the cases, while the patient’s own choice accounted for 36.50% (n = 23) of the cases. None of the patients in this study received information about herbal medicine use from their attending physicians.
Types of plants
Thirteen different traditional substances were used by the patients. Twenty-nine patients (46.03%) used turmeric as an herbal medicine, while 26 patients (41.2%) used fenugreek. Additionally, 32 patients (50.7%) used a combination of plants. The specific plant species used by the patients are detailed in Figure 1.
How patients take herbal medicine
Among patients, 33 (52.38%) began using phytotherapy before starting chemotherapy, 17 (26%) started during chemotherapy and 15 (23.8%) used it after completing chemotherapy, despite the potential risk of interaction with oncological treatment. Only six patients (10%) reported taking the herbal remedies daily (Table 4).
Patients’ beliefs about herbal medicine
More than 50% of the patients believed that herbal remedies were harmless. Sixteen patients (25%) using phytotherapy believed that these plants have a synergistic effect with their treatment. Only 3% believed that herbal medicine is more effective than conventional treatment, while 31 patients (49%) believed it is safe. Twenty-four patients (38%) of patients believed that these plants are beneficial in alleviating the side effects of chemotherapy, with 27 patients (42%) reporting alleviation of pain and 7 patients (10%) reporting a decrease in nausea and vomiting. Five patients (7%) reported an improvement in diarrhea after using herbal medicine. Only one patient declared that she takes the plants to avoid the risk of neutropenia and to prevent postponing chemotherapy sessions. Twenty-two patients (35%) received sufficient information on the effectiveness and safety of herbal medicine before using it. 30 patients (43.47%) reported no benefit from using herbal medicine, while 8 patients (11.57%) reported a disappearance of pain and 5 patients (7.2%) reported feeling better and healthier after using it. Three patients reported a reduction in the size of their tumours (Table 4).
Adverse effects
Six patients exhibited an impact on their liver function tests, and two of them required a dosage reduction according to the recommendations. Among the patients, 19 patients (31%) reported adverse effects, with weight loss being the most commonly reported at 11 patients (17%), followed by anorexia at 8 patients (11.5%) (Table 4).
Discussion
Herbal remedies or phytotherapy, are commonly used by cancer patients worldwide as CAM. Patients often self-medicate with natural products made from whole or part of plants [15–17]. Although these remedies contain potentially active components, their interactions with conventional medications may increase side effects or decrease the effectiveness of BC treatments. This can lead to compromised treatment outcomes and increased health risks for patients [18].
Table 4. Pattern and perception of herbal medicine use among BC patients at Mohammed VI Oncological Center, Marrakech (October 2021–January 2022).
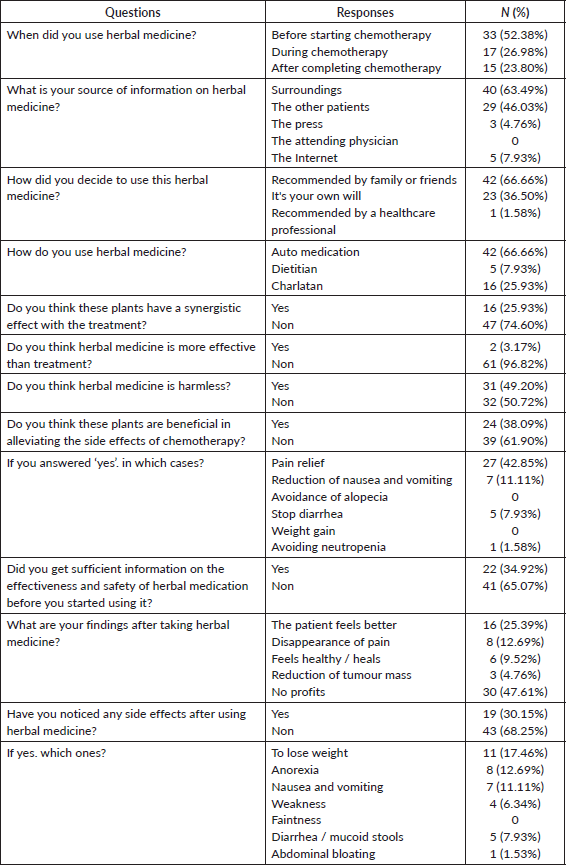
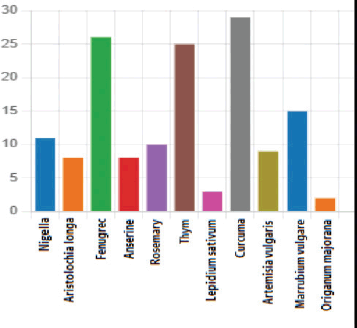
Figure 1. The different plant species used by BC patients at the Mohammed VI Oncological Center in Marrakech (October 2021–January 2022).
