Locally advanced breast cancer is the number of dissected nodes after neoadjuvant chemotherapy important?
Josepmilly Del Valle Peña Colmenares1,a, Wladimir José Villegas Rodríguez1,b, Osama Bahsas Zaky1,c, Carlos Eduardo Martínez1,d and Douglas José Angulo Herrera2,e
1General Surgeon/Oncologic Surgeon, Department of Breast Pathology, Hospital Oncology Service of the Venezuelan Institute of Social Security (SOH-IVSS), Caracas, Venezuela
2School of Statistics and Actuarial Sciences, Central University of Venezuela (UCV), Caracas 1040, Venezuela
a https://orcid.org/0000-0002-1114-6289
b https://orcid.org/0000-0001-8999-9751
c https://orcid.org/0000-0003-2051-7077
d https://orcid.org/0009-0006-1714-0984
e https://orcid.org/0009-0003-5506-0297
Abstract
Objective: To assess whether the number of lymph nodes (LN) in breast cancer (BC) patients undergoing axillary dissection (AD) after neoadjuvant chemotherapy affects disease-free survival (DFS) and overall survival (OS).
Methods: Descriptive, retrospective, longitudinal cut-off study (2011–2020).
Results: 391 patients, 176 patients in the <10 LNs group and 215 ≥10 dissected LGs. The mean number of dissected nodes was 6.2 and 13.8 in the < or ≥ 10 LN groups, respectively. The <10 LN group had a higher proportion of stage IIIB (p = 0.012) and ypN0 (p = 0.001) patients and higher frequency in the phenotypes: luminal A 23.5%, TN 24.1% and HER 2 18.7% when compared with patients with ≥10 LN. Patients with ≥10 LN retrieved had a higher mean OS compared to the group of patients <10 LN with no statistical association (p = 0.184) (hazard ratio = 1.91 95% CI: 0.73–4.98) and a survival probability at 120 months (both groups) of 96.2%. There was also no statistical difference in the DFS when comparing the two groups of patients, indicating that the number of nodes removed is not associated with a differential risk of relapse, with a survival probability at 120 months of 63.3%.
Conclusion: The results of the study indicate that the number of nodes removed during AD does not affect survival (OS and EFS) in patients with neoadjuvant (ypN0/ypN+) BC. Axillary staging remains a key factor in the management of BC; therefore, an individualised approach considering the response to triple negative breast cancer and tumour burden in therapeutic decision making is recommended.
Keywords: breast cancer, axillary lymphadenectomy, neoadjuvant chemotherapy
Correspondence to: Josepmilly del Valle Peña Colmenares
Email: josepmillyp@yahoo.com
Published: 30/01/2026
Received: 03/06/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Axillary lymph node (LN) metastasis remains an important prognostic factor in breast cancer (BC). The Danish Breast Cancer Cooperative Group [1] studied 13,851 patients with a diagnosis of BC more than 30 years ago, concluding that the number of LGs removed had to be at least 10 LGs to exclude misclassification of LN-positive patients as node negative and this in turn correlated with a very significant improvement in prognosis. Kiricuta and Tausch [2] and Veronesi et al [3], also evaluated the LN thresholds needed to ensure a node-negative axilla (10 LN). Insufficient dissection can lead to inaccurate staging of the axilla, and removal of more nodes allows a more accurate determination of the node LN status [2]. It was then established in the 1990s that the minimum number of nodes to be retrieved for more accurate staging of the axilla should include ten nodes.
Axillary dissection (AD) was considered diagnostic, prognostic and therapeutic in the 1980s and 1990s; however, it is not without complications that can affect the quality of life of patients. Although the prognostic value of axillary staging for MCC has been well established and AD has been considered the standard procedure in patients with MCC, with or without positive nodes, until the end of the last century. However, time and different clinical trials have demonstrated a diverse range of possibilities in different scenarios, such as: patients with positive nodes that do not warrant dissection (ACOZ0011) [4] or patients receiving neoadjuvant chemotherapy (NCT), the latter being a reason for discussion about the different staging techniques and/or treatment to the axilla in patients with negative nodes after NCT.
