Is using the Gail model to calculate the risk of breast cancer in the Venezuelan population justified?
Josepmilly del Valle Peña Colmenares1,a, Carmen Cristina García2,b, Yazmin José Velásquez Velásquez1,c, Leider Arelis Campos Pino1,d, Álvaro Gómez Rodríguez1,e, Wladimir José Villegas Rodríguez1,f, David José González Vargas3,g and Douglas José Angulo Herrera4,h
1Servicio Patología Mamaria del Servicio Oncológico Hospitalario (SOH), Instituto Venezolano del Seguro Social (IVSS), Caracas 1040, Venezuela
2Cátedra de Patología General y Fisiopatología, Escuela Luis Razetti, Facultad de Medicina, Caracas 1050, Venezuela
3Servicio Oncológico Hospitalario (SOH), Instituto Venezolano del Seguro Social (IVSS), Caracas 1040, Venezuela
4Escuela de Estadística y Ciencias Actuariales, Universidad Central de Venezuela, Caracas 1050, Venezuela
ahttps://orcid.org/0000-0002-1114-6289
bhttps://orcid.org/0000-0002-7889-9445
chttps://orcid.org/0000-0003-3307-2564
dhttps://orcid.org/0000-0002-0907-8467
ehttps://orcid.org/0000-0003-3740-0238
fhttps://orcid.org/0000 0001-8999-9751
ghttps://orcid.org/0000-0001-8071-3139
hhttps://orcid.org/0009-0003-5506-0297
Abstract
Objective: To evaluate the accuracy of the Gail model (GM) in women who already have a diagnosis of breast cancer (BC) from the Breast Pathology Service, Hospital Oncology Department of the Venezuelan Social Security Institute (SOH-IVSS) in the period 2004–2014. To compare the accuracy of the GM in women aged above and below 40 years with a diagnosis of BC.
Method: Descriptive, retrospective, cross-sectional, 830 records of patients diagnosed with BC were reviewed between 2004 and 2014.
Results: The mean age for diagnosis of the disease was 46 ± 13 years; menarche age was 13 years ± 2; age at first birth 22 ± 5 years, with a history of biopsy 32 ± 11, the percentage of relatives with a primary history of BC reported (PHBC) 9.3%. Only 41% of women with a diagnosis of BC reported Gail >1.67 (positive Gail). In the dichotomous logistic regression that related positive Gail with the independent variables, it was observed: greater probability of positive Gail if menarche age <11 years (p < 0.036), PHBC (p = 0.005), previous biopsy (p = 0.007), age at first birth 25–29 years (p = 0.019). When stratifying by age, unlike the bivariate analysis, women over 40 years of age are more likely to have a positive Gail in menarche age <11 years (p = 0.008), PHBC (p = 0.001), previous biopsy (p = 0.025) when compared with younger women, the age at first birth between 25 and 29 years was statistically significant for both groups; however, the probability was higher in younger women (p = 0.008).
Conclusion: There is no conclusive evidence to consider that the GM is applicable to Venezuelan women due to its low precision since it only identified 41% of the patients who had BC as high risk; however, when the factors are analysed separately, we found a higher probability of a positive Gail with statistical significance in EM <11 years, PHBC, previous biopsy and age at first birth 25–29 years; When stratifying by age, we observed that the age at first birth 25–29 years in women aged 40 or less increases the probability of a positive Gail. It is necessary to develop new risk assessment models that are adapted to our female population.
Keywords: Gail model, breast cancer, young women, risk factor
Correspondence to: Josepmilly del Valle Peña Colmenares
Email: josepmillyp@yahoo.com
Published: 21/08/2023
Received: 27/03/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer (BC) is the most common cancer in women, with high morbidity and mortality rates [1]. In 2020, Globocan figures showed that BC accounted for 15.5% of cancer incidence and 10.5% of cancer deaths in Venezuela. The standardised incidence by age was 52.6 per 100,000 residents [2]. For over three decades, reports have described risk assessment models that make it possible to estimate the absolute risk an individual will develop BC and identify women at high risk (HR). That data make it possible to design prevention trials in HR population subgroups that can help identify detection and prevention strategies [3, 4].