Numerous studies have examined the prevalence and knowledge of phytotherapy among patients with BC. An evaluation report indicated patients from the United States, Europe, China and Australia. The prevalence rates ranged from 40.3% to 94.7%. Among the causes for this wide range noted by the authors are disparities in the definitions of CAM, different modalities of CAM, as well as variations in research methods (self-reporting or interviews) and geographic plus cultural factors together with attitude toward acceptance of CAM [19]. The rate of herbal medicine use among women with BC differs from one study to another. These studies show that a large number of women with BC use herbal products in addition to other therapies [9, 18, 20]. Brazilian cancer patients turn to herbal remedies up to 48.12%, attracted by the promise of relief from symptoms, among other benefits [21]. At the same time, a German study revealed that 30.1% of patients treated for breast or gynecological cancer by systemic therapy used herbal medicine in addition to conventional treatment [22]. A study conducted in Nigeria reports a prevalence of herbal medicine use of 34.5%, which is similar to the finding of 37%. Our study population had an average age of 51.3 years, consistent with previous studies in Fez [23] (49.1 years), France [21] (57.2 years), Nigeria [25] and others (51.9 years). These findings suggest that a significant number of BC patients use phytotherapy as a complementary therapy.
Phytotherapy may potentially improve or decrease the efficacy of conventional BC treatments. Among the plants used by the patients, curcumin was the most commonly used at 46.03% (n = 29). It is a natural compound found in turmeric, which is widely cultivated in Southeast Asia [26, 27]. Curcumin exhibits potential anti-cancer properties by influencing several biological mechanisms. A meta-analysis of randomised clinical trials indicates that curcumin effectively reduces TNF-α levels, a pro-inflammatory cytokine linked to chronic inflammation and various cancers. By lowering TNF-α, curcumin may help mitigate chronic inflammation, potentially slowing or preventing cancer progression driven by inflammation [28]. In vitro and animal studies reveal that curcumin can inhibit Stat3, a protein crucial for cancer cell growth and survival. It also suppresses matrix metalloproteinases, which degrade the extracellular matrix and facilitate cancer spread, and vascular endothelial growth factor, which supports tumour blood vessel formation [29, 30]. Curcumin promotes cancer cell apoptosis through caspase-dependent and mitochondrial pathways [31, 32]. Additionally, it negatively regulates cyclin-dependent kinases, which control cell cycle progression, thereby slowing cancer cell division [32]. In BC, curcumin might inhibit chemotherapy-induced apoptosis by blocking the c-Jun NH2-terminal kinase pathway and generating reactive oxygen species. This suggests curcumin could protect normal cells during chemotherapy, although more research is needed to fully understand its effects on cancer cells and its overall impact [33]. In an animal experimental study, the bioactive compounds in turmeric like curcumin C3 complex and bisdemethoxycurcumin have been shown to attenuate glial activation, oxidative stress and mitochondrial dysfunction, including mechanical hypersensitivity in neuropathic pain conditions [34]. Curcumin is known to inhibit cytochrome P450 enzymes, potentially affecting the metabolism of certain medications, though its clinical relevance remains unclear [35–38]. In a study of 60 BC patients, 2 g/day of curcumin caused only minor, clinically insignificant changes in paclitaxel pharmacokinetics [39, 40]. Additionally, curcumin inhibits doxorubicin-induced apoptosis in BC cell lines and impedes cyclophosphamide-induced tumour regression in animal studies, but the clinical significance of these effects is still unknown [33]. Turmeric has shown promise in relieving musculoskeletal pain. In a pilot, randomised clinical trial, Studies have highlighted the potential benefits of turmeric extracts for relieving knee joint pain [41, 42], with formulations containing water-soluble turmeric extracts demonstrating analgesic effects and potential inflammation reduction [42]. Additionally, A systematic review has explored the effects of turmeric on musculoskeletal pain, indicating a significant reduction in pain levels comparable to nonsteroidal anti-inflammatory drugs like ibuprofen [43].
The second most commonly used plant was fenugreek at 41.2% (n = 26). Fenugreek is an herbaceous plant of the Fabaceae family native to the Middle East and India. It is mainly used as a medicinal and condiment plant, and its active substance is isolated in seeds, which are still found in the form of seeds included in preparations. Fenugreek has been found to have anticancer effects, and its chemical components are known to cause apoptosis. A study by Khoja et al [44]. has demonstrated that MCF-7 breast cells can be effectively killed by chloroform seed extract, and according to Amin et al [45], this little plant has a protective effect on BC cells and prevents MDA 231 from inducing mammary hyperplasia. In our country, fenugreek is used as a tonic, for hair care, as a hypoglycemic and against heart palpitations. These findings suggest that phytotherapy may have synergistic effects with conventional BC treatments, potentially improving treatment outcomes. However, more clinical studies are needed to validate the effectiveness and safety of phytotherapy in combination with conventional treatments.