However, at present, the ideal number of nodes dissected in BC patients with residual disease in the axilla after neoadjuvant therapy is not defined. The National Comprehensive Cancer Network defines adequate AD as recovery of ≥10 LGs to accurately stage the axilla [5] and recommends performing AD at Berg levels I and II, in patients with residual disease in the axilla post-NCT [6]. We are currently awaiting the results of the various ongoing studies that define the treatment of the axilla in the presence of residual disease limited to the sentinel node in patients receiving triple negative breast cancer (TNBC).
The aim of this study was to evaluate whether the number of LGs removed in patients with BC who received TNBC with subsequent AD affects disease-free survival (DFS) and overall survival (OS).
Methods
Descriptive, retrospective, longitudinal study of 2,633 patients diagnosed with BC between January 2011 and December 2020, treated in the Breast Pathology and Medical Oncology Services of the Hospital Oncology Service of the Venezuelan Institute of Social Security (SOH-IVSS), 1,517 were operated on and 391 met the inclusion criteria: 1. Female patients over 18 years of age with histological and immunohistochemical diagnosis of stage II and III BC, with cytology demonstrated as positive axillary node and/or axillary ultrasound with suspicion of malignancy. The tumours were classified according to the parameters of the American Joint Committee on Cancer (AJCC eighth edition) [7]. 2. They had received standard NCT treatment, approved by the Medical Oncology Service and surgery in the Breast Pathology Service of the SOH-IVSS. 3. The patients underwent DA dissection after NCT. Evaluation of the surgical specimen by anatomic pathology specialists, using the Miller and Payne classification [8, 9] in all specimens. Patients' complete pathological response (PCR) was defined as: ypT0ypN0 or ypTisypN0 and by Miller and Payne classification (Grade V for complete breast response/ A and D for axillary response). 5. Two groups were analysed for the study, one group had <10 LN after DA and the other had ≥10 LN. Of note, the sample reviewed included patients with locally advanced BC and where selective sentinel node lymphadenectomy post-NCT was in the study protocol in our centre from 2014 to 2019 (all patients had DA completed). In total, 84.65% of the patients received adjuvant radiotherapy (RT).
In the present study, the Helsinki Declaration belonging to the World Medical Association was considered as the ethical basis. This research protocol was approved by the Ethics Committee of the IVSS SOH [10].
Objective
To evaluate whether the number of LN in patients with MS undergoing DA after NCT affects EFS and OS.
Specific objectives
To determine whether the number of nodes retrieved (<10 or ≥10 LN) is related to recurrence when performing AD. 2. To identify the clinical and pathological characteristics of the patients in the study group. 3. To identify whether in patients with (ypN+) or without (ypN0) nodal residual disease, the number of retrieved LGs affects OS and DFS.
Statistical analysis
The mean and standard deviation of the quantitative variables were calculated; in the case of qualitative variables, their frequencies and percentages were calculated. The results were arranged in unidimensional tables and contingency tables. Differences between groups, <10 nodes and ≥nodes, in the case of qualitative variables, were performed with Pearson's chi-square test; in the case of quantitative variables, the Student's t-test for independent samples was applied, after normality check, with the Shapiro–Wilk test. OS and DFS were determined using the Kaplan–Meier procedure, and the differences between the survival curves were determined using the log-rank test. A value was considered statistically significant if p < 0.05. Data were tabulated with R Studio version 2024.12.0+467. The hazard ratio (HR) was calculated using a Cox proportional hazards model. Relevant independent variables were adjusted for and model assumptions, including proportional hazards, were checked. The HR was interpreted as the change in the risk of event occurrence between groups, holding all other variables constant. A value was considered statistically significant if p < 0.05. Data were tabulated with R Studio version 2024.12.0+467.
