Prevalence of human papilloma virus infection and risk of cervical intraepithelial neoplasia among female sex workers in Mumbai, India
Vandita Pahwa1a, Sharmila A Pimple2b, Gauravi A Mishra2c, Parishi Majmudar2, Sanjay K Biswas3d and Kedar Deodhar4e
1Department of Preventive Oncology, Homi Bhabha Cancer Hospital & Research Center, New Chandigarh, Punjab, India
2Department of Preventive Oncology, Centre for Cancer Epidemiology (CCE), Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India
3Department of Microbiology, Tata Memorial Hospital, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India
4Department of Pathology, Tata Memorial Hospital, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Mumbai, India
ahttps://orcid.org/0000-0002-3492-4418
bhttps://orcid.org/0000-0003-2976-9107
chttps://orcid.org/0000-0002-0154-7302
dhttps://orcid.org/0000-0002-9802-0848
ehttps://orcid.org/0000-0002-6338-8191
Abstract
Introduction: Cervical cancer, mostly caused by human papilloma virus (HPV), has disproportionately high incidence in developing countries. HPV infection being essentially a sexually transmitted infection, high-risk behaviour women with multiple sexual contacts like female sex workers (FSWs) are at higher risk of co-infection with HPV and of developing cervical precancer and cancer.
Objective: This study aimed to determine the prevalence and determinants of HPV infection and cervical intraepithelial neoplasia (CIN) among FSWs in Mumbai, India.
Methods: A cross-sectional study was conducted among 448 FSWs, between the ages of 18–50 years, by collaborating with local non-government organizations working for the health and welfare of FSW communities at sexually transmitted diseases clinics. All FSWs were screened for HPV DNA by hybrid capture II followed by reference diagnosis of colposcopy and/or cervical biopsy.
Results: The prevalence of HPV DNA positivity was 35.5% and CIN was 2.2%. Factors significantly associated with HPV DNA positivity were age group younger than 30 years odds ratio (OR = 2.098, 95% confidence interval (CI) 1.408–3.127), Illiteracy (OR = 2.015, 95% CI 1.305–3.112), being single (OR = 2.409, 95% CI 1.558–3.724), less than 18 years of age at time of initiating work as FSW (OR = 3.718, 95% CI 3.718–2.392), having more than five clients per day (OR = 2.078, 95% CI 1.301–3.318), been working as a FSW for more than 5 years (OR = 2.321, 95% CI 1.455–3.701), not using barrier contraception methods (OR = 5.155, 95% CI 3.395–7.827) and having no exposure to human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) education program (OR = 29.153, 95% CI 15.385–55.240). FSWs with a positive HPV DNA test are substantially more likely to have CIN compared to those with a negative test (OR = 7.6, 95% CI 1.59–36.25).
Conclusion: The prevalence of HPV infection and CIN was high among FSWs. FSWs with a positive HPV DNA test had a seven times higher risk of developing CIN. The persistence of HPV infection is expected to significantly raise the risk of cervical cancer in the future. It is suggested to have an integrated approach towards cervical cancer screening and HIV/AIDS control activities.
Keywords: female sex workers, human papilloma virus (HPV), cervical cancer, CIN, screening, precancer
Correspondence to: Dr Sharmila A Pimple
Email: drsharmilapatil@yahoo.com
Published: 19/09/2024
Received: 23/12/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Globally, cancer of the cervix ranks fourth among the most common cancers in females [1]. In addition, it has been observed that cervical cancer is disproportionally high in developing countries [2]. In India, incidence of cervical cancer is next only to breast cancer [3]. It is preventable due to vaccination and screening programs, it is curable if diagnosed early and treated in time. The most important causative agent for cervical cancer is infection with oncogenic human papilloma virus (HPV) [4, 5].
HPV is the most common sexually transmitted infection (STI). Out of the several known types of HPV, 14 are known to be carcinogenic [6]. Around two-third of carcinoma of the cervix are caused by HPV subtypes 16 and 18 [7]. Most HPV infections are transient without any symptom and resolve on their own [8]. Cervical cytological abnormalities and cancer is known to occur only with persistent HPV infection [9]. With advances in detection of HPV DNA technologies including genotyping, we can identify subgroups of population with increased risk of infection [10].
India has a dual burden of human immunodeficiency virus (HIV) infection and high cervical cancer rates. Several studies have shown that HIV positive women are at an increased risk of developing cervical squamous intraepithelial lesions (SIL) and cervical cancer [11–13]. The usual course of HPV infection is modified among HIV women. The lesions revert to normal in lesser percentage of HIV positive individuals and often expedites to severe and cancerous forms [14].
Women with high-risk behaviour (multiple sexual contacts) are at an increased risk of co-infection by HPV and intraepithelial neoplasia of the cervix. Studies reveal that HPV prevalence depends largely on age and on sexual practices [15, 16].
Studies show that female sex workers (FSWs) are known to have a very high prevalence of HPV infection primarily due to early inception age of sexual activity and prevalence of multiple sexual partners [17]. Compared to low-risk population groups, FSWs have higher vulnerability to HPV infection, thus resulting in abnormal pap smears and cervical cancer [18–20]. There is also widespread ignorance about infection spread dynamics, preventive measures and screening for cancer of the cervix [21].
