Acute leukaemia in children with Down syndrome in a low middle-income country
Rahat Ul-Aina, Mahwish Faizan, Saadia Anwar, Shazia Riaz, Alia Ahmad, Huma Zafar and Wasila Shamim
Department of Pediatric Hematology/Oncology & Bone Marrow Transplant, University of Child Health Sciences, Children’s Hospital, Lahore, Punjab 54600, Pakistan
ahttps://orcid.org/0000-0001-8492-1293
Abstract
Down syndrome (DS) is the commonest chromosomal disorder and is considered to be the most common syndrome associated with acute leukaemia. The objective of this study was to determine the characteristics of acute leukaemia in children with DS in Pakistan. It was a retrospective, cohort study conducted over a 2-year period, and the data was analysed in SPSS 20.0 in terms of descriptive statistics. Nineteen DS patients with acute leukaemia were enrolled. The proportion of DS-acute leukaemia was found to be 1.84% among all cases of paediatric acute leukaemia. The mean age of presentation was 5.5 years ± 4.3 SD with a male to female ratio of 1.1:1. The precursor B-cell ALL was found in 13 (68.4%) and acute myeloid leukaemia was found in 6 (31.6%) patients of DS. Thirteen patients (68.4%) completed treatment, while 6 (31.6%) expired due to treatment-related toxicity. Mean overall survival was 38 months ± 5.34 SD. The status of diagnosis of DS before presentation with acute leukaemia was the only statistically significant factor associated with the outcome. Few distinct characteristics of DS-acute leukaemia have been found in our population. Treatment toxicity was the sole cause of treatment failure.
Keywords: Down syndrome, acute leukaemia, childhood leukaemia, low middle-income country
Correspondence to: Rahat Ul-Ain
Email: dr.rkashif@yahoo.com
Published: 13/04/2022
Received: 12/01/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Down syndrome is the most common chromosomal disorder with an incidence of 1 in 700 births in the United States. It is associated with an increased risk for certain medical conditions including childhood leukaemia [1]. There is a 10–20-fold increased risk of developing acute leukaemia in children with DS, with a cumulative risk of 2.1% at the age of 5 years [2, 3]. Leukaemia is one of the most common causes of mortality in patients with DS [3]. The prognosis of Down syndrome-acute lymphoblastic leukaemia (DS-ALL) has been considered poorer as compared to non-DS-ALL [5–7], but the recent trials have shown excellent long-term survival of children with DS-ALL and increased rate of toxicity [8, 9]. Down syndrome-acute myeloid leukaemia (DS-AML), on the other hand, is known for its favourable outcome [10–15]. In the past few decades, the life expectancy of DS has dramatically increased in the developed world [1]. Developing countries, like Pakistan, are yet far away from these goals. The most important milestone lacking, in this regard, is the basic statistics of the disease and research. There are limited data available from developing countries regarding DS with childhood acute leukaemia.
The objective of this study was to determine the frequency of DS-associated paediatric leukaemia among all paediatric leukaemia cases, including the outcome, factors associated with outcome and the causes of mortality in patients with DS with paediatric acute leukaemia in our country.
Materials and methods
This was an observational; retrospective, cohort study conducted in the Department of Paediatric Haematology/Oncology at the Children’s Hospital, Lahore, Pakistan. Approval from the institutional ethical committee was taken. The data was collected retrospectively over a 2-year period, i.e., from January 2017 to December 2018. All children of DS, aged more than 1 year and less than 16 years, newly diagnosed with acute leukaemia, from January 2017 to December 2018, were included in the study. Patients not having DS, aged less than 1 year and/or with transient abnormal myelopoiesis/transient myeloproliferative disorder were excluded from the study. The data was collected retrospectively from ward records and the study cohort was followed up till July 2021 regarding the outcome of the cohort in terms of disease-free survival/overall survival. The data were analysed in terms of descriptive statistics with SPSS version 20.0. The range of the observation time was 31–54 months after diagnosis and the mean time of observation was 42.5 months after diagnosis. Chi-square test was applied for the determination of p-values, and the survival outcome was analysed with the Kaplan–Meier method and the log-rank test.