Results
Thirty-nine hundred and ninety-one patients met the inclusion criteria. The mean age at disease diagnosis was 51 years ± 11 (ranging from 25 to 87 years), 176 patients in the <10 LN group and 215 ≥ 10 dissected nodes. The average number of dissected LGs was 6.32 and 13.8 in the groups and the proportion of positive LGs 0.23 and 0.20< or ≥ 10 LGs, respectively. The rate of PCR 19.3%, PCR in the breast (CPRR) 22.7% and axillary pathological response (APR) 55.7% when <10 LN were retrieved, when dissected or retrieved ≥10 LN the found rates of PCR, CPRR and APR reported 16.3%, 19.5% and 45.1%, respectively.
Table 1 shows the characteristics of the patients according to the number of LGs. The three patients with stage IIIC in the <10 LN group corresponded to the AJCC classification before NCT.
Table 1. Distribution of patients by number of LNs and clinical and surgical variables.
In the analysis of mortality and recurrence as a function of the number of nodes removed, it was identified that the mortality rate was higher in patients with ≥10 resected nodes, with 12 (5.6%) deaths compared to 5 (2.8%) in the group with <10 nodes, although the difference was not statistically significant (p = 0.186). The proportion of relapses (local, regional and/or distant) was similar in the two groups; in the <10 nodes group, it was 17.6% with 13.6% distant relapses, while in the ≥10 group 16.7% relapses, of which 13.5% included distant relapses with no statistical association (p = 0.820)
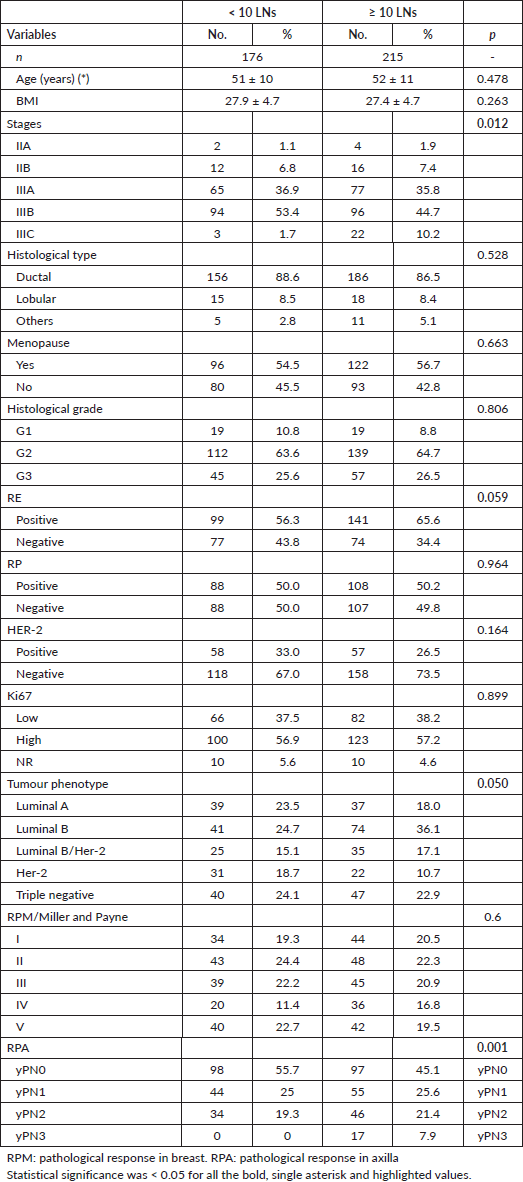
Kaplan-Meier OS curve analysis evaluated the number of salvaged LGs. The log-rank test yielded a c2 = 1.765 (p = 0.184). Clinical interpretation of the results indicates that there was no statistical association in OS in patients with BC who received NCT to whom DA < or ≥ 10 LN was performed. Patients with ≥10 LN had a longer median survival compared to the group of patients <10 LN, with no statistical association and both groups with a 120-month survival probability of 96.2%. The HR for patients with ≥10 nodes removed was 1.91 (95% CI: 0.73–4.98) Figure 1.