The objectives of this work are to describe the determinants of, and report prevalence of high-risk HPV infection and subsequent risk of cervical intraepithelial neoplasia (CIN) among FSWs in Mumbai, India.
An increasing proportion of the FSWs in Mumbai originate from various parts of the country as well as neighbouring countries. This also introduces differences in their sociodemographic characteristics, sexual plus health-seeking behaviour and HPV prevalence and types. The knowledge of these characteristics is essential to design appropriate preventive and curative strategies for women with high-risk behaviour patterns.
Methods
This cross-sectional study was conducted among 448 FSWs, recruited by collaborating with local non-government organizations (NGO’s) working for the health and welfare of the FSW communities at sexually transmitted diseases (STDs) clinics in Mumbai. All FSWs aged between 18 and 50 years were invited to participate in the study. Apparently, healthy FSW, non-pregnant with an intact uterus and no history of cervical cancer or debilitating physical and mental illness were recruited in the study. All FSWs who were pregnant, with a record of cervical cancer or hysterectomy, or with a debilitating condition that prevents a pelvic examination were excluded from the study.
Anticipating an HPV prevalence of 30%–50% from studies in different geographical areas, the accrual of 448 women was required in the study to assess the true prevalence of HPV among FSW at a 95% confidence interval (CI) with 80% statistical power. The study was reviewed and approved by the scientific and ethical committee of the Institutional Review Board (IRB) of Tata Memorial Hospital, Mumbai, India, and was conducted in compliance with the medical research regulations involving human subjects set by the IRB.
In the first phase of the study, the NGOs working for the health and welfare of the FSW community were identified. The study participants were recruited from community based programmes conducted by these NGO’s and attended by the FSWs. Cervical cancer awareness sessions highlighting the risk of cervical cancer due to high-risk behaviour were conducted in these programs by the medical social worker (MSW), after which the women were invited to participate in the screening programme. The FSWs were explained about the study by giving a participant information sheet. A written informed consent in the vernacular language (Hindi/Marathi) was obtained from the participant and a unique participant identification number was assigned to the eligible women.
Design of the questionnaire
The questionnaire was designed keeping in mind the study objectives and the high-risk study population. A thorough literature review was performed, and the questions were drafted to cover most of the risk factors. The questions were then translated into the native language and reverse translated. The questionnaire was then pilot tested and validated before administering it to the study population. MSW then conducted interviews with the consenting FSWs using the structured questionnaire. Data on socio demographic characteristics (age, place of birth and marital status), sexual and health seeking behaviour, reproductive health (number of pregnancies, children, termination of pregnancies, type of contraceptives and barriers methods used), tobacco habits and so on was collected from them.
Screening of FSWs
The screening clinics were conducted at the STD Clinics once in 15 days to recruit participants.
A gynaecologic examination was performed on all participants. Per speculum examinations were conducted to obtain cervical cells for HPV DNA assay by scraping the cervix. Detection of precancer lesions was done by visual inspection with 5% acetic acid (VIA) and cervical smears were obtained from all participating women on the same day they were interviewed.
HPV DNA hybrid capture II (HC II)
HC II assay kits were procured from the USA. The specimens were collected and stored at −20º until further processing at the HPV laboratory at the tertiary hospital using the HC II microtiter assay in accordance with the manufacturer’s instructions. Cervical samples were classified as positive for DNA from the high-risk HPV types (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) if the relative light unit reading obtained from the luminometer of the HC-II assay equipment was equal or greater than the mean of the positive control values supplied by the HC II kit.
Visual inspection with 5% acetic acid
The visual screening test VIA was administered by trained health care workers by application of 5% acetic acid to the cervix and visualizing the cervix with the help of a halogen focus lamp. VIA was considered to be positive if definite acetowhite lesions were visualized close to the squamocolumnar junction.
Colposcopy with or without biopsy
Colposcopy with or without biopsy was administered to all the participants irrespective of the status of VIA screening test. After the collection of cervical samples for HPV testing and VIA, colposcopy was performed by trained doctors and the colposcopy impression was noted down along with a punch biopsy from the acetowhite area on the cervix.
The true disease was defined as a histologically confirmed high-grade Squamous Intraepithelial Lesion. The consensus category includes cervical intraepithelial lesion (CIN) 1, 2 and 3 and/or carcinoma in situ.
Post-test counselling
At the end of all testing procedures, posttest counseling was done by the doctor and MSW to explain the significance and the results of the testing procedures performed. In addition, tobacco users were offered tobacco cessation counseling by the MSW. The importance of follow-up visits to understand the results of HPV or cervical biopsy were explained to the women.
Data management and analysis
Data were entered and analyzed using Statistical Package for the Social Sciences v 29. Data were regularly checked for consistency, safety and analysis at regular intervals. Frequencies of sociodemographic, reproductive and sexual behaviour attributes were determined. Prevalence of HPV infection, disease spectrum of CIN and risk factors for acquiring HPV infection with 95% CI were estimated.
Results
A total ten NGOs were identified out of which six consented for participation in the project. Around 12 sensitization programs and 39 cervical cancer awareness programs were held at community settings. A total of 736 eligible FSWs were contacted, counselled and were invited for cervical cancer awareness and screening. A total of 448 (60.8%) FSWs consented to the program.