The Paediatric Haematology/Oncology Unit at the Children’s Hospital, Lahore, is Pakistan’s largest, public sector, 100-bedded subspecialty unit. It offers treatment and palliative care, free of cost, to paediatric patients with all kinds of malignant disorders, from all over the country, as well as from the neighbouring country, Afghanistan.
DS-ALL patients were treated as per UKALL2011 trial [16], while DS-AML patients were treated as per COG A2971 [17] protocol. Supportive care was given to all patients as per the treatment protocols. Tumour lysis syndrome prophylaxis was given to all patients on admission and pneumocystis jiroveci prophylaxis (trimethoprim–sulfamethoxazole) was given to all patients throughout the treatment. Antibiotic prophylaxis with fluoroquinolones was not given to any patient.
Operational definitions
Down syndrome: Patients with DS were diagnosed based on clinical features and/or karyotype suggestive of DS.
Acute leukaemia: Patients with acute leukaemia were diagnosed based on flow cytometry with immunophenotype suggestive of various types of acute leukaemia.
Sepsis: Sepsis was defined as clinical or laboratory evidence of infection with systemic inflammatory response syndrome.
Invasive Fungal Infection: It was diagnosed based on the presence of neutropenia or any other immunocompromised state, along with sufficient clinical evidence consistent with invasive fungal disease (without mycological support).
Results
A total of 1,035 paediatric patients with acute leukaemia were registered over the 2-year study period (871 with ALL and 164 with AML). Nineteen (1.84%) patients were found to have DS and therefore included in the study (Figure 1). The median age of presentation of DS-acute leukaemia was 5.5 years ± 4.3 SD, with an age range of 14.00 years, and a male to female ratio of 1.1:1. DS-associated precursor B-cell ALL (B-ALL) had a proportion of 1.5% among all paediatric ALL cases while DS-AML cases were 3.7% of all paediatric AML cases. None of the patients with DS presented with T-cell ALL or mature B-cell immunophenotype. The median age at diagnosis of DS-ALL was 4 years, while that of DS-AML was 6 years. Twelve patients (63%) were already diagnosed as DS and were on regular follow-up with their primary physicians, while the remaining seven patients (37%) were diagnosed with DS at the time of presentation with acute leukaemia. Regarding the outcome of the study cohort, 13 patients (68.4%) completed treatment successfully and are alive, while 6 patients (31.6%) expired during treatment (Figure 1). The mean overall survival of all of the DS patients with acute leukaemia was 38.6 months ± 5.25 SD. The mean overall survival in children with DS-ALL was 35.5 months and 42.8 months in DS-AML (Figure 2). The log-rank test showed that there was no statistically significant difference in the overall survival distributions between the two groups, i.e., DS-ALL and DS-AML (p-value = 0.388).
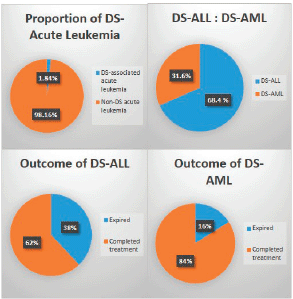
Figure 1. Proportion, types, and outcome of paediatric acute leukaemia with Down syndrome.
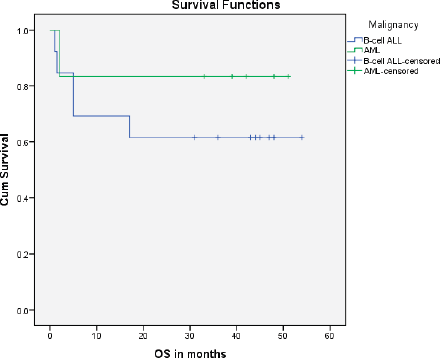
Figure 2. Survival curve for DS-AL patients.