The univariate and multivariate logistic regression analyses of extracted variables related to phytotherapy usage among BC patients after treatment showed that widowed women (AOR 4.95, 95% CI 1.16–20.90, p = 0.03) had a four times higher probability of using herbal remedies than those who were single or divorced. In a Nigerian study, this percentage was close to our average (77.9%) [25]. Üstünda et al [46] investigated the relationship between civil status and the prevalence of the use of herbal medicines, but the results were statistically insignificant (p = 0.78). Research indicates that married/divorced women are more likely to use herbal medicine daily, with a 4.4-fold increase compared to unmarried individuals [47]. Widowed women tend to use herbal remedies more than those who are single or divorced due to various factors. Research shows widows are more independent and have a stronger work ethic. They also put in more hours and make all of the decisions regarding providing for their families [48]. Moreover, women are burdened by societal expectations, which makes them susceptible during widowhood especially when they do not have time for self-care [49]. Additionally, herbal medicines have traditionally been used to alleviate symptoms and improve the quality of life in different stages of women’s lives, including after menopause, which could contribute to the preference of widowed women for herbal remedies [50]. In general, the combination of cultural beliefs, perceived benefits and historical usage of herbal remedies likely influences widowed women to use these natural treatments more frequently.
In our study, it was found that illiterate women (AOR: 0.18, 95% CI: 0.052–0.65, p = 0.009) or those who attended Koranic school (AOR: 0.04, 95% CI: 0.004–0.47, p = 0.01) were less likely to use herbal medicine. A study in Istanbul found that highly educated individuals preferred herbal therapy more, highlighting a potential correlation between education level and herbal medicine use [51]. In Germany, where plant remedies are commonly used alongside conventional care, medical students showed a high knowledge and usage of phytotherapy, suggesting that education promotes herbal medicine use [52]. Illiterate women and those who attended Koranic schools may have less access to written information about herbal medicines, including their uses, benefits and potential risks. This limited access can reduce their likelihood of using phytotherapy [46]. Additionally, The use of herbal medicines is common among people seeking alternative treatments for BC, with significant belief in their effectiveness [52, 53]. Educated women can try herbal medicine because of its promising anti-cancer properties and the possibility of alleviating the side effects of conventional treatments [53]. According to other research reports, illiterate women who do not have access to or do not believe in current health systems generally receive herbal remedies based on recommendations from other locals or traditional herbalists, without scientific training [54]. The use of alternative medicine is generally more practiced by individuals with a low level of education, because they may have a negative view of conventional medicine (considering it contrary to alternative methods) or have the feeling that doctors do not support their preferred choice due to their noncompliance with alternative methods [51]. These discrepancies could be attributed to cultural differences, access to healthcare services and varying perceptions of herbal medicine across different regions.
The results of our study indicated that individuals residing in urban regions were more likely to utilise herbal medicine than those residing in rural areas (AOR: 2.02, 95% CI: 1.002–4.09, p = 0.049), which contrasts with the common assumption that individuals in rural areas would use them more frequently. These findings are consistent with those of previous studies [55,56]. Access to information is indeed higher in urban areas, potentially leading to increased awareness of herbal remedies among city dwellers [57]. Urban environments, characterised by cultural diversity, are more cosmopolitan and open to integrating herbal medicinal practices from various cultures [58]. Studies show that urban residents tend to have better access to health information through various channels such as social networks and interpersonal communication, which can influence their beliefs about herbal medicine [59]. However, there is a need for improved health education emphasising herbal products and socio-economic factors to promote herbal consumption among urban populations [60]. This highlights the importance of tailored strategies for urban areas to leverage their information access and cultural diversity for promoting herbal medicinal practices.
The primary source of information regarding phytotherapy was found to be the individual’s social circle, accounting for 74.3% of cases, as observed in a study conducted in Fez, as well as in subsequent studies in France, Nigeria and a European meta-analysis [24, 25, 61, 62]. Another significant source of information, as reported in an American study, was the Internet [63]. However, it is important to warn patients that not all information available in search engines can be relied upon. In Morocco, despite the increasing availability of internet access, our study found that it only accounted for 7.93% of the sources of information for patients.
BC patients often use phytotherapy to alleviate the side effects of conventional treatments, such as chemotherapy-induced nausea and vomiting (CINV), fatigue and pain. Based on the research provided, it is evident that ginger has been extensively studied for its effectiveness in reducing CINV in cancer patients [64–67]. Multiple randomised controlled trials have shown that ginger can significantly decrease the severity and frequency of CINV, without causing serious side effects, making it a safe option for managing this distressing side effect of chemotherapy [64–66]. Similarly, ginseng, another plant-based drug, has been found to improve fatigue in patients with BC who undergo chemotherapy [68]. Although some studies suggest that ginseng extracts may have estrogenic effects, particularly at low concentrations, which could potentially stimulate the growth of oestrogen receptor positive BC cells [69], other research indicates that ginseng can induce apoptosis in estrogen receptor-positive BC cells and enhance the effects of tamoxifen, a common treatment for this type of cancer [70]. Overall, the evidence is mixed, suggesting that some forms of ginseng may exhibit estrogen-like effects, while others might provide benefits in the treatment of hormone-sensitive BC.