Sociodemographic characteristics
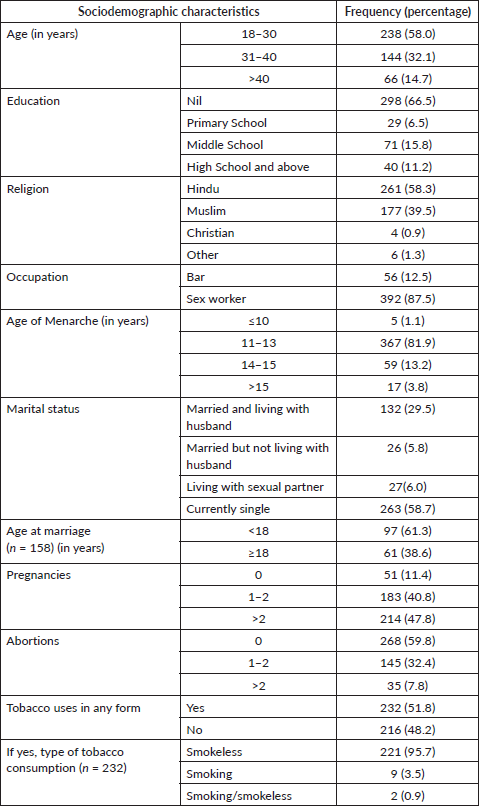
Table 1 shows the socio-demographic details of the study population. More than half of the of the FSWs were in the age bracket of 18–30 years (58.0%), single (58.7%) with no formal education (66.5%) and practiced Hinduism (58.3%). Tobacco consumption habit was found in most of the FSWs (51.8%), of which 95.7% were smokeless tobacco consumers. The majority (87.5%) had worked as sex workers and the rest (12.5%) worked in bars.
Table 1. Sociodemographic profiling of FSWs (N = 448).
Sexual behaviour profiling
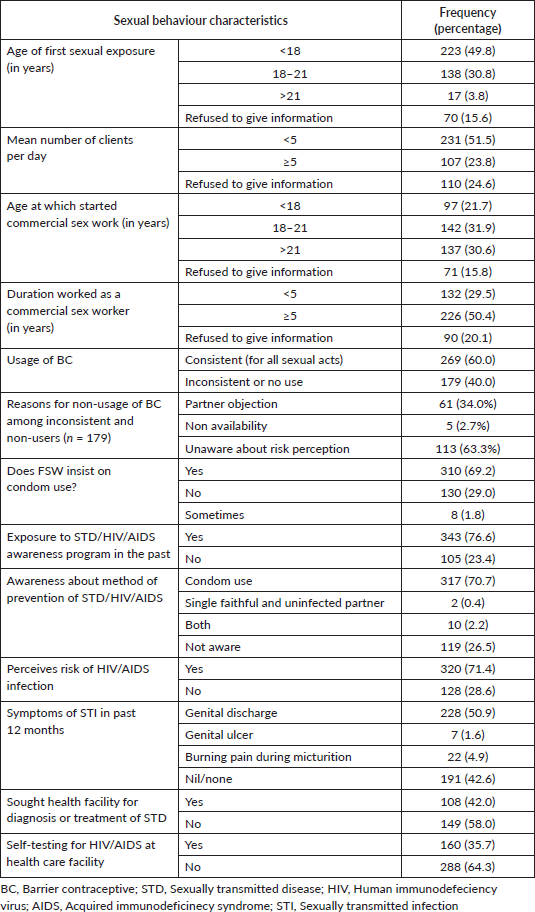
Table 2 shows sexual behaviour profiling of the study population. Around half of the participants were aged less than 18 years when they had their first sexual exposure (49.8%). A sizeable percentage had started work as commercial sex workers at less than 18 years of age (21.7%). The mean number of clients seen per day was more than four for 23.8% of the participants. Almost half (50.4%) of these workers have been working as FSWs for more than 4 years.
Barrier contraception was consistently used by 60.0% of the FSWs for all sexual acts. Among those who used barrier contraceptives (BCs) inconsistently, the most common reason for non-use was the lack of awareness of the risks associated with not using barrier contraception (63.3%) followed by an objection by the partner (34.0%) for use.
Regarding awareness of STDs, the majority (76.6%) had attended an awareness program for the prevention of these diseases. However, among those who had themselves suffered from symptoms of STI (57.4%), most did not seek any treatment (58%) nor did they ever get themselves tested for HIV-acquired immunodeficiency syndrome (AIDS) at a healthcare facility (64.3%).
Table 2. Sexual behaviour profiling of FSWs (N = 448).
Results of screening, histopathology tests
Table 3 shows the results of screening and histopathology tests performed for cervical cancer. The prevalence of HPV DNA positivity was 35.5%. The VIA (visual inspection by acetic acid) was positive in 12.5% FSWs. Histopathology examination of the cervix revealed CIN I in four participants, CIN II in five participants and CIN III in one participant. The overall prevalence of CIN was 2.2%.