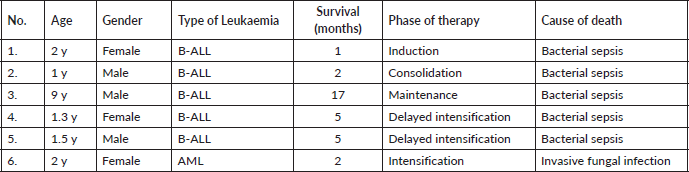
Regarding the cohort of patients who expired, five patients (83%) had B-ALL and one patient (17%) had AML (38% of DS-B-ALL and 16% of DS-AML expired). All the patients had treatment-related mortality and none of the patients expired due to resistant disease, relapse, or early death due to advanced disease at presentation. Five out of six patients (83%) had the disease in remission, while one patient (17%) expired during induction chemotherapy before remission (Table 1).
Various factors, like age, gender, type of malignancy, and the status of diagnosis of DS before presentation, were analysed in terms of the outcome of the study cohort, but the status of diagnosis of DS before presentation was the only statistically significant factor found to be associated with the outcome (p-value = 0.004).
Discussion
The first case of DS-associated acute leukaemia was published in 1930 [18]. DS is now considered the most common syndrome of leukaemia predisposition, and DS-leukaemia has exceptional clinical features with noteworthy differences in prognosis and treatment toxicity [19, 20]. There have been particular immunophenotypic characteristics of DS-ALL in the literature, with almost all patients exhibiting B-cell phenotype and rarely with T-cell or mature B-cell (Burkitt’s leukaemia) phenotype [21–23]. The same pattern of immunophenotype in DS-ALL cases has been observed in this study cohort. This study highlights the high incidence of life-threatening treatment-related toxicities in DS-acute leukaemia patients [4, 20]. The peak age of DS-ALL was also found to be the same [24], but the peak age at diagnosis of DS-AML in this study differed from the published literature [25, 26].
We also found a slightly lesser proportion of DS-associated acute leukaemia among all patients of paediatric acute leukaemia as compared to the data from HICs [27]. The ratio of B-ALL to AML in children with DS was found to be much higher (3.17) in this study as compared to the ratio (1.7) reported in previous studies from high-income countries (HICs) [3, 28]. The mean overall survival of children with DS-ALL was found to be lower (35.5 months) than children with DS-AML (42.8 months), which consolidates the findings that the overall survival rate in DS-ALL is worse than DS-AML [29].
The reasons behind the few differences observed in children with DS-acute leukaemia in this study could be the different demographical characteristics, like genetics, social and environmental factors, or it could be due to discrepancies in the data. The major limitations in this study are the retrospective study design, lack of electronic data available in our setting and small sample size. A cancer registry database is not present in our country and all of the patients’ records are kept manually in individual centres. Therefore, the data recorded retrospectively in this study might be incomplete and inadequate. Moreover, small sample size and the short study duration might not extract significant data for conclusions. But the strength of this study lies in the fact that this is the first study conducted in DS-associated paediatric acute leukaemia patients in Pakistan, and despite the innate hurdles of collection of retrospective data in a resource-limited setting, an effort has been made to publish the available data. This study calls for further prospective studies to be conducted in this special group of patients in Pakistan to draw significant conclusions, gain a better understanding of the clinical manifestations and the challenges in the management of children of DS with acute leukaemia and improve the prognosis of these patients in this part of the world too.
Table 1. Mortality analysis of patients with Down syndrome with acute leukaemia.
Conclusion
When compared to the data from HICs, the notable differences seen in childhood acute leukaemia with DS in our setting show a lesser proportion of DS-associated childhood acute leukaemia among all cases of paediatric acute leukaemia, an older peak age of DS-AML at diagnosis and a higher ratio of DS-ALL to DS-AML. Treatment-related mortality was the sole cause of treatment failure in children of DS with acute leukaemia in our setting. Further prospective studies should be conducted on children with DS-associated acute leukaemia in developing countries.
List of abbreviations
ALL Acute lymphoblastic leukaemia
AML Acute myeloid leukaemia
DS Down syndrome
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding
The authors declare that they have not received any funding for this research/article.