In our study, 43.7% of patients reported no perceived benefit from using herbal medicine. These findings are consistent with a study conducted in Nigeria, which found that 58.3% of the patients reported a reduction in symptoms after using herbal medicine [71]. However, it is challenging to assess the effectiveness of phytotherapy since patients in our study used it alongside conventional treatment. Despite the potential benefits of phytotherapy in the treatment of BC, it is crucial to note that plant-based medicines and supplements can also have adverse effects and interact with conventional treatments. For example, St. John’s wort, a commonly used plant-based supplement, can interact with chemotherapy drugs, reducing their efficacy [72].
In our study, we observed the impact of herbal medicines on liver function tests in six patients and 31% (n = 19) reported experiencing adverse effects. The liver function of these patients improved after stopping herbal medicine. Among these, weight loss was the most frequently reported at 17% (n = 11), followed by anorexia at 11.5% (n = 8) [73]. These results are consistent with findings from previous studies. For instance, a study conducted in Japan found that 62.2% of patients did not experience adverse effects, while 5.3% reported side effects such as nausea, diarrhea, constipation, rash and liver dysfunction [74]. A European meta-analysis showed that only 4.4% of the patients reported side effects from complementary therapies, such as stomach upset and nausea, pruritus, headache and deterioration of kidney function [62].
This study highlights the significant prevalence of the use of herbal medicine by BC patients. Healthcare providers should be aware of this and discuss herbal medicines with their patients to ensure that they are taking care of them completely and that there are no other conventional treatments that interact with them. However, patients often do not inform their physician about their use of herbal medicine, as they may feel uncomfortable or uncertain about how their physician will react. This miscommunication between the oncologist and the patient often arises because the former is primarily concerned with the potential toxic effects and the risk that the patient may abandon conventional treatment in favour of CM, while the patient is more focused on the benefits of phytotherapy. Further research could focus on specific herbal remedies often used by women with BC, evaluating how they work, their risk profiles as well as possibilities for interaction with traditional therapies. Longitudinal surveys could also examine herb use treatment outcomes and quality of life in BC patients.
Study limitations
In our cross-sectional study, the main objective was to assess the prevalence of herbal medicine use among BC patients, as well as the factors associated with this use. We collected information on the general use of herbal products, but due to the design of the study, it was difficult to obtain specific details on aspects such as dose, frequency and duration of use. The participants were chosen conveniently rather than through a random or representative process. The results of the study may not be generalisable to the entire population.
Conclusion
The prevalence of phytotherapy in cancer patients is high, as shown by the results of our study and supported by data from the literature. The effectiveness and safety of phytotherapy in the context of BC treatment remain unclear. More research is needed to determine its potential benefits and risks. It is essential for BC patients using phytotherapy to seek advice from their healthcare providers and be aware of possible interactions with conventional treatments. In our study, more than half of patients believe that natural products are harmless, and there is an urgent need to educate patients about the potential risks associated with medicinal plants. As healthcare providers, it is crucial to discuss the use of CAM therapies with BC patients and provide evidence-based information to help them make informed decisions about their treatment options. Understanding these factors will help oncologists provide better care to BC patients and avoid potentially harmful interactions between herbal remedies and standard treatments.
List of abbreviations
AOR, Adjusted odds ratios; BC, Breast cancer; CAM, Complementary and alternative medicine; CINV, chemotherapy-induced nausea and vomiting; COR, Crude odds ratios.
Acknowledgments
Not applicable.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
None.
Ethics approval and consent to participate
The Ethics Review Committee of the Marrakech Faculty of Medicine and Pharmacy granted its approval for data access. It was not necessary to obtain the patient’s informed permission. Before analysis, patient records were anonymised to ensure confidentiality. All methods were performed by the relevant guidelines and regulations.
Consent for publication
Not applicable.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on a reasonable request.
Author contributions
Conception and design: Anass Baladi, Mohammed El Fadli, Hassan Abdelilah Tafenzi, Rhizlane Belbaraka, Ismail Essaadi
Statistical analysis: Anass Baladi, Hassan Abdelilah Tafenzi
Data interpretation: All Authors
Financial support: All Authors
Administrative support: Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech, Morocco & Medical Oncology Department, Mohammed VI University Hospital, Marrakech, Morocco, Bioscience and Health Laboratory.
Provision of study materials or patients: Anass Baladi, Hassan Abdelilah Tafenzi
Drafting: Anass Baladi
Review, revise and approve the manuscript: All authors.