The Kaplan–Meier DFS curve analysis evaluated the number of LGs. The log-rank test yielded a c2 = 0.038 (p = 0.845). Clinical interpretation of the results indicated that DFS in patients with ≥10 had a mean 164 months at the end of follow-up; however, there was no statistical difference when compared with DA <10 LN. The HR in the group with ≥10 nodes was 0.95 (95% CI: 0.59–1.54), indicating that the number of nodes removed was not associated with a differential risk of relapse. This reinforces the need for a comprehensive assessment of axillary status and tumour biomarkers to better predict disease progression Figure 2.
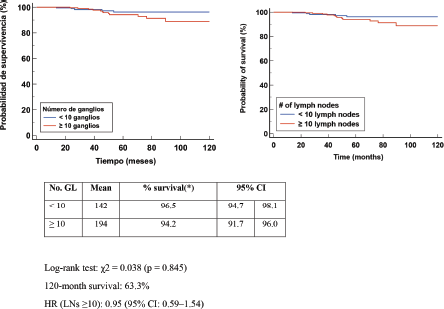
Figure 1. Kaplan Meier OS curve according to LN number (No.).
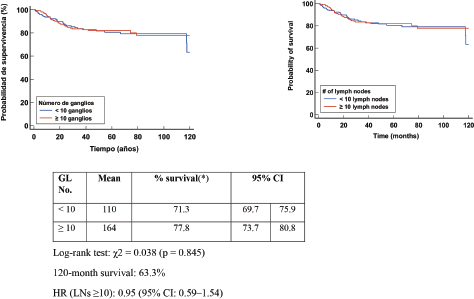
Figure 2. Kaplan Meier DFS curve according to LN number.
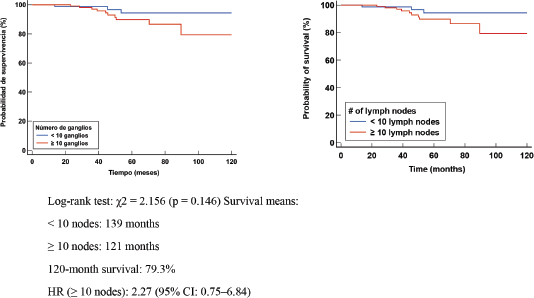
Figure 3. Kaplan Meier OS curve according to number of positive LN (+).
Patients with post-NCT residual nodal disease (ypN1,ypN2,ypN3)
In patients with lymphonodal metastases or residual nodal disease, the median OS was 139 months in those with <10 nodes removed and 121 months in those with ≥10 nodes. Survival at 120 months was 79.3%. No significant differences were identified in the log-rank test (χ² = 2.156, p = 0.146). However, the HR in the group with ≥10 nodes was 2.27 (95% CI: 0.75–6.84), suggesting a possible higher mortality risk in this group. This could be explained by the larger tumour volume present in these cases or by the persistence of residual disease after TNBC (Figure 3).
In patients with lymphnodal involvement (ypN+), median EFS was 103 months in those with <10 nodes removed and 94 months in those with ≥10 nodes. Survival at 120 months was 55.9%. The log-rank test showed no significant difference (χ² = 0.011, p = 0.918). The HR was 1.03 (95% CI: 0.57–1.89) (Figure 4). The absence of differences in DFS suggests that the number of excised LGs does not significantly modify the risk of relapse in patients with axillary involvement. These findings reinforce the importance of tumour biological factors and response to NCT as major determinants of prognosis.
Patients with no residual nodal disease following NCT
In patients with axillary pathologic complete response (APCR), no significant difference in OS or EFS was observed. These results suggest that in patients with ypN0 or APCR, the number of retrieved LGs does not appear to significantly influence OS, supporting the possibility of performing axillary de-escalation or reduced axillary surgery in this group of patients Figure 5. The presence of a PRC suggests that the number of LNs removed does not significantly influence the risk of relapse in patients without axillary involvement Figure 6.