Predictors of prevalence of HPV infection
Table 4 shows the predictors of positive HPV infection among the participants. Factors significantly associated with HPV DNA positivity were age group younger than 30 years (odds ratio (OR) = 2.098, 95% CI 1.408–3.127), Illiteracy (OR = 2.015, 95% CI 1.305–3.112), being single (OR = 2.409, 95% CI 1.558–3.724), less than 18 years of age at the time of initiating work as FSW (OR = 3.718, 95% CI 3.718–2.392), having more than five clients per day (OR = 2.078, 95% CI 1.301–3.318), been working as an FSW for more than 5 years (OR = 2.321, 95% CI 1.455-3.701), not using barrier contraception methods (OR = 5.155, 95% CI 3.395–7.827) and having no exposure to HIV/AIDS education program (OR = 29.153, 95% CI 15.385–55.240).
Table 3. Results of screening and histopathology tests done among FSWs (N = 448).
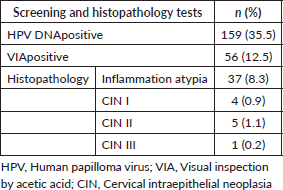
Table 4. Predictors of prevalence of HPV infection among FSWs: unadjusted bivariate logistic regression analysis (N = 448).
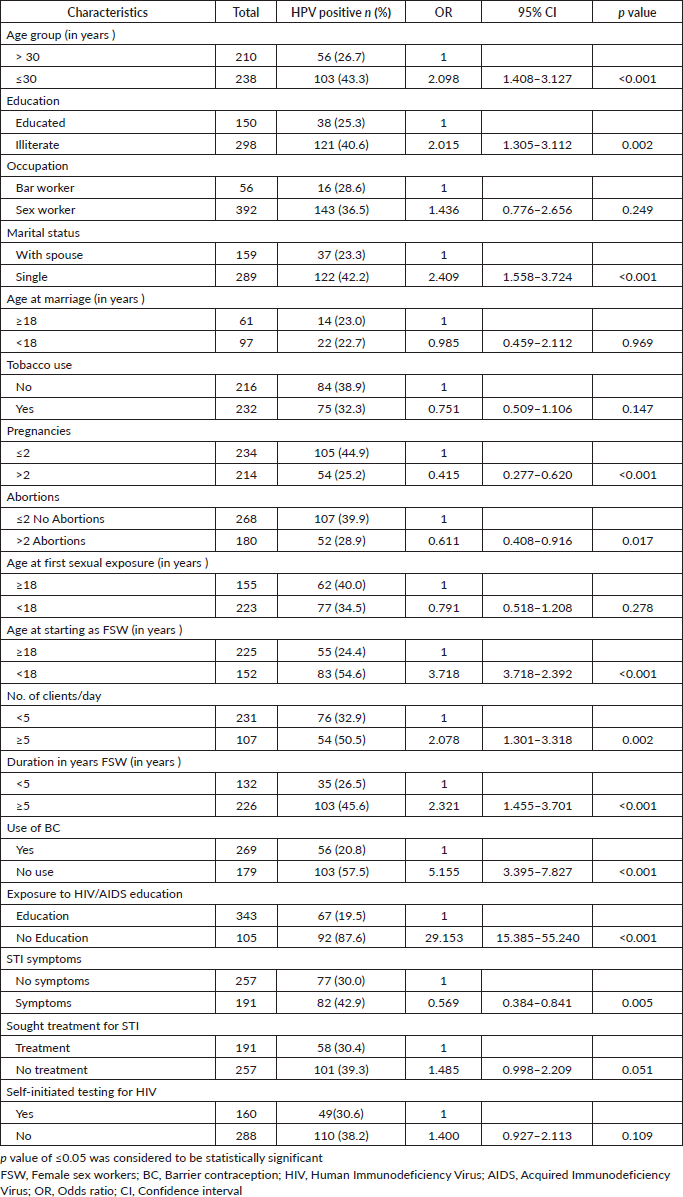
Table 5. Risk of CIN among HPV positive FSWs.
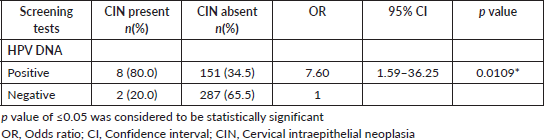
Risk of CIN among HPV-positive FSWs
Table 5 shows the risk of CIN among those who are HPV positive. As observed from the study results among those who were HPV positive, histopathology examination of the cervix found CINI in three participants, CIN II in four participants and CIN III in one participant. A significant association between a positive HPV DNA test and the presence of CIN is also observed. Individuals with a positive HPV DNA test are substantially more likely to have CIN compared to those with a negative test. The OR with 95% C.I. of 7.6 (1.59–36.25), with a statistically significant p-value of 0.0109 shows the effectiveness of the HPV DNA test as a screening tool for CIN.
Discussion
This study found the overall prevalence of HPV DNA positivity rate among sample as 35.5%. This is higher than the prevalence found in studies done in other parts of India. A study by Sarkar et al [22] in West Bengal found an HPV prevalence of 25% and Singh et al [23] in Chandigarh reported HPV prevalence of 27.5%. Similarly, studies conducted in African countries such as Ghana (26%) and Togo (32.9%) have also reported a lower prevalence [24, 25]. However, higher HPV prevalence was noted in the studies done in other countries: Vietnam (85.0%), Cambodia (41.1%), Belgium (41.7%) and Dominican Republic (43.4%) [26–29]. The systematic review by Soohoo et al [30] and Wu et al [31] show a higher prevalence of HPV at 42.7% and 39.5%, respectively. Detailed discussion on major predictors of the prevalence of HPV among respondents of this study are as follows:
In this study, more than half (58.0%) of the females were in the age group of 18–30 years. It is observed that those who are HPV positive were two times more likely to be less than 30 years of age (OR = 2.098, 95% CI 1.408–3.127). These results are consistent with those reported in a number of other studies [22, 28, 30]. This is also in accordance with the literature which shows that the probability of HPV decreases significantly with growing age [32]. The study conducted in Vietnam and Mexico, however, did not show any change in HPV prevalence by age [26, 33].