Quantitative risk estimation is possible because multiple risk factors (RFs) can be combined into a comprehensible risk expression. The Gail and Claus models are the most widely used tools for estimating quantitative risk [5]. Gail et al [3, 6, 7] described a risk-assessment model that focuses mainly on non-genetic RFs, with limited information on family history. The Gail model (GM) was first designed to determine eligibility for a BC prevention trial and was one of the first models for estimating BC risk, but reports have described its limitations. The GM has since been modified and validated in different settings [3, 6, 8–9].
Current models can give a fairly accurate estimate of the risk of developing BC and can inform women with sufficient certainty if they have an increased lifetime risk. But it remains impossible to determine which patients will develop cancer and which patients will not [5]. Risk-estimation algorithms therefore now include more parameters, such as mammographic density [10], various single nucleotide polymorphisms [11] and other lifestyle-related factors to improve their accuracy. But more studies with large patient cohorts are vital for validating these new criteria.
We must remember that all risk-estimation tools have limitations, and while various BC risk models have emerged in the last 20 years, their use is not common in Venezuelan clinical practice. Nonetheless, we can establish appropriate guidelines for applying these models as their use grows more common. Our study applied the GM to a group of patients already diagnosed with BC, allowing us to evaluate the accuracy of the GM and if applying it to the Venezuelan population is feasible.
General objectives
1) To evaluate the accuracy of the GM in women with a BC diagnosis from the Breast Pathology Service, Hospital Oncology Department, Venezuelan Institute of Social Security (SOH-IVSS).
2) To compare the accuracy of the GM in women over and under age 40 with a BC diagnosis.
3) To analyse if the variables that the GM include – percentage of relatives with a primary history of BC reported (PHBC), age at menarche (AM), age at first delivery (AFD) and previous biopsy (PB) – were associated with a higher BC risk.
Method
This is a descriptive, retrospective, cross-sectional study of 830 patients with a BC diagnosis at the Breast Pathology Service, SOH-IVSS, covering two periods and including two patient groups. From 2004 to 2014, the study included 415 women aged 40 and below, and from 2008 to 2012, the study included 415 women aged 41 and over. Risk estimates were calculated using the computerised GM [12]. A Gail risk >1.66% was considered high and designated Gail positive, while a Gail risk of ≤1.66 Gail was designated Gail negative. BC risks of all patients were calculated separately using the GM.
Statistical analysis
Means and SD were calculated for continuous variables and frequencies and percentages were calculated for nominal variables. The McNemar test was applied to verify differences in the GM between white and black patients. The estimate of Gail-positive probability was determined using a dichotomous logistic regression model, and the validity of that model was verified using the Hosmer-Lemeshow test. Confidence intervals were adjusted to infinity using the Rindskopf procedure. A value of p < 0.05 was considered statistically significant. Study data were tabulated and processed using SPSS 25.
Results
Eight hundred and thirty patients met the inclusion criteria in the Breast Pathology Service, SOH-IVSS from 2004 to 2014. The average age at BC diagnosis was 46 ± 13 years. The average ages for GM variables were AM 13 ± 2 years, AFD 22 ± 5 years and PB 32 ± 11 years; 23% of patients (191) had relatives with a history of breast cancer (HBC); 40.3% of those relatives were first degree; and 26.5% had a history of other cancers. Notably, only 41% of the women with BC diagnosis had a Gail score >1.66. Of all patients, 92.2% with a PHBC, 72.2% with a PB, 54% with AM <11 years and 82.11% with AFD from 25 to 29 years were Gail positive.
Relationship between the GM and the different variables
A comparison of patients' ages ≤40 with patients’ ages ≥41 found no difference between a positive or negative Gail score, but four other GM variables – AM, PHBC, PB and AFD – were all statistically significant. No difference was found when including patients who had relatives with another type of cancer (Table 1).
Bivariate analysis: Gail and odds ratio
Performing a bivariate analysis and relating the independent variables (AM, PHBC, PB, AFD and others) with the GM revealed a higher probability of being Gail positive with a lower AM (<11 years OR: 2.01/p < 0.001), PHBC (OR: 5.85/p < 0.01), PB (OR: 1.79/p < 0.006) and AFD >25 years (25–29 years OR: 4.48/p < 0.007; ≥ 30 years OR: 7.87/p < 0,001), with all variables statistically significant. But for patients with AM ≥14 years (OR: 0.22/p < 0.001), no PB (OR: 0.47/p < 0.039) and AFD ≤24 years (OR: 0.21/p < 0.008), the probability of being Gail positive decreased 0.22 times, 0.47 times and 0.21 times, respectively. The bivariate analysis found no statistical association between being Gail positive and age (≤ or >40 years). This analysis included other variables, such as second-degree relatives with BC and other cancers, for which no relationship was found (Table 2).
