Ionizing radiation-induced cancer: perplexities of the bystander effect
Lakshmi Gopinathan1 and C Gopinathan1,2
1Independent consultant, Navi Mumbai 400703, India
2Ex-Head, Chemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai 400085, India
Abstract
Ionizing radiation (IR) is a carcinogen. This has been established beyond doubt from many years of studies such as those conducted among the survivors of the atomic bomb attacks on Hiroshima and Nagasaki and later from the Chernobyl accident. Despite immense progress in the field of carcinogenesis, complete understanding of the underlying mechanisms behind IR-induced cancer remains elusive. In particular, the long gestation period between exposure to IR and the onset of cancer, frequently unpredictable, and sometimes lasting for many years, remains poorly understood. The centrality of DNA damage and misrepair in carcinogenesis research has not entirely benefited IR-induced cancer research and the past decade has seen a shift in understanding radiation-driven cellular mechanisms beyond simplistic models of targeted DNA damage. This paper presents a viewpoint on the gaps in our knowledge of IR-induced cancer with a focus on the non-targeted bystander effect, the mechanisms underlying which may be key to radiotherapeutic advances.
Keywords: cancer, ionizing radiation, bystander effect, non-targeted effect
Correspondence to: Lakshmi Gopinathan and C Gopinathan
Email: lakshmigopinathan@gmail.com and chakrapanygopinathan@gmail.com
Published: 20/07/2023
Received: 12/06/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (
Introduction
In a nuclear weapon, several switches have to be activated in the right sequence for the bomb to explode. Carcinogenesis is similarly a multi-stage process where information from various damage centres has to be recalled in the right sequence for the cell to transition into a cancerous mode. Once the various damage sites have been created, the system is like a ticking time bomb waiting to explode. The series of sequential events such as tumour initiation, promotion and progression involving activation of oncogenes and inactivation of tumour-suppressing genes are well studied and have been extensively reviewed [1, 2]. The long latent period between exposure to ionizing radiation (IR) and the onset of cancer, which in the case of humans could run into decades, could be attributed to the duration of this sequence. However, the mutations and growth characteristics of tumours induced by radiation appear indistinguishable from spontaneous or other carcinogen-induced tumours that have quicker onset or similar multi-stage progression [3]. Further, the delayed occurrence of genetic aberrations in the progeny of irradiated cells and the occurrence of DNA damage in non-irradiated cells near the site of irradiation (Figure 1) strongly challenge a direct causal connectivity from radiation to DNA damage to cancer. In this scoping review, we discuss a few established as well as evolving insights that support a multifarious model of radiation carcinogenesis. We are hopeful that our perspective on IR-induced non-targeted effects can help researchers identify poorly understood aspects in the field and incite further research on the mechanisms of IR-induced cancer with potential implications in the radiotherapeutic management of cancer.
Methodology
We searched PubMed using the terms ‘IR-induced cancer’, ‘IR-induced non-targeted effects’, ‘IR-induced non-targeted bystander effect’, and ‘IR carcinogenesis’ with no time limit to identify relevant studies. Taking into consideration our aim to provide a focused review, we have cited as many studies as possible, both original as well as review articles that offer a historical perspective of knowledge evolution as well as current understanding.
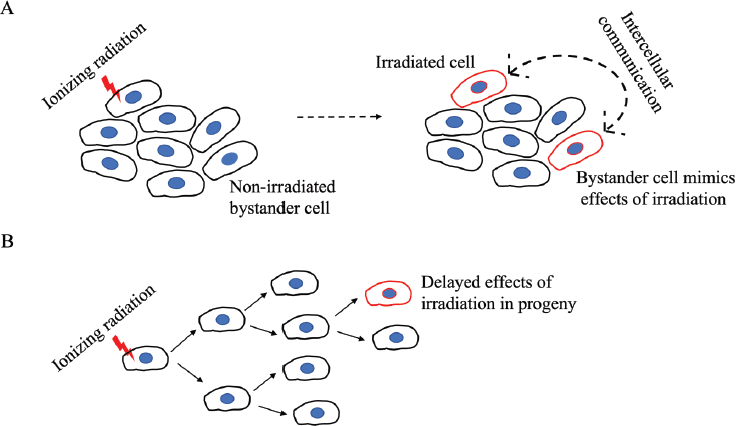
Figure 1. A schematic representation of the non-targeted effects of IR on bystander cells. (a): An irradiated cell communicates stress stimuli to a neighbouring non-irradiated cell which then mimics the outcome of IR. The bidirectional arrow indicates that the communication can be reciprocal such as the adaptive response seen in radiotherapy where bystander cells can diminish the damage response in irradiated cells. (b): The effects of IR are seen only in distant progeny.