This study found that 66.5% of the FSWs with no formal education. Similar proportions of illiteracy were reported in the study by Sarkar et al [22] in West Bengal (62.9%), Singh et al [23] in Chandigarh (60.8%) and Hernandez and Nguyen [26] in Vietnam (63%). Our study shows that HPV positivity is two times more common among Illiterates (OR = 2.015, 95% CI 1.305–3.112), similar to the study of Hernandez and Nguyen [26]. Lack of education among women makes them less receptive to health awareness programs and prevents them from taking informed decisions about their sexual and reproductive health, thereby predisposing them to HPV infection. We also observed that 40.0% of FSWs did not use BCs consistently for all commercial sexual acts. This percentage is lesser than that reported in studies by Hernandez and Nguyen [26] (84%), Couture et al [27] (90.8%) and Singh et al [23] (71.6%). Our findings also show that participants with HPV DNA positivity were unlikely to be using barrier contraception methods (OR = 5.155, 95% CI 3.395–7.827) and have had any exposure to HIV/AIDS education program (OR = 29.153, 95% CI 15.385–55.240).
Literature shows that tobacco causes diminished antibody reaction in HPV16/18-afflicted young females [34]. Tobacco use in our study is reported as 51.8%, of which 95.7% were smokeless tobacco consumers. However, no association was found between HPV status and tobacco use in this study. Similar results have been reported by Jia et al [35]. However, a study by Singh et al [23] shows higher HPV positivity among FSWs who smoked tobacco (36.7% versus 24.4%, OR = 4.11, p = 0.05).
It is observed in the study that younger age at first sexual exposure was not significantly associated with HPV positivity, but it was associated with participants who started as FSW at an age younger than 18 years. This could be because age at first sexual contact is more likely to be a single sexual partner exposure. However, it is likely that when participants started working as FSWs the exposure to multiple sexual partners because of commercial sex work resulted in increased HPV positivity in this group. Sarkar et al [22] showed found that sex workers beginning their work at ≤20 years of age had the highest HPV prevalence (29.7%), followed by older age groups. Females with early initiation of sexual intercourse may get infected with the HPV virus earlier in their life course thus giving the virus more time to persist and progress to initiate precancer changes.
We found a positive association between more than five clients per day and HPV-positive status (OR = 2.078, 95% CI 1.301–3.318). Likewise, Sarkar et al [22] observed that sex workers having the usual number of four or more clients per day were nearly four times more likely of getting HPV (OR = 3.9; 95% CI 1.6–9.4). Couture et al [27] noted a higher number of sexual partners (AOR 1.05; 95% CI: 1.01–1.09) to be associated with HPV infection. Contrary to this, Hernandez and Nguyen [26] in their study noted that HPV infection was lower among FSWs attending to more clients per day. They suggested that constant, recurrent contact with HPV enhances the immunogenic reaction locally. Hence, FSWs with the most clients are relatively more protected from getting infected by the new HPV.
Taking into consideration the length of time as a sex worker, our study found that FSWs working for more than 5 years had a significantly greater risk of acquiring HPV infection (OR = 2.321, 95% CI 1.455–3.701). This could be due to acquired immunity gained against HPV antigen over time. This is contrary to the findings by Sarkar et al [22] which show sex workers with a lesser duration of work are at an increased chance of HPV acquisition (OR = 3.3, 95% CI 1.455–7.6).
Histopathology examination of the cervix revealed CIN I in 0.9%, CIN II in 1.1% and CIN III in 0.2% participants, overall prevalence being 2.2%. The findings are higher compared to the study by Sarkar et al [22] where 1% of the studied FSW population suffered from a pre-cancerous lesion caused by high-risk HPV. However, the CIN prevalence in our study was lower compared to the study done in Pune, Ahmednagar and Sangli districts of Maharashtra, India (8.3%) by Joshi et al [36]. This may be because these districts have a high prevalence of HIV infection as well. The CIN prevalence in our study was lower when compared to those reported from Kenya (5.5%), Cameroon (5.9%) and China (16.82%) [36, 27, 38]. Our study found that those who were HPV Positive had seven times (OR = 7.60, 95% CI 1.59–36.25) increased risk of getting CIN. The review published by Muñoz et al [39] which analysed data from 11 case control studies across 9 countries showed that while the odds of developing cervical cancer vary with the type of HPV infection, the overall risk of cervical cancer is much higher (OR = 158.2, 95% CI, 113.4 to 220.6) with oncogenic type [39]. Our study shows a high prevalence of HPV among FSWs and its strong association with CIN emphasizing the need for enhanced preventive measures and cervical cancer screening for this high-risk population.