Bivariate analysis: GM > and <40 years with odds ratio (Table 3)
Notably, stratifying patients by age with a cutoff at age 40 showed that women under 40 were more likely to be Gail positive (OR: 2.48/p = 0.001) with an AM ≤11 years. In contrast, there was no statistical association between age and AM amongst women aged ≥41 years (OR: 1.55/ p = 0.072).
Also notably, younger women (≤40) with a PHBC (OR: 7.47/p = 0.002) had an 88% probability of being Gail positive compared to older women (OR: 2.89/p = 0.002), which was statistically significant. Similarly, women aged £40 years with a PB for any disease were 2.32 times more likely to be Gail positive (OR:2.32/p = 0.048).
Table 1. Relationship between the GM and the different variables.
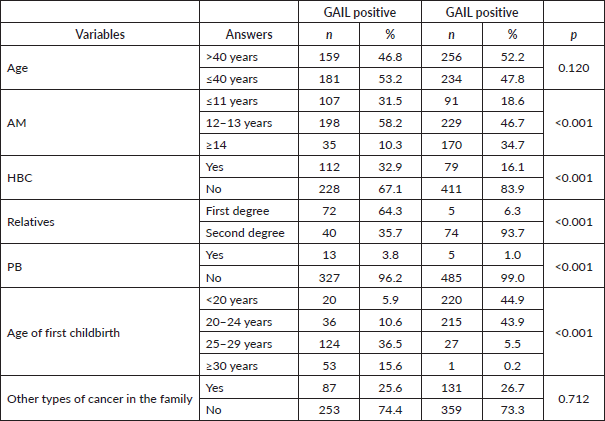
Table 2. Gail and odds ratio (bivariate analysis).
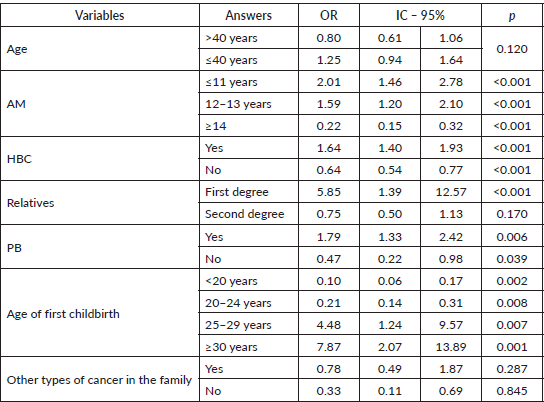
Table 3. GM > and <40 years with odds ratio (bivariate analysis).
This analysis also included AFD, another GM variable, and found that women >40 years with an AFD >30 years were 7.14 times more likely to be Gail positive (p = 0.001). Women aged ≤40 years were 5.87 times more likely to be Gail positive (p = 0.013). The bivariate analysis also found that while the probability of being Gail positive fell with a lower AFD, the difference was greater in women aged>40 years, with a statistical association (AFD 20–24 OR: 0.33/p = 0.002).
Dichotomous logistic regression relating the GM with independent variables
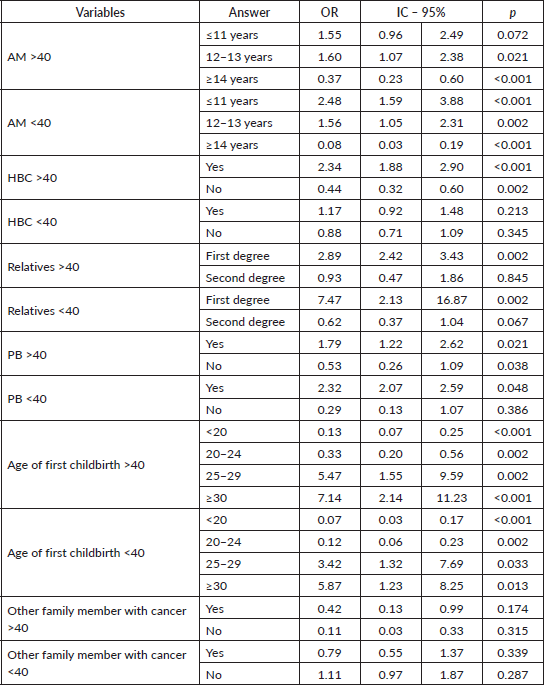
The multivariable regression analysis found that women with AM <11 years, relatives with a family HBC, PHBC, PB and AFD 25–29 years were 3.98 (p = 0.036), 2.99 (p = 0.014); 4.06 (p = 0.005), 3.09 (p = 0.007) and 4.05 (p = 0.019) times more likely to be Gail positive than women with AM >11 years with no family HBC or PB or whose AFD <25 years. All these findings were statistically significant (Table 4).