IR-induced non-targeted effects
Mechanistic radiobiology studies for a long time focused on IR-induced DNA damage and misrepair as initiators of cancer. However, given the protracted gestation period for cancer development following IR exposure, radiation-induced initiating events are unlikely to be effects on specific genes that typify-targeted cancer onset. Rather, a delayed or an enduring event(s) with eventual tumorigenic implications is a more likely culprit. To this end, persistent genomic instability induced by radiation has gained acceptance over the last decade as the grounds for mutagenic events that are tumorigenic [4]. Mutagenic damage as such an indirect effect of radiation exposure is supported by experimental studies demonstrating the normal division of irradiated cells over several generations, with mutagenic change appearing only in distant progeny [5]. This presents a further challenge to the assumption of cancer initiation by radiation-induced direct DNA damage and is a curious phenomenon termed the bystander effect. In contrast to its use in psychology where the term refers to an apathetic state, the bystander effect in radiation biology refers to the active involvement of ‘bystander’ non-irradiated cells, located near the site of irradiation, in the development of cancer. Revealing an intricate web of cell-cell communication, irradiated cells convey stress stimuli to non-irradiated cells which then mimic the outcomes of radiation exposure in a delayed manner, sometimes only after several generations (Figure 1). Initially identified as chromosomal aberrations in the progeny of cells irradiated with a-particles [6, 7], the radiation-induced bystander effect (RIBE) or radiation-induced non-targeted effect, is now known to involve the gamut of IR-induced modifications such as mutations, chromosomal instability, apoptosis, epigenetic changes and altered cell signalling, thereby associating RIBE closely with several hallmarks of cancer [8, 9]. In contrast to the direct effects of radiation, RIBE exhibits a non-linear dose-response with effects seen at very low doses and may be linked to secondary cancers in patients who have undergone radiation treatment. These systemic long-range effects of irradiation, occurring at sites distant from the irradiated volume within the same organism are referred to as abscopal effects, and have led to the identification of inhibitors of RIBE to minimise irradiation risks for patients [10–12].
Despite extensive research, RIBE remains a perplexing phenomenon and the underlying molecular pathways are not fully understood. Key mechanisms involve communication via intercellular gap junctions [13–15] or directly by the release of diffusible factors by irradiated cells [16, 17, reviewed in 18] (Figure 2). Gap-junction-mediated signaling can occur via ions such as calcium or small molecules such as nitric oxide (NO), while secretions into the extracellular compartment include cytokines such as transforming growth factor-β chemokines and reactive oxygen species (ROS) [19–22]. In addition, exosomes, a form of extracellular vesicles, have been proposed as transmitters of RIBE [23]. Early studies reported increased levels of Fas ligand, also called the ‘death ligand’, on extracellular vesicles in response to IR [24]. Since then, many types of exosome cargo such as mitochondrial DNA (mtDNA), non-coding RNAs, immune players, lipids and protein mediators have been implicated in RIBE (Figure 2) [25]. The persistent longevity of exosome-mediated RIBE was well demonstrated when non-irradiated cells treated with exosomes derived from the progeny of bystander cells also exhibited RIBE [26].
A demonstration of a role for mtDNA in RIBE came from experiments where exosomes or conditioned medium derived from irradiated cells depleted of mtDNA were unable to induce DNA damage in non-irradiated cells [27, 28]. In comparison to its nuclear counterpart which is protected by histones, mtDNA has been reported to be more sensitive to certain oxidative damages due to its proximity to the electron transport chain [29, 30]. The criticality of mitochondria in energy production and cellular metabolism suggests that exosome-mediated communication of mitochondrial dysfunction using mtDNA could represent an important facet of RIBE. In more recently identified mechanisms, biophotons, which are photons of light in the ultraviolet and low visible light range, have been proposed as intercellular communicators of radiation-induced stress and have been shown to incite bystander cells to release exosomes and disrupt mitochondrial oxidative phosphorylation [31, 32].
