Background: Regorafenib is a therapeutic alternative for patients with metastatic colorectal cancer (MCRC) resistant to conventional therapies. The reported toxicity is relevant and there is no data on Latin American patients. The objective was to evaluate the overall survival (OS), progression-free survival (PFS) and quality of life (QoL) in a prospective cohort of Latin American patients treated with an adjusted dose of regorafenib.
Methods: We prospectively recruited patients with MCRC that progressed to standard therapy. A dose escalation algorithm was used. OS, PFS, response rate and QoL were evaluated.
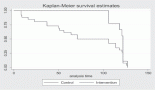
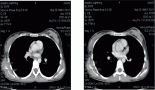
Results: We recruited 13 patients between June and November 2015. The median age was 60 years. Median OS was 8.6 months and median PFS was 2.2 months. The response rate was 8%. Grade 3–4 toxicities included grade 3 palmoplantar erythrodysesthesia in 23% and grade 3 fatigue in 12% of patients.
Conclusion: Regorafenib treatment is effective in Latin American patients with conventional therapy resistant MCRC.