Table 4. Dichotomous logistic regression relating the GM with independent variables.
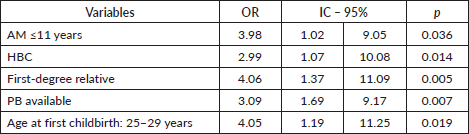
Table 5. Dichotomous logistic regression relating Gail positive result age.
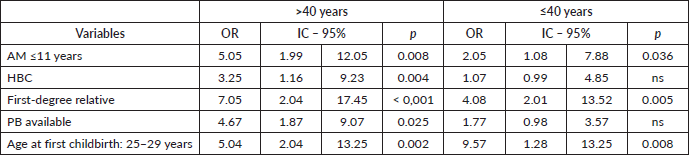
Dichotomous logistic regression relating the GM to age ( > and ≤ 40 years)
Using dichotomous regression analysis to compare the GM to the different variables by age (> or ≤40 years) again found that women aged ≥41 years with AM ≤11 years OR:5.05/p = 0.008), PHBC (OR:7.05/p = 0.001), PB (OR:4.67/p = 0.025) were more likely to be Gail positive than younger women. The difference between the two groups was statistically significant for all variables, though PHBC in women under the age of 40 years was less significant. However, younger women were significantly more likely to be Gail positive, despite having an AFD from 25 to 29 years (Table 5).
Notably, only 41% of our study population was classified as HR. Evaluating all the factors used in this calculation, we note that both the bivariate analysis and the multivariable regression model found significant differences in all factors the GM includes. Unlike the bivariate analysis, the dichotomous logistic regression model found, when stratifying by age, that women aged >40 years were more likely to be Gail positive and had a lower AM, a PHBC and a PB when compared with younger women. AFD from 25 to 29 years was statistically significant for both groups.
Discussion
The accuracy of a risk model depends on identifying RFs, estimating their effects in specific populations and knowing the medical, family and demographic history of women [13]. However, the socioeconomic situation in Venezuela and Latin America is also important when considering the use of risk models. The regional context justifies using methods considered outdated in developed countries, where advanced technologies such as artificial intelligence are more common and make the GM useful in studying populations such as Venezuelan women.
Notably, the GM statistical algorithm was developed after a screening study of 280,000 women ages 35–74 and has since undergone multiple modifications. Researchers have also noted that the GM does not consider racial or ethnic differences, BRCA genetic variants, tamoxifen use, or women excluded from the GM who already had a confirmed diagnosis of ductal or lobular carcinoma of the breast in situ. Nonetheless, research has shown that the GM is a reasonable tool for estimating BC risk in white women, and other researchers have enhanced the GM to provide accurate risk assessments for African-American, Hispanic and Asian women. The Venezuelan population is characterised by a mix of three ethnic groups (white, Indian and black) for which the GM has not been validated [5, 13–15].
BC is a complex disease resulting from the interaction of multiple environmental, hormonal and lifestyle factors, plus the genome of each patient. Clinicians and researchers consider a broad range of RFs but cannot determine the causative agent in 75% of BC cases [16]. Carcinogenesis results from a complex combination of genome and host factors and the response of the body to DNA and to cell damage [16–19].
There are reports that years of exposure to oestrogens and the increase in ovulatory cycles associated with early AM is an RF for BC [3, 14, 17, 18]. Pregnancy at an early age has also been defined as an RF, and pregnancy before age 20 is associated with a markedly reduced BC risk [13, 20]. Some researchers have suggested that this ‘protector’ effect is found only when pregnancies end in the birth of a viable foetus [5]. In contrast, nulliparity and AFD >30 years are associated with an increased risk of subsequent BC [5, 20].