One of the most notable advances in understanding the role of non-coding RNAs in IR-induced cancer has been their detection in exosomes from irradiated cells [33]. Among the various classes of non-coding RNA, long non-coding RNA (lncRNA), microRNA (miRNA) and circular RNA have been studied the most in IR-induced carcinogenesis [34, 35]. Because of distinct changes in their expression upon radiation exposure, and due to their stability and abundance in serum and other biofluids, several of these non-coding RNAs have been proposed as diagnostic and prognostic biomarkers of radiation damage. While most research has focused on their utility in assessing response to radiotherapy, their roles in IR-induced cancer are less clear ([36–38] for lncRNA; [39–42] for miRNA; and [43–45] for circular RNA). Non-coding RNAs have been shown to respond to IR by modulating key pathways related to apoptosis, the cell cycle, DNA damage repair, glycolysis and autophagy [46–49]. One of the most abundant and conserved miRNAs regarded as an important cancer biomarker, miR-21, is a well-studied example whose increased expression in bystander cells is likely mediated through endocytic uptake by exosomes [50].

Figure 2. A schematic representation of the mechanisms of intercellular communication between an irradiated cell and a bystander cell. An irradiated cell can communicate stresses to a bystander cell via NO-and ROS-based signalling pathways, by the release of diffusible factors such as cytokines and chemokines, or by exosome (Exo)-mediated transport of cargo such as mtDNA, miRNA and protein mediators. In addition to mimicking IR-induced changes such as genetic changes, epigenetic changes and genomic instability, bystander cells may also exhibit adaptive responses such as those seen in response to radiotherapy.
Implications of radiation-induced non-targeted effects in cancer therapy
In addition to the studies that have focused on the damaging features of RIBE, some have also described alternate aspects such as stress resistance, adaptive responses to radiotherapy, increased cell proliferation and enhanced cell differentiation [5, 51, 52]. In this regard, it is important to note that the communication between irradiated and bystander cells can be reciprocal, whereby bystander cells can diminish the damage response in irradiated cells (Figure 1a) [10, 53]. Although studies on RIBE have yet to clearly define a mechanism, studying RIBE in relation to radiotherapy has revealed its immunomodulatory nature. Apart from cytokine or exosome-mediated immune modulation, T-cell activation via the presentation of tumour-associated antigens from IR-damaged cancer cells is an important consideration that is a likely feature of abscopal effects. The recruitment of leucocytes, maturation of antigen-presenting cells and activity of cytotoxic lymphocytes are key immune aspects shown to be modulated by radiotherapy [10]. The integration of this knowledge with cancer immunotherapy may prove valuable for therapeutic advances by assessing radiation risk and improving patient responses to radiotherapy. Greater understanding of immunomodulatory abscopal effects has led to studies investigating the combinatorial efficacy of cancer immunotherapy and radiotherapy in an effort to enhance abscopal antitumor effects, which are normally rare to occur [54, 55]. These include studies on the concurrent use of IR with checkpoint inhibitors, cytokines and cytotoxic immune therapy.
Conclusion
We have strived to provide a critical overview that can clarify concepts and identify gaps in the current understanding of the mechanisms underlying IR-induced carcinogenesis. While evidence has accumulated for the criticality of non-targeted effects of IR, a comprehensive understanding of the molecular events leading to IR-induced cancer is lacking. How radiation-induced non-targeted effects influence the immune cell machinery and manifest as abscopal effects is also poorly understood. A deeper understanding of these concepts may prove instrumental in advancing the immunotherapeutic and radiotherapeutic management of cancer.
Conflicts of interest
The authors do not have any conflict of interest to declare.
Funding
The authors do not have any financial declaration.