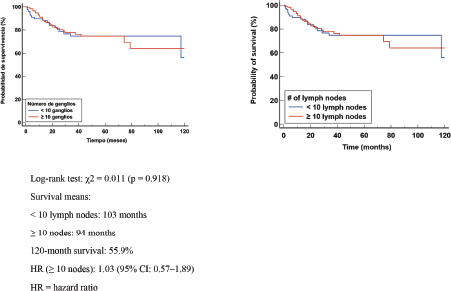
Figure 4. Kaplan Meier DFS curve according to number of positive (+) GLs.
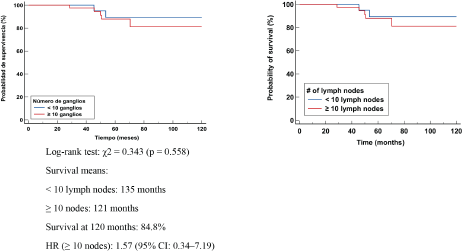
Figure 5. Kaplan Meier OS curve according to number of negative LN (-).
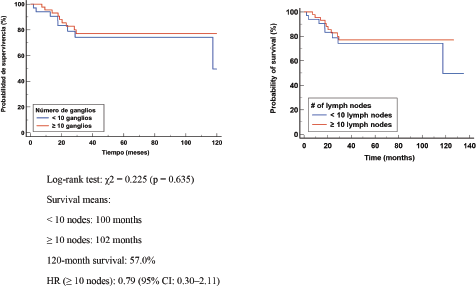
Figure 6. Kaplan Meier DFS curve according to negative LN numbers (-).
Discussion
The number of positive GLs is strongly associated with the AJCC N (nodal) stage, having positive GLs is a critical factor in determining strategies in adjuvant systemic therapy [11, 12]. DA still continues to be the treatment of choice for patients with post-CTN nodal residual disease.
Rosenberger et al [13], in their cohort study including 129,685 patients from the National Cancer Data Base divided the sample into two groups according to treatment sequence: initial surgery 102,200 (78.8%) or NCT 27,485 (21.2%) to identify the optimal yield of LN DA among LN positive patients concluding that removal of approximately 20 LGs may improve survival through more accurate nodal staging and increased use of adjuvant therapy. Wang et al [14], also studied the optimal threshold of LGs examined for BC patients with >3 positive LGs after modified radical mastectomy (MRM), with the threshold being number 12 for improved cancer-specific survival, in contrast to Neuman et al [15], who report that a recovery of less than ten nodes in patients who have received TNBC may not necessarily imply inadequate surgical staging.
In our review, we evaluated oncologic outcomes in relation to the number of resected nodes (<10 or ≥ 10) after AD in patients with ALMC who received TNBC, in the Kaplan Meier analysis there was no significant difference in relation to the number of resected nodes in terms of EFS and OS; as Choi et al [16], who in their research, did not find statistical significance in OS between the two groups studied (<10 and ≥10 dissected nodes). In our study, patients with residual nodal disease, the removal of ≥ 10 nodes seems to be associated with a tendency to worse prognosis, although without statistical significance. This suggests that axillary tumour volume (residual disease) and tumour biology could be more determined than the number of dissected nodes in the evolution of the disease.
However, in the subgroup reported by Choi et al [13, 16], patients ypN0 and ypN2, the group with <10 nodes had a lower SCE than the group with ≥10 nodes and Rosenberger et al [13], retrieving less than 8 LN subsequent NCT decreased OS, which increased (OS) with retrieval between 8 and 22 LN, high versus low LN retrieval was also associated with a slightly higher receipt of adjuvant RT (p = 0.002).
In our study there was no statistical association between the number of retrieved LGs and OS in patients who were LN negative (ypN0) which could be interpreted that APCR patients the number of retrieved LGs does not modify OS, which supports reduced axillary surgery in this type of patients in contrast to the data provided by Chen et al [17], whose findings associated better OS with greater retrieval ≥10 LGs, even in a population of women with pathologically negative nodes who received TNBC followed by MRM. However, some authors describe that the number of dissected LGs in BC patients who receive TNBC may be lower than those who do not and this may not always be attributed to inadequate AD and it should be considered that TNBC may reduce the number of LGs [15, 18, 19].