BC has always been considered a multifactorial disease. However, having relatives with an HBC is another variable that has been studied, and has been called a non-modifiable RF. The risk of developing BC is strongly associated with the number and type of relatives affected, and their age at diagnosis [5, 13, 20, 21]. In this study, 23% of patients had relatives with an HBC, and 40.3% of them were first-degree relatives. Notably, just 9.2% of study patients had a PHBC, but 92.2% of those patients were Gail positive.
To date, few studies have evaluated risk-estimation tools in patients who already have a BC diagnosis. Pastor et al [15] used the National Surgical Adjuvant Breast and Bowel Project (NSABP) model, an adaptation of the GM, to estimate the 5-year risk of developing BC in 186 incident BC cases. That study found the NSABP would only have identified 40% of women with a BC diagnosis as HR. Ateş et al [22] reported similar results, which showed the risk analysis method they studied did not detect 67.48% of BC patients. That finding was similar to our study, which reported only 41% of patients as Gail positive. The GM would thus not have identified 59% of our patients as HR before their diagnosis, compared to the average risk of the population of the same age.
Comparison of the GM in women > or <40 years of age
To date, few studies have evaluated the GM as a population screening tool. Nickson et al [23] used data from 40,158 women aged 50–69 years who participated in the BreastScreen Australia Programme. The authors examined the association between Gail scores and future invasive BC, comparing the observed and expected results for groups classified by Gail score. That study confirmed that the GM can effectively stratify a population aged 50–69 by the risk of future invasive BC.
Several methods for determining BC risk have been identified, and the two most common are the GM, including modified versions, and the National Cancer Institute (NCI) method. Studies have found these models valid in many European racial and ethnic groups, but some of those studies did not include women under age 35, and neither does the NCI model [8, 12, 13].
Mackarem et al [24] evaluated 124 patients under the age of 40 years treated for carcinoma in situ and invasive cancer from 1983 to 1995. This study also compared this group with two other cohorts of women under age 40 years and verified that the GM provided little useful information in this age group. Similar to our study, Mackarem et al [24] found the relative risk of developing BC rose both as AM fell and in women with a PB. However, BC risk level depends on whether the patient is over or under the age of 50 years. Our bivariate analysis found that patients under the age of 40 years with an AM <11 years, a PHBC and a PB were more likely to be Gail positive than women over the age of 40 years. AM, PHBC and PB were all statistically significant in both groups, but the dichotomous logistic regression model found that AFD from 25 to 29 years (OR: 9.57/p = 0.008) in women under the age of 40 years increased the probability of being Gail positive. Notably, we found no significant difference between the two study groups when we used age as the cohort cutoff point.
The GM was designed using a population of cases and controls from the breast cancer detection project (BCDP). Women under the age of 40 years were only 3.8% of the BCDP population with BC. However, BC frequency in young women in developed countries varies from 5% to 6%, while in developing countries, it varies from 18% to 20%. This difference could explain why the GM has not been validated in women under the age of 40 years [3, 24–31].
It seems logical that all risk models apply to women with higher BC risk, and to various research programmes. Projects or studies in populations of women under the age of 40 years, in which BC percentage is under 20% [26, 30–34], would thus lead us to ask ‘Will this study also fail to validate the GM in this patient subgroup? Will BC detection and prevention continue to exclude young women?’
Risk assessment
Assessing BC risk requires a deep knowledge of RFs, family tree analysis, comparative statistics, genetic susceptibility testing and probability science [5].
Calculations of absolute individual BC risk are not common but providing appropriate clinical management for women requires knowing that risk [35]. BC has been the leading cause of cancer morbidity and mortality in Venezuelan women since 2009 [32]. Venezuela lacks updated registries and prevention policies and thus needs risk-estimation methods that allow us to predict the probability a woman will develop BC relative to the general population.
All patients in our study already had a BC diagnosis, and the GM could thus not identify 59% as HR. We could thus conclude that using the GM in Venezuelan women seems inappropriate.
Risk assessment and risk management involve scientific and public beliefs and issues of power and trust. Policymakers who want to promote educational and interventional strategies to reduce health risks must understand the different ways the public and health professionals perceive risks [13, 36].