Reference
1. National Down Syndrome Society (2021) Down syndrome facts [http://www.ndss.org/Down-Syndrome/Down-SyndromeFacts/] Data accessed: 06/08/21
2. Krivit W and Good RA (1957) Simultaneous occurrence of mongolism and leukemia: report of a nationwide survey AMA J Dis Child 94 289–293 https://doi.org/10.1001/archpedi.1957.04030040075012 PMID: 13457660
3. Hasle H, Clemmensen IH, and Mikkelsen M (2000) Risks of leukaemia and solid tumours in individuals with Down’s syndrome Lancet 355 165–169 https://doi.org/10.1016/S0140-6736(99)05264-2 PMID: 10675114
4. Day SM, Strauss DJ, and Shavelle RM, et al (2005) Mortality and causes of death in persons with Down syndrome in California Dev Med Child Neurol 47 171–176 https://doi.org/10.1017/S0012162205000319 PMID: 15739721
5. Zeller B, Gustafsson G, and Forestier E, et al (2005) Acute leukaemia in children with Down syndrome: a population-based Nordic study Br J Haematol 128 797–804 https://doi.org/10.1111/j.1365-2141.2005.05398.x PMID: 15755283
6. Maloney KW, Carroll WL, and Carroll AJ, et al (2010) Down syndrome childhood acute lymphoblastic leukemia has a unique spectrum of sentinel cytogenetic lesions that influences treatment outcome: a report from the Children’s Oncology Group Blood 116 1045–1050 https://doi.org/10.1182/blood-2009-07-235291 PMID: 20442364 PMCID: 2938126
7. Seewald L, Taub JW, and Maloney KW, et al (2012) Acute leukemias in children with Down syndrome Mol Genet Metab 107 25–30. https://doi.org/10.1016/j.ymgme.2012.07.011 PMID: 22867885
8. Matloub Y, Rabin KR, and Ji L, et al (2019) Excellent long-term survival of children with Down syndrome and standard-risk ALL: a report from the Children’s Oncology Group Blood Adv 3(11) 1647–1656 https://doi.org/10.1182/bloodadvances.2019032094 PMID: 31160295 PMCID: 6560340
9. Athale UH, Puligandla M, and Satevenson KE, et al (2018) Outcome of children and adolescents with Down syndrome treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium protocols 00-001 and 05-001 Pediatr Blood Cancer 65(10) e27256 https://doi.org/10.1002/pbc.27256 PMID: 29878490
10. Gamis AS, Woods WG, and Alonzo TA, et al (2003) Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children’s Cancer Group Study 2891 J Clin Oncol 21(18) 3415–3422 https://doi.org/10.1200/JCO.2003.08.060 PMID: 12885836
11. Creutzig U, Reihardt D, and Diekamp S, et al (2005) AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity Leukemia 19(8) 1355–1360 https://doi.org/10.1038/sj.leu.2403814 PMID: 15920490
12. Kudo K, Kojima S, and Tabuchi K, et al (2007) Prospective study of a pirarubicin, intermediate-dose cytarabine, and etoposide regimen in children with Down syndrome and acute myeloid leukemia: the Japanese Childhood AML Cooperative Study Group J Clin Oncol 25(34) 5442–5447 https://doi.org/10.1200/JCO.2007.12.3687 PMID: 18048827
13. Taga T, Shimomura Y, and Horikoshi Y, et al (2011) Continuous and high-dose cytarabine combined chemotherapy in children with Down syndrome and acute myeloid leukemia: report from the Japanese Children’s Cancer and Leukemia Study Group (JCCLSG) AML 9805 Down Study Pediatr Blood Cancer 57(1) 36–40 https://doi.org/10.1002/pbc.22943 PMID: 21557456
14. Abildgaard L, Ellebaek E, and Gustafsson G, et al (2006) Optimal treatment intensity in children with Down syndrome and myeloid leukaemia: data from 56 children treated on NOPHO-AML protocols and review of the literature Ann Hematol 855 275–280 https://doi.org/10.1007/s00277-005-0045-5
15. Rao A, Hills RK, and Stiller C, et al (2006) Treatment for myeloid leukaemia of Down syndrome: population-based experience in the UK and results from the Medical Research Council AML 10 and AML 12 trials Br J Haematol 1325 576–583 https://doi.org/10.1111/j.1365-2141.2005.05906.x