Strengths and limitations
Our study demonstrates several strengths of the program. The study was conducted aligning with the other health program and welfare measures targeted towards the FSW community by the local non-government organisations. Peer educators from the FSW community were identified and sensitised for encouraging and counselling the FSW to participate in the cervical cancer screening program. The study thus parallelly demonstrates the feasibility of introducing cervical cancer screening along with interventions for HIV/AIDS and STD control and prevention during gynaecological examinations targeted for STD intervention. We report the following limitations of our study. Since HPV detection was undertaken by a qualitative test (HC II) therefore the high-risk HPV genotype could not be ascertained among this at-risk population. Also, the HIV status of this high-risk population could not be captured due to reasons of confidentiality and hence the association between the HIV status and HPV infection and vice versa could not be established. Due to the cross sectional nature of the study, the risk of progression to CIN and cervical cancer could not be ascertained.
Conclusion
FSWs have a high prevalence of HPV infection and are at increased risk of cervical cancer. Cervical cancer awareness and screening is not part of any health care interventions currently targeted towards this high-risk group. Because of their similar epidemiological determinants, the scope of the current national program for STD/HIV AIDS prevention and control should be expanded to cover cervix cancer prevention and screening which will be highly cost-effective to decrease the burden of cervical cancer among FSW.
List of abbreviations
BC, Barrier contraceptive; CI, Confidence interval; CIN Cervical intraepithelial neoplasia; FSWs, Female sex workers; HC II, Hybrid capture II; HIV/AIDS, Human immunodeficiency virus/acquired immunodeficiency syndrome; HPV, Human papilloma virus; IRB, Institutional Review Board; MSW, Medical social worker; NGO, Non-governmental organization; OR, Odds ratio; SIL, Squamous intraepithelial lesions; STD, Sexually transmitted diseases; VIA, Visual inspection by acetic acid.
Acknowledgments
This study was supported by the intramural research funds from Women’s Cancer Initiative (WCI), Tata Memorial Centre. The authors express our gratitude toward the sex workers who volunteered to be a part of this study and the representatives of the organizations working for the health and welfare of the FSWs. The authors thank our MSW, Ms Parishi Majumdar, for her invaluable support in motivating the participants, the Statistical Department at the Clinical Research Secretariat of Tata Memorial Hospital for undertaking the Statistical analysis of the study. The authors acknowledge the contribution of Ms Nicole Aguirre for coordinating with the statistical team.
Conflicts of interest
The authors declare no conflicts of interest in this work.
Author contributions
SP designed and implemented the study, interpreted the data and prepared the manuscript; VP prepared the manuscript; GM contributed in data analysis and interpretation; SKB and KD were involved with HPV and histology reporting, respectively, and interpretation of data and preparation of the manuscript.
Funding declaration
The study was supported by the intramural research funds from Women’s Cancer Initiative (WCI), Tata Memorial Centre.
References
1. International Agency for Research on Cancer GLOBOCAN 2020 New Global Cancer Data [GLOBOCAN 2020: New Global Cancer Data|UICC] Date accessed: 16/09/21
2. Randall TC and Ghebre R (2016) Challenges in prevention and care delivery for women with cervical cancer in Sub-Saharan Africa Front Oncol 6 160 https://doi.org/10.3389/fonc.2016.00160 PMID: 27446806 PMCID: 4923066
3. International Agency for Research on Cancer GLOBOCANIndia 2020 [356-india-fact-sheets.pdf(iarc.fr)]. Date accessed: 20/04/21
4. Muñoz N and Bosch FX (1996) The causal link between HPV and cervical cancer and its implications for prevention of cervical cancer Bull Pan Am Health Organ 30 362–377 PMID: 9041748