Usefulness of GM in the era of artificial intelligence
In the last two decades, BC research has focused on early detection methods and sought treatments, achieving significant progress. However, BC remains the leading cause of morbidity and mortality in women in Venezuela and globally [5, 15]. One of the newest scientific and technological advances is the global development of various artificial intelligence tools for diagnosis (screening, artificial intelligence based on computer-aided diagnosis, imaging control and dose reduction, radiological-pathological correlations, etc.) and establishing the probability of determining the risk a woman will develop BC [37, 38]. The validity of using these tools either individually or with traditional risk-assessment methods is the subject of debate. Venezuela has suffered a steady decline in its economy and basic social services amid a humanitarian crisis worsened by the COVID-19 pandemic. The country thus lacks public health policies and its public health facilities either lack new technologies for their specialist physicians to use, or those technologies arrive late. Consequently, the benefits of technology do not reach most Venezuelans, who rely on public health facilities for care.
Venezuela also lacks a working BC screening programme. We know that risk-stratified BC detection protocols require accurate risk estimates that draw on data that is easily accessible from population-based programmes [39]. Venezuela lacks clear policies for monitoring and caring for BC patients, and such policies require guidelines for BC screening [16].
It is possible to obtain the data the GM requires, so implementing the GM broadly in many healthcare centres that serve low-income patients remains vital. And notably, other BC risk-estimation models are based on the GM, specifically the breast cancer risk assessment tool, which is available in the website of the NCI of the U.S. National Institutes of Health.
It is therefore critical to create a new BC prevention strategy. Our study opens a line of research in Venezuela that relies on using simple predictive methods for obtaining data, variables, or other factors the GM incorporates that could be useful in developing new risk algorithms. This line of research could prevent Venezuela from having to adapt guidelines for Venezuelan women and instead lead to similar studies in public and private healthcare facilities. That research could examine other independent variables and help create a new risk model appropriate for Venezuelan women.
Observations
Our study has several limitations: a) It was a retrospective study that collected data from patients diagnosed with BC, not a prospective study conducted in a population screening context. This study did not include healthy women with follow-up to assess observed/expected cases. b) We know of other models or new BC risk calculation algorithms, specifically the Tyrer Cuzick Calculator, but we used the GM because it was based on the only data obtained from histories of breast disease at the hospital where we conducted the study. That hospital lacks genetic counselling, a geneticist, and genetic testing, which were thus unavailable for our study. We also could not consider mammographic density because patients had mammograms in different health centres. These and other limitations forced us to use one of the earliest GM methods, which some doctors might consider obsolete compared to more recent technologies. c) Some authors caution that a cutoff point at age 40 in the GM is not useful and recommend against it. Our study used the age of 40 years as a cutoff point and had the same number of patients in the two age groups, as we tried to stratify our study population by age to determine any differences. Risk models usually exclude women under the age of 40 years due to the low incidence of BC in that subgroup. d) Our study data are not generalisable as the study sample is from a single hospital, though that hospital is a national breast carcinoma referral centre.
Conclusion
Risk models are used to help advise women about their risk or to identify patients who need a referral to genetic or HR specialists. Venezuela lacks studies that allow us to evaluate the various predictive BC risk models. It is thus useful to apply the GM in our population, as risk-estimation tools are important for decision-making in BC prevention and detection.
The GM only identified 41% of patients with BC as HR. Given such low accuracy, the GM does not seem appropriate for Venezuelan women. However, by analysing RFs separately, we found a greater probability of a positive Gail score, with statistical significance in AM <11 years, PHBC, PB and AFD 25–29 years.
We know that currently used risk models have been developed in other latitudes and were validated in populations that do not necessarily have the same characteristics as ours. Developing a new risk-assessment model is thus essential. That model should include the aforementioned variables, add other RFs that the GM risk calculation does not include and contain enough cases to allow us to set appropriate guidelines for Venezuelan women.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Disclosure
The authors have no financial interest to declare related to the disclosure of the content of this article. Their only interest focuses on opening a line of research on the topic of the study in Venezuela.
Funding
No financial support was received for writing this article, for data collection or analysis, or for preparing or approving this current manuscript.