A higher number of LGs examined after MRM could provide more accurate information on TNM stage and adjuvant therapy. However, a higher number of LGs examined is associated with a higher risk of adverse outcomes [20, 21]. Several authors [13, 14, 17] report that obtaining a greater number of nodes on DA in patients with stages I, II or III post-TNM stage I, II or III BC with negative or positive LNs increases EFS and/or OS and that this is probably due to a reduction in false-negative staging, as well as a greater extent of DA may be an indicator of more aggressive overall care with increased receipt of adjuvant RT and/or chemotherapy, but as well defined by Roseberger et al [13], does not interpret these data to suggest a therapeutic survival benefit due to additional axillary clearance, but in the possible influence on adjuvant treatment decision making. In our review, no significant difference was found; all our patients were advanced stages and most of them received adjuvant treatment (systemic therapy and/or RT), where the number of dissected LGs (<10 or ≥10) did not influence OS, EFS or the frequency of local, regional and/or distant recurrence rate.
When dissecting < However, the exact percentage of underestimation when less than ten nodes are obtained is not expressly quantified [1, 2, 22, 23] in the aforementioned works, in general the importance of recovering as many nodes as possible to reduce the rate of false negatives in axillary staging is described, it would be thoughtless or daring to conclude that regional or distant recurrence in these patients is inversely proportional only to the number of resected LN, since MS is considered a systemic disease and it is the intratumoural heterogeneity, the biomolecular differences in the tumour microenvironment, the use of targeted therapies and the various randomised trials that have allowed us to acquire a better understanding of the various characteristics of tumour behaviour.
In the same vein, when reviewing relapse rates in various series with less than ten retrieved nodes, Neuman et al [15] stated that axillary recurrence is not determined in patients during their 72-month follow-up after removal of <10 LN and proposed that the surgical technique they applied was sufficient. Uyan et al [18] did not observe axillary recurrence in any patient who received TNBC and had <10 LN removed during their 36-month follow-up. In our review, the loco-regional relapse rate (<10 retrieved LN) was 4.5% at the end of 120 months follow-up, as already mentioned, with no statistical association when compared to the group of >10 retrieved LN and no influence on survival.
Having more positive nodes implies a higher probability of local and distant relapse, higher stage disease, resecting more nodes or ‘increasing’ the number of nodes retrieved in the AD in LN positive patients will probably reduce the tumour burden and achieve regional control, but it will not increase survival. There are currently a large number of prospective trials involving the use of new chemotherapeutic agents, monoclonal antibodies and/or biological agents in smaller and smaller tumours in search of a better pathologic response rate. A la lustre of new studies, most or nearly all tumours will receive some form of targeted therapy for a specific tumour subtype, which proportionally increases the number of patients receiving neoadjuvant systemic therapy, more selective sentinel node lymphadenectomies, more targeted ADs and less DA.
If there are positive LNs post-NCT without evidence of distant progression, our recommendation, like most authors so far, remains DA of levels I and II.
However, it is important to note that the presence of extensive axillary positive LGs (N2 or N3a) is related to aggressive tumour biology and therefore may indicate the need for more systemic treatment and/or RT and not necessarily more extensive surgery, as this does not compensate for adverse tumour biology, making it clear that in patients with positive LGs after TNCT, DA is still part of the multidisciplinary treatment.
Conclusion
The results of the study indicate that the number of nodes removed during DA is not significantly associated with OS or EFS in TNBC patients treated with TNBC. This suggests that axillary tumour burden and tumour biology may be more determined than the number of nodes dissected during disease progression.
The goal of BC surgery is currently to reduce treatment without affecting survival. Time and trials demonstrated that RT replaces or is as effective as AD in patients with limited sentinel LN involvement; however, there is still no evidence on the results of de-escalated axillary staging after NCT (LN positive). The ingrained ideology that more surgery is always better over time is probably changing, PCR increasingly translates into a prognostic factor and reduced axillary surgery becomes more relevant (so the number of resected nodes in ycN0 patients would not be the main target). Ongoing trials such as: ALLIANCE 202, ADARNAT trial [24] will confirm or not the efficacy and safety of axillary RT on DA in patients with limited GC involvement after NCT, probably give us the answer of axillary de-escalation or escalation in patients who receive NCT; however, until we have more evidence, patients with positive LN following neoadjuvant, DA remains a key factor in the management of BC, an individualised approach is recommended, considering the response to TNBC and tumour burden in therapeutic decision making.