5. Montero JA, Larkin JA, and Houston SH, et al (1997) Examining complex relationship of human papillomavirus to cervical dysplasia and carcinoma Medscape Women’s Health 2(6) 1
6. World Health Organization (2020) Human Papillomavirus (HPV) and Cervical Cancer: Fact Sheet World Health Organization [https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer]
7. Petrosky E, Bocchini JA Jr, and Hariri S, et al (2015) Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices MMWRMorb Mortal Wkly Rep 64(11) 300–304
8. Steben M and Duarte-Franco E (2007) Human papillomavirus infection: epidemiology and pathophysiology J Gynecol Oncol 107(2 Suppl 1) S2–S5 https://doi.org/10.1016/j.ygyno.2007.07.067
9. Castellsagué X (2008) Natural history and epidemiology of HPV infection and cervical cancer Gynaecol Oncol 110(3) S4–S7 https://doi.org/10.1016/j.ygyno.2008.07.045
10. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2007) Human Papillomaviruses (Lyon: International Agency for Research on Cancer) (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 90.) pp 1 Human Papillomavirus (HPV) Infection [https://www.ncbi.nlm.nih.gov/books/NBK321770/]
11. Chambuso RS, Shadrack S, and Lidenge SJ, et al (2016) Influence of HIV/AIDS on cervical cancer: a retrospective study from Tanzania J Glob Oncol 3(1) 72–78 https://doi.org/10.1200/JGO.2015.002964
12. Du P (2019) Human papillomavirus infection and cervical cancer in HIV+women Cancer Treat Res 177 105–129 https://doi.org/10.1007/978-3-030-03502-0_5
13. Denny L, Boa R, and Williamson A-L, et al (2008) Human papillomavirus infection and cervical disease in human immunodeficiency virus-1–infected women Obstet Gynecol 111(6) 1380–1387 PMID: 18515522
14. Clarke B and Chetty R (2002) Postmodern cancer: the role of human immunodeficiency virus in uterine cervical cancer Mol Pathol v.55(1) 19–24 https://doi.org/10.1136/mp.55.1.19
15. Sasagawa T, Tani M, and Yasuda H, et al (2005) Sexual behavior and highrisk human papillomavirus infections in Japanese women Sex Transm Infect; 81 280–282 https://doi.org/10.1136/sti.2004.011411 PMID: 15923306 PMCID: 1744986
16. International Collaboration of Epidemiological Studies of Cervical Cancer (2009) Cervical carcinoma and sexual behavior: collaborative reanalysis of individual data on 15,461 women with cervical carcinoma and 29,164 women without cervical carcinoma from 21 epidemiological studies Cancer Epidemiol Biomarkers Prev 18(4) 1060–1069 https://doi.org/10.1158/1055-9965.EPI-08-1186 PMID: 19336546
17. del Amo J, González C, and Losana J, et al (2005) Influence of age and geographical origin in the prevalence of high-risk human papillomavirus in migrant female sex workers in Spain Sex Transm Infect 81(1) 79–84 https://doi.org/10.1136/sti.2003.008060 PMID: 15681729 PMCID: 1763723
18. Vallès X, Murga GB, and Hernandez G, et al (2009) High prevalence of human papillomavirus infection in the female population of Guatemala Int J Cancer 125 1161–1167 https://doi.org/10.1002/ijc.24444 PMID: 19415744
19. Leung KM, Yeoh Gary PS, and Cheung HN, et al (2013) Prevalence of abnormal Papanicolaou smears in female sex workers in Hong Kong Hong Kong Med J 19(3) 203–206 PMID: 23650200
20. Mak R, Van Renterghem L, and Cuvelier, C (2004) Cervical smears and human papillomavirus typing in sex workers Sex Transm Infect 80 118–120 https://doi.org/10.1136/sti.2002.003749 PMID: 15054172 PMCID: 1744807
21. Kietpeerakool C, Phianmongkhol Y, and Jitvatcharanun K, et al (2009) Knowledge, awareness, and attitudes of female sex workers toward HPV infection, cervical cancer, and cervical smears in Thailand Int J Gynaecol Obstet 107(3) 216–219 https://doi.org/10.1016/j.ijgo.2009.07.023 PMID: 19716556
22. Sarkar K, Bhattacharya S, and Bhattacharyya S, et al (2008) Oncogenic human papilloma virus and cervical pre-cancerous lesions in brothel-based sex workers in India J Infect Public Health 1(2) 121–128 https://doi.org/10.1016/j.jiph.2008.09.001 PMID: 20701853
23. Singh MP, Kaur M, and Gupta N, et al (2016) Prevalence of high-risk human papilloma virus types and cervical smear abnormalities in female sex workers in Chandigarh, India Indian J Med Microbiol 34(3) 328 https://doi.org/10.4103/0255-0857.188325 PMID: 27514955