References
1. GLOBOCAN: estimated cancer incidence, mortality and prevalence worldwide in 2012 [http://globocan.iarc.fr/Pages/summary_table_pop_sel.Aspx] (Date accessed: 31/01/17)
2. Venezuela, BOLIVARIAN REPUBLIC OF – International Agency for Research on Cancer (Global Cancer Observatory) [https://gco.iarc.fr/today/data/factsheets/populations/862-venezuela-bolivarian-republic-of-fact-sheets.pdf] (Date accessed: 02/06/23)
3. Gail MH, Brinton LA, and Byar DP, et al (1989) Projecting individualized probabilities of developing breast cancer for white females who are being examined annually J Natl Cancer Inst 81 1879–1886 https://doi.org/10.1093/jnci/81.24.1879 PMID: 2593165
4. Wang X, Huang Y, and Li L, et al (2018) Assessment of performance of the Gail model for predicting breast cancer risk: a systematic review and meta-analysis with trial sequential analysis Breast Cancer Res 18(20) 1–19
5. Sakorafas GH, Krespis E, and Pavlakis G (2002) Risk estimation for breast cancer development; a clinical perspective Surg Oncol 10(4) 183–192 https://doi.org/10.1016/S0960-7404(02)00016-6 PMID: 12020673
6. Costantino JP, Gail MH, and Pee D, et al (1999) Validation studies for models projecting the risk of invasive and total breast cancer incidence J Natl Cancer Inst 91(18) 1541–1548 https://doi.org/10.1093/jnci/91.18.1541 PMID: 10491430
7. Evans G, Brentnall A, and Harvi M, et al (2014) Breast cancer risk in young women in the national breast screening programme: implications for applying NICE guidelines for additional screening and chemoprevention Cancer Prev Res 7(10) 993–1001 https://doi.org/10.1158/1940-6207.CAPR-14-0037
8. Euhus DM, Leitch AM, and Huth JF, et al (2002) Limitations of the Gail model in the specialized breast cancer risk assessment clinic Breast 8(1) 23–27 https://doi.org/10.1046/j.1524-4741.2002.08005.x
9. Claus EB, Risch N, and Thompson WD (1993) The calculation of breast cancer risk for women with a first-degree family history of ovarian cancer Breast Cancer Res Treat 28 115–120 https://doi.org/10.1007/BF00666424 PMID: 8173064
10. Judkins T, Rosenthal E, and Arnell C, et al (2012) Clinical significance of large rearrangements in BRCA1 and BRCA2 Cancer 118(21) 5210–5216 https://doi.org/10.1002/cncr.27556 PMID: 22544547 PMCID: 3532625
11. Kast K, Schmutzler RK, and Rhiem K, et al (2014) Validation of the manchester scoring system for predicting BRCA1/2 mutations in 9,390 families suspected of having hereditary breast and ovarian cancer Int J Cancer 135(10) 2352–2361 https://doi.org/10.1002/ijc.28875 PMID: 24700448
12. Detailed breast cancer risk calculator [
13. Seyednoori T, Pakseresht S, and Roushan Z (2012) Risk of developing breast cancer by utilizing Gail model Women & Health 52(4) 391–402 https://doi.org/10.1080/03630242.2012.678476
14. McTiernan A, Kuniyuki A, and Yasui Y, et al (2001) Comparisons of two breast cancer risk estimates in women with a family history of breast cancer Cancer Epidemiol Biomarkers Prev 10(4) 333–338 PMID: 11319173
15. Pastor I, Morales M, and Llopis A, et al (2005) Aplicación del método de Gail de cálculo de riesgo de cáncer de mama a la población valenciana Clin Transl Oncol 7(8) 336–343 [https://pesquisa.bvsalud.org/portal/resource/pt/ibc-040784] https://doi.org/10.1007/BF02716549
16. Peña CJ, Pérez J, and Jahon J, et al (2017) Consenso de detección Temprana de Cáncer de Mama. Pesquisa oportunista. Pesquisa en Cáncer de mama Rev Venez Oncol 29 141–159 [https://www.redalyc.org/journal/3756/375650363011/375650363011.pdf]
17. McTiernan A (2003) Behavioral risk factors in breast cancer: can risk be modified? Oncologist 8(4) 326–334 https://doi.org/10.1634/theoncologist.8-4-326 PMID: 12897329
18. Prentice RL, Caan B, and Chlebowski RT, et al (2006) Low-fat dietary pattern and risk of invasive breast cancer: the women’s health initiative randomized controlled dietary modication trial JAMA 295(6) 629–642 https://doi.org/10.1001/jama.295.6.629 PMID: 16467232