References
1. Ali Salman R (2023) Prevalence of women breast cancer Cell Mol Biomed Rep 3(4) 185–196 https://doi.org/10.55705/cmbr.2023.384467.1095
2. Wilkinson L and Gathani T (2022) Understanding breast cancer as a global health concern Br J Radiol 95(1130) 20211033 https://doi.org/10.1259/bjr.20211033 PMCID: 8822551
3. Khanikar D, Kamalasanan K, and Krishnamurthy A, et al (2022) Fundamentals in Gynaecologic Malignancy [Internet] (Singapore: Springer Nature Singapore) pp 133–181 https://link.springer.com/10.1007/978-981-19-5860-1_10 Date accessed: 10/05/24
4. Sharma R (2021) Global, regional, national burden of breast cancer in 185 countries: evidence from GLOBOCAN 2018 Breast Cancer Res Treat 187(2) 557–567 https://doi.org/10.1007/s10549-020-06083-6 PMID: 33515396
5. Patial A, Kumar P, and Sunita S, et al (2023) Herbal medicine used in cancer treatment Res J Pharmacogn Phytochem 33–38
6. Hutten RJ, Weil CR, and King AJ, et al (2023) Multi-institutional analysis of cancer patient exposure, perceptions, and trust in information sources regarding complementary and alternative medicine JCO Oncol Pract 19(11) 1000–1008 https://doi.org/10.1200/OP.23.00179 PMID: 37722084
7. Guerra-Martín MD, Tejedor-Bueno MS, and Correa-Casado M (2021) Effectiveness of complementary therapies in cancer patients: a systematic review Int J Environ Res Public Health 18(3) 1017 https://doi.org/10.3390/ijerph18031017 PMID: 33498883 PMCID: 7908482
8. Hutten RJ, Weil CR, and Barney BM, et al (2022) Complementary and alternative medicine exposure in oncology (CAMEO) study: a multi-institutional cross-sectional analysis of patients receiving cancer treatment J Clin Oncol 40(16_suppl) e18739 https://doi.org/10.1200/JCO.2022.40.16_suppl.e18739
9. Anago AD, Gaetan Segbo JA, and Gnangnon F, et al (2023) Some medicinal plants with anti-breast cancer activity and the input of phytotherapy in the treatment of breast cancer Eur Sci J ESJ 19(18) 66
10. Thiam K, Emhemmed F, and Diop A, et al (2024) Ethnobotanical survey and biological activities of plants used for cancer treatment in traditional Senegalese medicine Eur J Chem 15(1) 17–24 https://doi.org/10.5155/eurjchem.15.1.17-24.2501
11. Lutoti S, Kaggwa B, and Kamba PF, et al (2023) Ethnobotanical survey of medicinal plants used in breast cancer treatment by traditional health practitioners in Central Uganda J Multidiscip Healthc 16 635–651 https://doi.org/10.2147/JMDH.S387256 PMID: 36919184 PMCID: 10008314
12. Zeggwagh AA, Lahlou Y, and Bousliman Y (2013) Enquete sur les aspects toxicologiques de la phytotherapie utilisee par un herboriste à Fes, Maroc Pan Afr Med J [Internet] 14 125 [http://www.panafrican-med-journal.com/content/article/14/125/full/] Date accessed: 10/05/24 https://doi.org/10.11604/pamj.2013.14.125.1746
13. Cui Y, Shu XO, and Gao Y, et al (2004) Use of complementary and alternative medicine by Chinese women with breast cancer Breast Cancer Res Treat 85(3) 263–270 https://doi.org/10.1023/B:BREA.0000025422.26148.8d PMID: 15111765
14. Ambrosone CB, Zirpoli GR, and Hutson AD, et al (2020) Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221) J Clin Oncol Off J Am Soc Clin Oncol 38(8) 804–814 https://doi.org/10.1200/JCO.19.01203
15. Matriz J, Hamachi A, and Fukui JA (2024) The use of complementary and alternative medicine (CAM) among patients with cancer in Hawaii J Clin Oncol 42(16_suppl) e24070 https://doi.org/10.1200/JCO.2024.42.16_suppl.e24070
16. Pai GA, Lobo AR, and Biradar B, et al (2024) IJCM_34A: usage of complementary / alternative medicine (CAM) and perception towards it among cancer patients admitted in tertiary care hospitals Indian J Community Med 49(Suppl 1) S10 https://doi.org/10.4103/ijcm.ijcm_abstract34 PMCID: 11156153
17. Sharma AN, Dewangan HK, and Upadhyay PK (2024) Comprehensive review on herbal medicine: emphasis on current therapy and role of phytoconstituents for cancer treatment Chem Biodivers 21(3) e202301468 https://doi.org/10.1002/cbdv.202301468 PMID: 38206170
18. Lopes CM, Dourado A, and Oliveira R (2017) Phytotherapy and nutritional supplements on breast cancer BioMed Res Int 2017 7207983 https://doi.org/10.1155/2017/7207983 PMID: 28845434 PMCID: 5563402
19. Akpunar D, Bebis H, and Yavan T (2015) Use of complementary and alternative medicine in patients with gynecologic cancer: a systematic review Asian Pac J Cancer Prev 16(17) 7847–7852 https://doi.org/10.7314/APJCP.2015.16.17.7847 PMID: 26625809