24. Adams AR, Nortey PA, and Dortey BA, et al (2019) Cervical human papillomavirus prevalence, genotypes, and associated risk factors among female sex workers in greater Accra, Ghana J Oncol 2019 8062176 https://doi.org/10.1155/2019/8062176 PMID: 31275383 PMCID: 6582788
25. Ferré VM, Ekouevi DK, and Gbeasor-Komlanvi FA, et al (2019) Prevalence of human papillomavirus, human immunodeficiency virus and other sexually transmitted Infections among female sex workers in Togo: a national cross-sectional survey Clin Microbiol Infect 25(12) 1560.e1–1560.e7 https://doi.org/10.1016/j.cmi.2019.04.015 PMID: 31051265
26. Hernandez BY and Nguyen TV (2008) Cervical human papillomavirus infection among female sex workers in southern Vietnam Infect Agent Cancer 3(1) 7 https://doi.org/10.1186/1750-9378-3-7 PMID: 18433504 PMCID: 2405771
27. Couture MC, Page K, and Stein ES, et al (2012) Cervical human papillomavirus infection among young women engaged in sex work in Phnom Penh, Cambodia: prevalence, genotypes, risk factors and association with HIV infection BMC Infect Dis 12(1) 166 https://doi.org/10.1186/1471-2334-12-166 PMID: 22839728 PMCID: 3436768
28. Vorsters A, Cornelissen T, and Leuridan E, et al (2016) Prevalence of high-risk human papillomavirus and abnormal pap smears in female sex workers compared to the general population in Antwerp, Belgium BMC Public Health 16 477 https://doi.org/10.1186/s12889-016-3099-5 PMID: 27266509 PMCID: 4897854
29. Richards SD, Stonbraker S, and Halpern M, et al (2018) Cervical cancer screening among transactional female sex workers in the Dominican Republic Int J STD AIDS 29(12) 1204–1214 https://doi.org/10.1177/0956462418779662 PMID: 29966506 PMCID: 6089663
30. Soohoo M, Blas M, and Byraiah G, et al (2013) Cervical HPV infection in female sex workers: a global perspective Open AIDS J 7 58–66 https://doi.org/10.2174/1874613601307010058
31. Wu J, Ding C, and Liu X, et al (2021) Worldwide burden of genital human papillomavirus infection in female sex workers: a systematic review and meta-analysis Int J Epidemiol 50(2) 527–537 https://doi.org/10.1093/ije/dyaa289 PMID: 33517415
32. Akarolo-Anthony SN, Famooto AO, and Dareng EO, et al (2014) Age-specific prevalence of human papilloma virus infection among Nigerian women BMC Public Health 14 656 https://doi.org/10.1186/1471-2458-14-656 PMID: 24972674 PMCID: 4094683
33. Velazquez-Hernandez N, Sanchez-Anguiano LF, and Guerra-Infante FM, et al (2019) Human papillomavirus infection in female sex workers: a case control study J Clin Med Res 11(3) 196–201 https://doi.org/10.14740/jocmr3739 PMID: 30834042 PMCID: 6396781
34. Simen-Kapeu A, Kataja V, and Yliskoski M, et al (2008) Smoking impairs human papillomavirus (HPV) type 16 and 18 capsids antibody response following natural HPV infection Scand J Infect Dis 40(9) 745–751 https://doi.org/10.1080/00365540801995360 PMID: 19086247
35. Jia H, Wang X, and Long Z, et al (2015) Human papillomavirus infection and cervical dysplasia in female sex workers in Northeast China: an observational study BMC Public Health 15(1) 695 https://doi.org/10.1186/s12889-015-2066-x PMID: 26202513 PMCID: 4512111
36. Joshi S, Kulkarni V, and Darak T, et al (2015) Cervical cancer screening and treatment of cervical intraepithelial neoplasia in female sex workers using “screen and treat” approach Int J Womens Health 7 477–483 https://doi.org/10.2147/IJWH.S80624 PMID: 25999765 PMCID: 4427081
37. Muffih TP, Manjuha F, and Fai M, et al (2018) Cervical cancer screening of commercial sex workers in NW and SW Regions of Cameroon J Glob Oncol 4(2) 50s–50s https://doi.org/10.1200/jgo.18.66300
38. Sweet K, Bosire C, and Sanusi B, et al (2020) Prevalence, incidence, and distribution of human papillomavirus types in female sex workers in Kenya Int J STD AIDS 31(2) 109–118 https://doi.org/10.1177/0956462419884454 PMID: 31948341 PMCID: 7031817
39. Muñoz N, Bosch FX, and de Sanjosé S, et al (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer N Engl J Med 348(6) 518–527 https://doi.org/10.1056/NEJMoa021641 PMID: 12571259
Supplementary information: Prevalence and determinants of high-risk human papillomavirus and the risk of cervical intraepithelial neoplasia in female sex workers in Mumbai, India
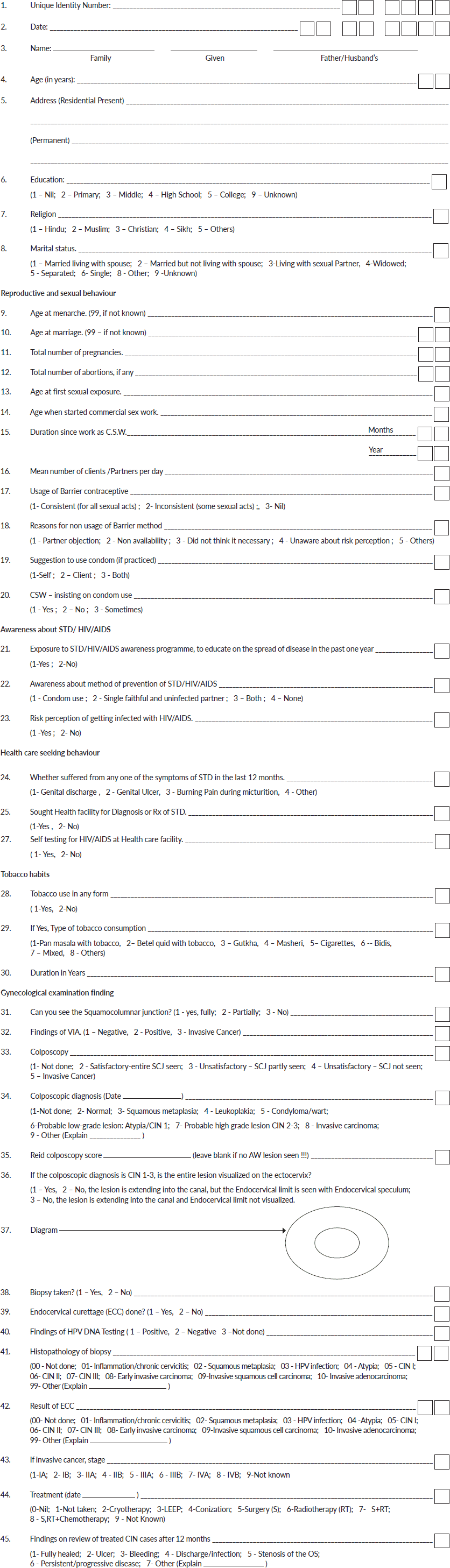
Questionnaire