19. Pruthi S, Brandt K, and Degnim A, et al (2007) A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis Mayo Clin Proc 82(8) 999–1012 https://doi.org/10.4065/82.8.999 PMID: 17673070
20. Vogel VG (2000) Breast cancer prevention: a review of current evidence CA Cancer J Clin 50(3) 156–170 https://doi.org/10.3322/canjclin.50.3.156 PMID: 10901739
21. Azzena A, Zen T, and Ferrar A, et al (1994) Risk factors for breast cancer. Case-control. Study results Eur J Gynaecol Oncol 15(5) 386–392 PMID: 7828610
22. Ateş E, Bozkurt B, and Çam R (2018) Sensitivities of the Gail, NSABP and NCI risk analysis models for Turkish women for breast cancer risk assessment Ankara Med J 18 31–39
23. Nickson C, Procopio P, and Velentzis L, et al (2018) Prospective validation of the NCI breast cancer risk assessment tool (Gail Model) on 40,000 Australian women Breast Cancer Res 20 155–166 https://doi.org/10.1186/s13058-018-1084-x PMID: 30572910 PMCID: 6302513
24. MacKarem G, Roche CA, and Hughes KS (2001) The effectiveness of the Gail model in estimating risk for development of breast cancer in women under 40 years of age Breast J 7(1) 34–39 https://doi.org/10.1046/j.1524-4741.2001.007001034.x PMID: 11348413
25. Baker LH (1982) Breast cancer detection demonstration project: five-year summary report CA Cancer J Clin 32(4) 194–225 https://doi.org/10.3322/canjclin.32.4.194 PMID: 6805867
26. Narod SA (2012) Breast cancer in young women Nat Rev Clin Oncol 9(8) 460–470 https://doi.org/10.1038/nrclinonc.2012.102 PMID: 22733233
27. Anders CK, Fan C, and Parker JS, et al (2011) Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 29(1) e18–e20 https://doi.org/10.1200/JCO.2010.28.9199 PMCID: 3055864
28. Paluch-Shimon S, Pagani O, and Partridge AH, et al (2017) ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3) Breast 35 203–217 https://doi.org/10.1016/j.breast.2017.07.017 PMID: 28822332
29. Partridge AH, Pagani O, and Abulkhair O, et al (2014) First international consensus guidelines for breast cancer in young women (BCY1) Breast 23(3) 209–220 https://doi.org/10.1016/j.breast.2014.03.011 PMID: 24767882
30. Peres J (2013) Advanced breast cancer in young women J Ntl Cancer Inst 105(17) 1257–1258 https://doi.org/10.1093/jnci/djt245
31. Franzoi M, Rosa D, and Zaffaroni F, et al (2019) Advanced stage at diagnosis and worse clinicopathologic features in young women with breast cancer in Brazil: a subanalysis of the AMAZONA III study (GBECAM 0115) J Glob Oncol 5 1–10 PMID: 31730380 PMCID: 6882517
32. Capote Negrín LG (2015) Resumen del cáncer en Venezuela 2012 Rev Venez Oncol 27 256–268 [https://www.redalyc.org/pdf/3756/375641011010.pdf]
33. Cazap E (2018) Breast cancer in Latin America: a map of the disease in the region Am Soc Clin Oncol Educ Book 38 451–456 https://doi.org/10.1200/EDBK_201315 PMID: 30231404
34. Villarreal-Garza C, Mohar A, and Bargallo-Rocha J, et al (2017) Molecular subtypes and prognosis in young Mexican women with breast cancer Clin Breast Cancer 17(3) e95–e102 https://doi.org/10.1016/j.clbc.2016.11.007 PMID: 28065398
35. Tyrer J, Duffy S, and Cuzick J (2004) A breast cancer prediction model incor- porating familial and personal risk factors Stat Med 23(7) 1111–1130 https://doi.org/10.1002/sim.1668 PMID: 15057881
36. Katapodi MC, Lee KA, and Facione NC, et al (2004) Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review Prev Med 38(4) 388–402 https://doi.org/10.1016/j.ypmed.2003.11.012 PMID: 15020172
37. Morgan MB and Mates JL (2021) Applications of artificial intelligence in breast imaging Radiol Clin North Am 59(1) 139–148 https://doi.org/10.1016/j.rcl.2020.08.007
38. Fernandez De Freitas M and Capecchi A (2021) Inteligencia artificial en la detección del cáncer de mama por tomosíntesis, ¿hacia dónde vamos? Rev Cient CMDLT 15(2) e-211066
39. Australian Institute of Health and Welfare (2017) Breast Screen Australia Monitoring Report 2014–2015. Cancer Series No. 106. Cat. No. CAN 105 (Canberra: AIHW)