References
1. Basu AK (2018) DNA damage, mutagenesis and cancer Int J Mol Sci 19(4) 970 https://doi.org/10.3390/ijms19040970 PMID: 29570697 PMCID: 5979367
2. Martínez-Reyes I and Chandel NS (2021) Cancer metabolism: looking forward Nat Rev Cancer 21(10) 669–680 https://doi.org/10.1038/s41568-021-00378-6 PMID: 34272515
3. National Research Council (2006) Radiation-induced cancer: molecular and cellular responses to ionizing radiation Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2, Chapter 2 (Washington: The National Academies Press) pp 43–64 https://doi.org/10.17226/11340
4. National Research Council (2006) Radiation-induced cancer: mechanisms, quantitative experimental studies, and the role of genetic factors Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2, Chapter 3 (Washington: The National Academies Press) pp 65–90 https://doi.org/10.17226/11340
5. Seymour CB and Mothersill C (2004) Radiation-induced bystander effects – implications for cancer Nat Rev Cancer 4(2) 158–164 https://doi.org/10.1038/nrc1277 PMID: 14964312
6. Nagasawa H and Little JB (1992) Induction of sister chromatid exchanges by extremely low doses of alpha-particles Cancer Res 52(22) 6394–6396 PMID: 1423287
7. Kadhim MA, Macdonald DA, and Goodhead DT, et al (1992) Transmission of chromosomal instability after plutonium alpha-particle irradiation Nature 355(6362) 738–740 https://doi.org/10.1038/355738a0 PMID: 1741061
8. Hu S and Shao C (2020) Research progress of radiation induced bystander and abscopal effects in normal tissue Radiat Med Prot 1(2) 69–74 https://doi.org/10.1016/j.radmp.2020.04.001
9. Heeran AB, Berrigan HP, and O'Sullivan J (2019) The radiation-induced bystander effect (RIBE) and its connections with the hallmarks of cancer Radiat Res 192(6) 668–679 https://doi.org/10.1667/RR15489.1 PMID: 31618121
10. Daguenet E, Louati S, and Wozny AS, et al (2020) Radiation-induced bystander and abscopal effects: important lessons from preclinical models Br J Cancer 123(3) 339–348 https://doi.org/10.1038/s41416-020-0942-3 PMID: 32581341 PMCID: 7403362
11. Yahyapour R, Salajegheh A, and Safari A, et al (2018) Radiation-induced non-targeted effect and carcinogenesis; implications in clinical radiotherapy J Biomed Phys Eng 8(4) 435–446 https://doi.org/10.31661/jbpe.v0i0.713 PMID: 30568933 PMCID: 6280111
12. Tremi A, Nowsheen S, and Aziz K, et al (2021) Chapter 13 Inflammation and oxidatively induced DNA damage: a synergy leading to cancer development Cancer – Oxidative Stress and Dietary Antioxidants 2nd edn, eds R Preedy and VB Patel pp 131–147 https://doi.org/10.1016/B978-0-12-819547-5.00013-4
13. Prise KM and O'Sullivan JM (2009) Radiation-induced bystander signalling in cancer therapy Nat Rev Cancer 9(5) 351–360 https://doi.org/10.1038/nrc2603 PMID: 19377507 PMCID: 2855954
14. Azzam E, de Toledo SM, and Little JB (2001) Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from α-particle irradiated to nonirradiated cells Proc Natl Acad Sci USA 98 473–478 https://doi.org/10.1073/pnas.98.2.473
15. Harada K, Nonaka T, and Hamada N, et al (2009) Heavy-ion-induced bystander killing of human lung cancer cells: role of gap junctional intercellular communication Cancer Sci 100(4) 684–688 https://doi.org/10.1111/j.1349-7006.2009.01093.x PMID: 19469013
16. Mothersill C and Seymour C (1997) Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of irradiated cells Int J Radiat Biol 71(4) 421–427 https://doi.org/10.1080/095530097144030 PMID: 9154145
17. Murphy JB, Liu JH, and Sturm E (1922) Studies on X-ray effects: IX. The action of serum from X-rayed animals on lymphoid cells in vitro J Exp Med 35(3) 373–384 https://doi.org/10.1084/jem.35.3.373 PMID: 19868613 PMCID: 2128138
18. Emerit I (1994) Reactive oxygen species, chromosome mutation, and cancer: possible role of clastogenic factors in carcinogenesis Free Radic Biol Med 16(1) 99–109 https://doi.org/10.1016/0891-5849(94)90246-1 PMID: 8300000
19. Wang R, Zhou T, and Liu W, et al (2018) Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy Oncotarget 9 18637–18647 https://doi.org/10.18632/oncotarget.24746 PMID: 29719632 PMCID: 5915099