16. Leukemia & Lymphoma Research [Internet] (2013) UKALL 2011 Trial: United Kingdom National RandomisedTrial For Children and Young Adults with Acute Lymphoblastic Leukaemia and Lymphoma 2011 [Online] (Birmingham: Children’s Cancer Trials Team, Cancer Research UK Clinical Trials Unit (CRCTU), University of Birmingham) [https://www.northerncanceralliance. nhs.uk/wp-content/ uploads/2019/01/UKALL2011-Protocol-v3.0-01- Oct-2013.pdf] Date accessed: 13/03/22
17. Sorrell AD, Alonzo TA, and Hilden JM, et al (2012) Favorable survival maintained in children who have myeloid leukemia associated with Down syndrome using reduced-dose chemotherapy on Children’s Oncology Group trial A2971: a report from the Children’s Oncology Group Cancer 118(19) 4806–4814 Epub 2012 Mar 5 https://doi.org/10.1002/cncr.27484 PMID: 22392565 PMCID: 3879144
18. Cannon HE (1930) Acute lymphatic leukemia: report of a case in an eleventh month Mongolian idiot New Orleans Med Surg J 94(3) 289–293
19. Xavier AC and Taub JW (2010) Acute leukemia in children with Down syndrome Haematologica 95(7) 1043–1045 https://doi.org/10.3324/haematol.2010.024968 PMID: 20595099 PMCID: 2895024
20. Lins MM, Mello MJC, and Ribeiro RC, et al (2019) Survival and risk factors for mortality in pediatric patients with acute myeloid leukemia in a single reference center in low–middle-income country Ann Hematol 98 1403–1411 https://doi.org/10.1007/s00277-019-03661-7 PMID: 30915498
21. Whitlock JA, Sather HN, and Gaynon P, et al (2005) Clinical characteristics and outcome of children with Down syndrome and acute lymphoblastic leukemia: a Children’s Cancer Group study Blood 106 4043–4049 https://doi.org/10.1182/blood-2003-10-3446 PMID: 16109782
22. Maloney KW (2011) Acute lymphoblastic leukaemia in children with Down syndrome: an updated review Br J Haematol 155 420–425 https://doi.org/10.1111/j.1365-2141.2011.08846.x PMID: 21933171
23. Chessells JM, Harrison G, and Richards SM, et al (2001) Down’s syndrome and acute lymphoblastic leukaemia: clinical features and response to treatment Arch Dis Child 85 321–325 https://doi.org/10.1136/adc.85.4.321 PMID: 11567943 PMCID: 1718934
24. Buitenkamp TD, Izraeli S, and Zimmermann M, et al (2014) Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group Blood 123(1) 70–77 https://doi.org/10.1182/blood-2013-06-509463 PMCID: 3879907
25. Hjalgrim LL, Rostgaard K, and Schmiegelow K, et al (2003) Age- and Sex-Specific Incidence of Childhood Leukemia by Immunophenotype in the Nordic Countries J Natl Cancer Inst Monogr 95(20) 1539–1544 https://doi.org/10.1093/jnci/djg064
26. Czogala M, Pawinska-Wasikowska K, and Ksiazek T, et al (2020) Retrospective Analysis of the Treatment Outcome in Myeloid Leukemia of Down Syndrome in Polish Pediatric Leukemia and Lymphoma Study Group From 2005 to 2019 Front Pediatr 8(277) 1–10 https://doi.org/10.3389/fped.2020.00277
27. Robinson LL, Nesbit Jr. ME, and Sather HN, et al (1984) Down syndrome and acute leukemia in children: A 10-year retrospective survey from Childrens Cancer Study Group J Pediatr 105(2) 235–242 https://doi.org/10.1016/S0022-3476(84)80119-5
28. Mateos MK, Barbaric D, and Byatt SA, et al (2015) Down syndrome and leukemia: insights into leukemogenesis and translational targets Transl Pediatr 4(2) 76–92 https://doi.org/10.3978/j.issn.2224-4336.2015.03.03
29. Madalina-Petronela S, Anca C, and Anca-Viorica I, et al (2021) Outcomes of patients with Down syndrome and acute leukemia. A retrospective observational study Medicine 100(40) e27459 https://doi.org/10.1097/MD.0000000000027459