20. Schnell-Inderst P, Steigenberger C, and Mertz M, et al (2022) Additional treatment with mistletoe extracts for patients with breast cancer compared to conventional cancer therapy alone – efficacy and safety, costs and cost-effectiveness, patients and social aspects, and ethical assessment Ger Med Sci 20 Doc10 https://www.egms.de/en/journals/gms/2022-20/000312.shtml
21. Monteiro CRDA, Schoueri JHM, and Turke KC, et al (2020) USO DE FITOTERÁPICOS EM PACIENTES COM CÂNCER NA REGIÃO DO GRANDE ABC Clin Oncol Lett [Internet] 4(1) https://www.clinicaloncologyletters.com/article/doi/10.4322/col.2019.001 Date accessed: 10/05/24
22. Drozdoff L, Klein E, and Kiechle M, et al (2018) Use of biologically-based complementary medicine in breast and gynecological cancer patients during systemic therapy BMC Complement Altern Med 18(1) 259 https://doi.org/10.1186/s12906-018-2325-3 PMID: 30249217 PMCID: 6154925
23. Bellakhdar J, Claisse R, and Fleurentin J, et al (1991) Repertory of standard herbal drugs in the Moroccan pharmacopoea J Ethnopharmacol 35(2) 123–143 https://doi.org/10.1016/0378-8741(91)90064-K PMID: 1809818
24. Träger-Maury S, Tournigand C, and Maindrault-Goebel F, et al (2007) Use of complementary medicine by cancer patients in a French oncology department Bull Cancer (Paris) 94(11) 1017–1025
25. Ezeome ER and Anarado AN (2007) Use of complementary and alternative medicine by cancer patients at the University of Nigeria Teaching Hospital, Enugu, Nigeria BMC Complement Altern Med 7 28 https://doi.org/10.1186/1472-6882-7-28 PMID: 17850665 PMCID: 2034592
26. Kumar P, Sujata, and Archana (2023) Medicinal importance of turmeric (Curcuma longa) and its natural products Frontiers in Natural Product Chemistry eds S Anjum (Bentham Science Publishers) [Internet] pp 1–31 https://www.eurekaselect.com/node/220398 Date accessed: 24/07/24
27. Verma RK, Kumari P, and Maurya RK, et al (2018) Medicinal properties of turmeric (Curcuma longa L.): a review Int J Chem Stud 6(4) 1354–1357
28. Sahebkar A, Cicero AFG, and Simental-Mendía LE, et al (2016) Curcumin downregulates human tumor necrosis factor-α levels: a systematic review and meta-analysis ofrandomized controlled trials Pharmacol Res 107 234–242 https://doi.org/10.1016/j.phrs.2016.03.026 PMID: 27025786
29. Alexandrow MG, Song LJ, and Altiok S, et al (2012) Curcumin: a novel Stat3 pathway inhibitor for chemoprevention of lung cancer Eur J Cancer Prev 21(5) 407–412 https://doi.org/10.1097/CEJ.0b013e32834ef194 PMCID: 3319490
30. Lin SS, Lai KC, and Hsu SC, et al (2009) Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF) Cancer Lett 285(2) 127–133 https://doi.org/10.1016/j.canlet.2009.04.037 PMID: 19477063
31. Chen QY, Lu GH, and Wu YQ, et al (2010) Curcumin induces mitochondria pathway mediated cell apoptosis in A549 lung adenocarcinoma cells Oncol Rep 23(5) 1285–1292 https://doi.org/10.3892/or_00000762 PMID: 20372842
32. Wu SH, Hang LW, and Yang JS, et al (2010) Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways Anticancer Res 30(6) 2125–2133 PMID: 20651361
33. Somasundaram S, Edmund NA, and Moore DT, et al (2002) Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer Cancer Res 62(13) 3868–3875 PMID: 12097302
34. Santos JM, Wang R, and Bhakta V, et al (2023) Turmeric bioactive compounds alleviate spinal nerve ligation-induced neuropathic pain by suppressing glial activation and improving mitochondrial function in spinal cord and amygdala Nutrients 15(20) 4403 https://doi.org/10.3390/nu15204403 PMID: 37892476 PMCID: 10610406
35. Zhang W and Lim LY (2008) Effects of spice constituents on P-glycoprotein-mediated transport and CYP3A4-mediated metabolism in vitro Drug Metab Dispos Biol Fate Chem 36(7) 1283–1290 https://doi.org/10.1124/dmd.107.019737 PMID: 18385293
36. Chen Y, Liu WH, and Chen BL, et al (2010) Plant polyphenol curcumin significantly affects CYP1A2 and CYP2A6 activity in healthy, male Chinese volunteers Ann Pharmacother 44(6) 1038–1045 https://doi.org/10.1345/aph.1M533 PMID: 20484172
37. Volak LP, Hanley MJ, and Masse G, et al (2013) Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers Br J Clin Pharmacol 75(2) 450–462 https://doi.org/10.1111/j.1365-2125.2012.04364.x PMCID: 3579260