Limitations
1. It should be noted that our hospital is a specialised oncology centre and our Breast Pathology service is made up of specialists in oncologic surgery and breast pathology, it is a teaching hospital setting, training oncologic surgery residents and although there are reports that there are higher rates of recovery of more than 10 LN from surgeons with an academic affiliation or in a teaching hospital setting, we have not performed such a review to compare whether the AD performed is higher or lower when performed by residents in training and monitored by attendings in our service and if there is any significant difference, so we do not know if this could have influenced the results. 2. This study was a retrospective review, from a single oncology centre, with a small sample, so the distribution of patients could be uneven, present certain population differences among the patients analysed and have some effect on the results of regional control. 3. We are aware that at the moment of performing the DA several factors influence, including the patient, the surgery, the processing of the anatomic pathology specimen, the NCT as some authors describe it, we do not have a central review for histopathological studies, so we do not rule out that these factors have influenced our review, besides being a retrospective study. 4. The data are not generalisable because the sample was limited to a single hospital centre, despite the fact that our hospital is considered a national reference for the treatment of MS.
Conflicts of interest
The authors declare that there is no conflicts of interest that could have affected the results of the study.
Funding
No financial support was received for the writing of this article, data collection or analysis or in the preparation or approval of the current manuscript.
Disclosure
The authors have no financial interest to declare in relation to the disclosure of the contents of this article, as their only interest is focused on opening a line of research in our country on the subject matter of the study.
References
1. Axelsson CK, Mouridsen HT, and Zedeler K (1992) Axillary dissection of level I and II lymph nodes is important in breast cancer classification Eur J Cancer 28A(8-9) 1415–1418 https://doi.org/10.1016/0959-8049(92)90534-9
2. Kiricuta CI and Tausch J (1992) A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma Cancer 69(10) 2496–2501 https://doi.org/10.1002/1097-0142(19920515)69:10<2496::AID-CNCR2820691018>3.0.CO;2-T PMID: 1568171
3. Veronesi U, Luini A, and Galimberti Marchini S, et al (1990) Extent of metastatic axillary involvement in 1446 cases of breast cancer Eur J Surg Oncol 16(2) 127–133 PMID: 2323409
4. Giuliano AE, Hunt KK, and Ballman KV, et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomised clinical trial JAMA 305(6) 569–575 https://doi.org/10.1001/jama.2011.90 PMID: 21304082 PMCID: 5389857
5. National Comprehensive Cancer Network (2018) NCCN, Invasive Cancer, Surgical Axillary Staging https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Date accessed: 4/12/2024
6. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) (2024) NCCN Guidelines for Breast Cancer V.1.2024 https://www.nccn.org/guidelines/guidelines-detail?id=1419 Date accessed: 14/01/2025
7. Amin MB, Edge SB, and Greene FL, et al (2018) American joint committee on cancer staging manual 8th edn (New York: Springer)
8. Ogston KN, Miller ID, and Payne S, et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival Breast 12(5) 320–327 https://doi.org/10.1016/S0960-9776(03)00106-1 PMID: 14659147
9. Wang H and Mao X (2020) Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer Drug Des Devel Ther 14 2423–2433 https://doi.org/10.2147/DDDT.S253961 PMID: 32606609 PMCID: 7308147