20. Shao C, Folkard M, and Prise KM (2008) Role of TGF-β1 and nitric oxide in the bystander response of irradiated glioma cells Oncogene 27(4) 434–440 https://doi.org/10.1038/sj.onc.1210653
21. Shao C, Prise KM, and Folkard M (2008) Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts Mutat Res 638(1–2) 139–145 https://doi.org/10.1016/j.mrfmmm.2007.09.007
22. Calveley VL, Khan MA, and Yeung IW, et al (2005) Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation Int J Radiat Biol 81(12) 887–899 https://doi.org/10.1080/09553000600568002
23. Jokar S, Marques IA, and Khazaei S, et al (2022) The footprint of exosomes in the radiation-induced bystander effects Bioengineering (Basel) 9(6) 243 https://doi.org/10.3390/bioengineering9060243 PMID: 35735486 PMCID: 9220715
24. Albanese J and Dainiak N (2000) Ionizing radiation alters Fas antigen ligand at the cell surface and on exfoliated plasma membrane-derived vesicles: implications for apoptosis and intercellular signaling Radiat Res 153(1) 49–61 https://doi.org/10.1667/0033-7587(2000)153[0049:irafal]2.0.co;2 PMID: 10630977
25. Diegeler S and Hellweg CE (2017) Intercellular communication of tumor cells and immune cells after exposure to different ionizing radiation qualities Front Immunol 8 664 https://doi.org/10.3389/fimmu.2017.00664 PMID: 28638385 PMCID: 5461334
26. Al-Mayah A, Bright S, and Chapman K, et al (2015) The non-targeted effects of radiation are perpetuated by exosomes Mutat Res 772 38–45 https://doi.org/10.1016/j.mrfmmm.2014.12.007 PMID: 25772109
27. Ariyoshi K, Miura T, and Kasai K, et al (2019) Radiation-induced bystander effect is mediated by mitochondrial DNA in exosome-like vesicles Sci Rep 9(1) 1–14 https://doi.org/10.1038/s41598-019-45669-z
28. Miranda S, Correia M, and Dias AG, et al (2020) Evaluation of the role of mitochondria in the non-targeted effects of ionizing radiation using cybrid cellular models Sci Rep 10(1) 6131 https://doi.org/10.1038/s41598-020-63011-w PMID: 32273537 PMCID: 7145863
29. Druzhyna NM, Wilson GL, and LeDoux SP (2008) Mitochondrial DNA repair in aging and disease Mech Ageing Dev 129(7–8) 383–390 https://doi.org/10.1016/j.mad.2008.03.002 PMID: 18417187 PMCID: 2666190
30. Rong Z, Tu P, and Xu P, et al (2021) The mitochondrial response to DNA damage Front Cell Dev Biol 9 669379 https://doi.org/10.3389/fcell.2021.669379 PMID: 34055802 PMCID: 8149749
31. Le M, Fernandez-Palomo C, and McNeill FE, et al (2017) Exosomes are released by bystander cells exposed to radiation-induced biophoton signals: reconciling the mechanisms mediating the bystander effect PLoS One 12(3) e0173685 https://doi.org/10.1371/journal.pone.0173685 PMID: 28278290 PMCID: 5344502
32. Le M, McNeill FE, and Seymour CB, et al (2018) Modulation of oxidative phosphorylation (OXPHOS) by radiation-induced biophotons Environ Res 163 80–87 https://doi.org/10.1016/j.envres.2018.01.027 PMID: 29427954
33. Shaw A and Gullerova M (2021) Home and away: the role of non-coding RNA in intracellular and intercellular DNA damage response Genes (Basel) 12(10) 1475 https://doi.org/10.3390/genes12101475 PMID: 34680868 PMCID: 8535248
34. May JM, Bylicky M, and Chopra S, et al (2021) Long and short non-coding RNA and radiation response: a review Transl Res 233 162–179 https://doi.org/10.1016/j.trsl.2021.02.005 PMID: 33582242 PMCID: 8475769
35. Gao J, Zong X, and Chen N, et al (2021) Research progress on three different types of noncoding RNAs related to ionizing radiation Radiat Med Prot 2(2) 83–87 https://doi.org/10.1016/j.radmp.2021.04.001
36. Jiang H, Hu X, and Zhang H, et al (2017) Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression Radiat Oncol 12(1) 65 https://doi.org/10.1186/s13014-017-0802-3 PMID: 28376901 PMCID: 5381027
37. Zhang N, Zeng X, and Sun C, et al (2019) LncRNA LINC00963 promotes tumorigenesis and radioresistance in breast cancer by sponging miR-324-3p and inducing ACK1 expression Mol Ther Nucleic Acids 18 871–881 https://doi.org/10.1016/j.omtn.2019.09.033 PMID: 31751910 PMCID: 6881674