38. Al-Jenoobi FI, Al-Thukair AA, and Alam MA, et al (2015) Effect of Curcuma longa on CYP2D6- and CYP3A4-mediated metabolism of dextromethorphan in human liver microsomes and healthy human subjects Eur J Drug Metab Pharmacokinet [Internet] 40(1) 61–66 Date accessed: 15/08/24 https://doi.org/10.1007/s13318-014-0180-2
39. Costa ML, Rodrigues JA, and Azevedo J, et al (2018) Hepatotoxicity induced by paclitaxel interaction with turmeric in association with a microcystin from a contaminated dietary supplement Toxicon Off J Int Soc Toxinol 150 207–211 https://doi.org/10.1016/j.toxicon.2018.05.022
40. Kalluru H, Mallayasamy SR, and Kondaveeti SS, et al (2022) Effect of turmeric supplementation on the pharmacokinetics of paclitaxel in breast cancer patients: a study with population pharmacokinetics approach Phytother Res 36(4) 1761–1769 https://doi.org/10.1002/ptr.7412 PMID: 35181963
41. Soudamini KK and Kuttan R (1992) Chemoprotective effect of curcumin against cyclophosphamide toxicity Indian J Pharm Sci 54(6) 213–217 https://www.ijpsonline.com/ Date accessed: 20/07/24
42. Calderón-Pérez L, Llauradó E, and Companys J, et al (2021) Acute effects of turmeric extracts on knee joint pain: a pilot, randomized controlled trial J Med Food 24(4) 436–440 https://doi.org/10.1089/jmf.2020.0074 PMCID: 8080919
43. Gaffey A, Slater H, and Porritt K, et al (2017) The effects of curcuminoids on musculoskeletal pain: a systematic review JBI Database Syst Rev Implement Rep 15(2) 486–516 https://doi.org/10.11124/JBISRIR-2016-003266
44. Khoja KK, Shaf G, and Hasan TN, et al (2011) Fenugreek, a naturally occurring edible spice, kills MCF-7 human breast cancer cells via an apoptotic pathway Asian Pac J Cancer Prev APJCP 12(12) 3299–3304 PMID: 22471470
45. Amin A, Alkaabi A, and Al-Falasi S, et al (2005) Chemopreventive activities of Trigonella foenum graecum (Fenugreek) against breast cancer Cell Biol Int 29(8) 687–694 https://doi.org/10.1016/j.cellbi.2005.04.004 PMID: 15936223
46. Üstündağ S and Demir Zencirci A (2015) Complementary and alternative medicine use among cancer patients and determination of affecting factors: a questionnaire study Holist Nurs Pract 29(6) 357–369 https://doi.org/10.1097/HNP.0000000000000113
47. https://media.neliti.com/media/publications-test/55949-more-frequent-use-of-herbal-medicine-dai-ba274f5f.pdf Date accessed: 10/05/24
48. Bland RD, Clarke TL, and Harden LB (1976) Rapid infusion of sodium bicarbonate and albumin into high-risk premature infants soon after birth: a controlled, prospective trial Am J Obstet Gynecol 124(3) 263–267 https://doi.org/10.1016/0002-9378(76)90154-X PMID: 2013
49. Meena G (2022) Women and widow’s health Manag J Nurs 12(2) 28
50. Wasnik SP, Chaudhari PM, and Popale SS (2023) Herbal remedies on hyperlipidemia Int J Sci Res Arch 8(2) 252–258 https://doi.org/10.30574/ijsra.2023.8.2.0239
51. Özçelik G and Toprak D (2015) Bitkisel Tedavi Neden Tercih Ediliyor? Why is phytotherapy preferred? Ank Med J 15(2) [Internet] Date accessed: 15/07/24 https://doi.org/10.17098/amj.05190
52. Ritschel ML, Hübner J, and Wurm-Kuczera R, et al (2023) Phytotherapy known and applied by head-neck cancer patients and medical students to treat oral discomfort in Germany: an observational study J Cancer Res Clin Oncol 149(5) 2057–2070 https://doi.org/10.1007/s00432-022-04200-0 PMCID: 10097744
53. Contarato AAPF, Bento FC, and Rampellotti LF (2016) Motivação dos pacientes com histórico de câncer de mama em buscar as terapias alternativas Extensio Rev Eletrônica Ext 13(24) 64 https://doi.org/10.5007/1807-0221.2016v13n24p64
54. Slama FB, Médini S, and Mansour NB, et al (2014) Perception of anemic women instead of herbal medicine and dietetics in treatment of nutritional anemia Food Nutr Sci 05(11) 971–976
55. Rossi E, Di Stefano M, and Firenzuoli F, et al (2017) Add-on complementary medicine in cancer care: evidence in literature and experiences of integration Med Basel Switz 4(1) 5
56. Hamilton AS, Miller MF, and Arora NK, et al (2013) Predictors of use of complementary and alternative medicine by non-hodgkin lymphoma survivors and relationship to quality of life Integr Cancer Ther 12(3) 225–235 https://doi.org/10.1177/1534735412449733
57. Mphafi K, Oyekale AS, and Ndou P (2023) Consumers’ preferences for local and imported culinary herbs in Gauteng province, South Africa Res Agric Livest Fish 10(1) 61–71 https://doi.org/10.3329/ralf.v10i1.66221