10. World Medical Association (1964) Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects [Internet] Declaration of Helsinki https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/ Date accessed: 24/01/2025
11. Escobar PF, Patrick RJ, and Rybicki LA, et al (2007) The 2003 revised TNM staging system for breast cancer: results of stage re-classification on survival and future comparisons among stage groups Ann Surg Oncol 14(1) 143–147 https://doi.org/10.1245/s10434-006-9147-0
12. Lin J, Li C, and Zhang C, et al (2017) Postmastectomy radiation therapy for breast cancer patients with one to three positive lymph nodes: a propensity score matching analysis Future Oncol 13(16) 1395–1404 https://doi.org/10.2217/fon-2017-0099 PMID: 28381104
13. Rosenberger LH, Ren Y, and Thomas SM, et al (2020) Axillary lymph node dissection in node-positive breast cancer: are ten nodes adequate and when is enough, enough? Breast Cancer Res Treatment 179 661–670 https://doi.org/10.1007/s10549-019-05500-9
14. Wang X, Ji C, and Chi H, et al (2018) How many ELNs are optimal for breast cancer patients with more than three PLNs who underwent MRM? A large population-based study Onco Targets Ther 11 1005–1011 https://doi.org/10.2147/OTT.S152936 PMID: 29520151 PMCID: 5833798
15. Neuman H, Carey LA, and Ollila DW, et al (2006) Axillary lymph node count is lower after neoadjuvant chemotherapy Am J Surg 191(6) 827–829 https://doi.org/10.1016/j.amjsurg.2005.08.041 PMID: 16720159
16. Choi HJ, Ryu JM, and Lee JH, et al (2022) Is Pathologic Axillary Staging Valid If Lymph Nodes Are Less than 10 with Axillary Lymph Node Dissection after Neoadjuvant Chemotherapy? J Clin Med 11(21) 6564 https://doi.org/10.3390/jcm11216564 PMID: 36362792 PMCID: 9656978
17. Chen S, Liu Y, and Huang L, et al (2014) Lymph node counts and ratio in axillary dissections following neoadjuvant chemotherapy for breast cancer: a better alternative to traditional pN staging Ann Surg Oncol 21(1) 42–50 https://doi.org/10.1245/s10434-013-3245-6
18. Uyan M, Koca B, and Yuruker S, et al (2016) Effect of neoadjuvant chemotherapy on axillary lymph node positivity and numbers in breast cancer cases Asian Pac J Cancer Prev 17(3) 1181–1185 https://doi.org/10.7314/APJCP.2016.17.3.1181 PMID: 27039745
19. Bélanger J, Soucy G, and Sidéris L, et al (2008) Neoadjuvant chemotherapy in invasive breast cancer results in a lower axillary lymph node count J Am Coll Surg 206(4) 704–708 https://doi.org/10.1016/j.jamcollsurg.2007.10.016 PMID: 18387477
20. Fleissig A, Fallowfield LJ, and Langridge CI, et al (2006) Post-operative arm morbidity and quality of life. Results of the ALMANACK randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer Breast Cancer Res Treatment 95(3) 279–293 https://doi.org/10.1007/s10549-005-9025-7
21. De Glas NA, Kiderlen M, and Bastiaannet E, et al (2013) Postoperative complications and survival of elderly breast cancer patients: a FOCUS study analysis Breast Cancer Res Treatment 138(2) 561–569 https://doi.org/10.1007/s10549-013-2462-9
22. Sun L, Li P, and Ren H, et al (2020) Quantifying the Number of Lymph Nodes for Examination in Breast Cancer J Int Med Res 48(2) 300060519879594 https://doi.org/10.1177/0300060519879594
23. Blancas I, García-Puche JL, and Bermejo B, et al (2006) Low number of examined lymph nodes in node-negative breast cancer patients is an adverse prognostic factor Ann Oncol 17(11) 1644–1649 https://doi.org/10.1093/annonc/mdl169 PMID: 16873428
24. Garcia-Tejedor A, Ortega-Exposito C, and Salinas S, et al (2023) Axillary lymph node dissection versus radiotherapy in breast cancer with positive sentinel nodes after neoadjuvant therapy (ADARNAT trial) Front Oncol 13 1184021 https://doi.org/10.3389/fonc.2023.1184021 PMID: 37621686 PMCID: 10446877