38. Liu SJ, Malatesta M, and Lien BV, et al (2020) CRISPRi-based radiation modifier screen identifies long non-coding RNA therapeutic targets in glioma Genome Biol 21(1) 83 https://doi.org/10.1186/s13059-020-01995-4 PMID: 32234056 PMCID: 7110660
39. Wang XC, Wang W, and Wang ZB, et al (2013) Overexpression of miRNA-21 promotes radiation-resistance of non-small cell lung cancer Radiat Oncol 8 146 https://doi.org/10.1186/1748-717X-8-146 PMID: 23777591 PMCID: 3698151
40. Huang X, Taeb S, and Jahangiri S, et al (2013) miRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1 Cancer Res 73(23) 6972–6986 https://doi.org/10.1158/0008-5472.CAN-13-1657 PMID: 24145350
41. Chen G, Zhu W, and Shi D, et al (2010) MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2 Oncol Rep 23(4) 997–1003 https://doi.org/10.3892/or_00000725 PMID: 20204284
42. Yan HL, Xu G, and Mei Q, et al (2009) Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis EMBO J 28(18) 2719–2732 https://doi.org/10.1038/emboj.2009.214 PMID: 19696742 PMCID: 2750010
43. Liu J, Xu N, and Guo Y, et al (2019) CircRNA_100367 regulated the radiation sensitivity of esophageal squamous cell carcinomas through miR-217/Wnt3 pathway Aging (Albany NY) 11(24) 12412–12427 https://doi.org/10.18632/aging.102580 PMID: 31851619 PMCID: 6949088
44. Guan Y, Cao Z, and Du J, et al (2020) Circular RNA circPITX1 knockdown inhibits glycolysis to enhance radiosensitivity of glioma cells by miR-329–3p/NEK2 axis Cancer Cell Int 20 80 https://doi.org/10.1186/s12935-020-01169-z
45. Niu H, Zhang L, and Chen YH, et al (2020) Circular RNA TUBD1 acts as the miR-146a-5p sponge to affect the viability and pro-inflammatory cytokine production of LX-2 cells through the TLR4 pathway Radiat Res 193(4) 383–393 https://doi.org/10.1667/RR15550.1 PMID: 32097101
46. Li Y, Ma X, and Li J, et al (2020) LncRNA gas5 regulates granulosa cell apoptosis and viability following radiation by X-ray via sponging miR-205-5p and Wnt/β-catenin signaling pathway in granulosa cell tumor of ovary Trop J Pharm Res 19(12) 1153–1159 https://doi.org/10.4314/tjpr.v19i6.5
47. Liu Y, Zhao J, and Zhang W, et al (2015) lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer Sci Rep 5 10159 https://doi.org/10.1038/srep10159 PMID: 25959498 PMCID: 4426700
48. Wang X, Arai S, and Song X, et al (2008) Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription Nature 454(7200) 126–130 https://doi.org/10.1038/nature06992 PMID: 18509338 PMCID: 2823488
49. Chen C, Wang K, and Wang Q, et al (2018) LncRNA HULC mediates radioresistance via autophagy in prostate cancer cells Braz J Med Biol Res 51(6) e7080 https://doi.org/10.1590/1414-431x20187080 PMID: 29694502 PMCID: 5937721
50. Du Y, Du S, and Liu L, et al (2020) Radiation-induced bystander effect can be transmitted through exosomes using miRNAs as effector molecules Radiat Res 194(1) 89–100 https://doi.org/10.1667/rade-20-00019.1 PMID: 32343639
51. Mothersill C and Seymour C (2006) Radiation-induced bystander effects: evidence for an adaptive response to low dose exposures? Dose Resp 4(4) 283–290 https://doi.org/10.2203/dose-response.06-111.mothersill
52. Goldberg Z and Lehnert BE (2002) Radiation-induced effects in unirradiated cells: a review and implications in cancer Int J Oncol 21 337–349 https://doi.org/10.3892/ijo.21.2.337 PMID: 12118330
53. Widel M, Przybyszewski WM, and Cieslar-Pobuda A, et al (2012) Bystander normal human fibroblasts reduce damage response in radiation targeted cancer cells through intercellular ROS level modulation Mutat Res 731 117–124 https://doi.org/10.1016/j.mrfmmm.2011.12.007 PMID: 22210495
54. Zhang Z, Liu X, and Chen D, et al (2022) Radiotherapy combined with immunotherapy: the dawn of cancer treatment Signal Transduct Target Ther 7(1) 258 https://doi.org/10.1038/s41392-022-01102-y PMID: 35906199 PMCID: 9338328
55. Dovedi SJ, Cheadle EJ, and Popple AL, et al (2017) Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal t-cell populations when combined with PD-1 blockade Clin Cancer Res 23(18) 5514–5526 https://doi.org/10.1158/1078-0432.CCR-16-1673 PMID: 28533222