58. Kenechukwu SA, Ogah A, and Ifeanyichukwu AC (2023) Health information and users’ beliefs: a study of herbal medicine users’ attitudes to efficacy of herbal medicine Int J Curr Res Humanit 26(1) 293–304 https://doi.org/10.4314/ijcrh.v26i1.17
59. Jiang K, Wen Y, and Li S, et al (2023) Differences in awareness of Chinese dietary guidelines among urban and rural residents: a cross-sectional survey in Southwest China Int J Public Health 68 1605344 https://doi.org/10.3389/ijph.2023.1605344 PMID: 36712819 PMCID: 9879960
60. Casagrande A, Ritter MR, and Kubo RR (2023) Traditional knowledge in medicinal plants and intermedicality in urban environments: a case study in a popular community in southern Brazil Ethnobot Res Appl [Internet] 25 https://doi.org/10.32859/era.25.35.1-34
61. Brahmi SA, El M’rabet FZ, and Benbrahim Z, et al (2011) Complementary medicine use among Moroccan patients with cancer: a descriptive study Pan Afr Med J 10 36 https://ethnobotanyjournal.org/index.php/era/article/view/4397 Date accessed: 11/05/24 PMID: 22187618 PMCID: 3240925
62. Molassiotis A, Fernández-Ortega P, and Pud D, et al (2005) Use of complementary and alternative medicine in cancer patients: a European survey Ann Oncol Off J Eur Soc Med Oncol 16(4) 655–663 https://doi.org/10.1093/annonc/mdi110
63. Navo MA, Phan J, and Vaughan C, et al (2004) An assessment of the utilization of complementary and alternative medication in women with gynecologic or breast malignancies J Clin Oncol Off J Am Soc Clin Oncol 22(4) 671–677 https://doi.org/10.1200/JCO.2004.04.162
64. Kim SD, Kwag EB, and Yang MX, et al (2022) Efficacy and safety of ginger on the side effects of chemotherapy in breast cancer patients: systematic review and meta-analysis Int J Mol Sci 23(19) 11267 https://doi.org/10.3390/ijms231911267 PMID: 36232567 PMCID: 9569531
65. Ansari Damavandi S, Nakhaei S, and Karimi M, et al (2019) Ginger relieve chemotherapy induced nausea and vomiting (CINV) in children: a randomized clinical trial Int J Pediatr [Internet] Date accessed: 16/06/24
66. Crichton M, Marshall S, and Marx W, et al (2019) Efficacy of ginger (Zingiber officinale) in ameliorating chemotherapy-induced nausea and vomiting and chemotherapy-related outcomes: a systematic review update and meta-analysis J Acad Nutr Diet 119(12) 2055–2068 https://doi.org/10.1016/j.jand.2019.06.009 PMID: 31519467
67. Panahi Y, Saadat A, and Sahebkar A, et al (2012) Effect of ginger on acute and delayed chemotherapy-induced nausea and vomiting: a pilot, randomized, open-label clinical trial Integr Cancer Ther 11(3) 204–211 https://doi.org/10.1177/1534735411433201 PMID: 22313739
68. Barton DL, Liu H, and Dakhil SR, et al (2013) Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2 J Natl Cancer Inst 105(16) 1230–1238 https://doi.org/10.1093/jnci/djt181 PMID: 23853057 PMCID: 3888141
69. Kim TH, Kwon SC, and Kim JN, et al (2020) Ginseng seed oil inhibits the growth of estrogen receptor-positive breast cancer cells Anticancer Res 40(8) 4529–4535 https://doi.org/10.21873/anticanres.14458 PMID: 32727783
70. King ML, Adler SR, and Murphy LL (2006) Extraction-dependent effects of American Ginseng (Panax quinquefolium) on human breast cancer cell proliferation and estrogen receptor activation Integr Cancer Ther 5(3) 236–243 https://doi.org/10.1177/1534735406291341 PMID: 16880429
71. Heidari Z, Salehzadeh A, and Sadat Shandiz SA, et al (2018) Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles 3 Biotech 8(3) 177 https://doi.org/10.1007/s13205-018-1199-x PMID: 29556431 PMCID: 5847643
72. Weiger WA, Smith M, and Boon H, et al (2002) Advising patients who seek complementary and alternative medical therapies for cancer Ann Intern Med 137(11) 889–903 https://doi.org/10.7326/0003-4819-137-11-200212030-00010 PMID: 12458989
73. Kunnumakkara AB, Anand P, and Aggarwal BB (2008) Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins Cancer Lett 269(2) 199–225 https://doi.org/10.1016/j.canlet.2008.03.009 PMID: 18479807
74. Hyodo I, Amano N, and Eguchi K, et al (2005) Nationwide survey on complementary and alternative medicine in cancer patients in Japan J Clin Oncol Off J Am Soc Clin Oncol 23(12) 2645–2654 https://doi.org/10.1200/JCO.2005.04.126






