Back pain in an adolescent: not just a sore spine!
Bothello Mark Richard1,a, Shilpa S Cholachagudda1,b, Pankhudi Priya2,c, Sidharth Totadri2 and Ranjini Srinivasan1,d
1Department of Paediatrics, St. John’s Medical College and Hospital, Bengaluru 560034, India
2Department of Pediatric Hematology Oncology and Bone Marrow Transplant, St. John’s Medical College and Hospital, Bengaluru 560034, India
a https://orcid.org/0009-0006-8016-9501
b https://orcid.org/0009-0008-1198-3248
c https://orcid.org/0009-0006-7577-3907
d https://orcid.org/0000-0001-8092-7882
Abstract
Acute lymphoblastic leukaemia (ALL) can mimic diverse musculoskeletal conditions, often resulting in diagnostic delays. Genetic predisposition to various cancer syndromes further complicates the clinical picture, influencing disease presentation and treatment response. We report an adolescent boy who presented with a 2-month history of episodic fever, persistent low back pain and non-migratory joint pain, with a history of growth failure, developmental delay and seizures since childhood. There was a history of malignancy in multiple family members. The clinical examination revealed no features suggestive of systemic involvement. The joint examination revealed swelling around the knee joints. Initial work-up for chronic infections and autoimmune diseases was negative. Magnetic resonance imaging spine findings of multiple T2 hyperintense lesions warranted a bone marrow examination, which confirmed the diagnosis of Ph+ B− ALL. Molecular analysis revealed a pathogenic heterozygous missense variant in the TP53 gene, leading to the diagnosis of Li-Fraumeni syndrome. This case highlights the importance of recognizing musculoskeletal symptoms as a potential presentation of ALL. Early consideration of leukaemia in the differential diagnosis can prevent delays in treatment, ultimately improving outcomes.
Keywords: adolescent, arthritis, leukaemia, spine
Correspondence to: Shilpa S Cholachagudda
Email: cshilpao9@gmail.com
Published: 20/01/2026
Received: 18/03/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Low back pain in children is a non-specific symptom but a fairly common problem with a lifetime prevalence ranging from 9% to 69% and thereby increasing with age [1]. The aetiology of low back pain varies from mechanical and inflammatory causes to infectious and more sinister neoplastic processes. Initial laboratory investigations, including blood counts, a peripheral smear and inflammatory markers, often give clues towards a probable aetiology and imaging may be used to provide additional insight into the diagnosis whenever indicated.
Although musculoskeletal (MSK) manifestations are well-documented in 40%–60% of acute lymphoblastic leukaemia (ALL) cases, the presence of arthritis or arthralgia without organomegaly and with a normal haematological profile can often result in misdiagnosis . A retrospective analysis of 63 children with MSK presentation who underwent bone marrow examination reported findings suggestive of leukaemia in 30.15% patients [2]. Misdiagnosis of a neoplastic process not only causes treatment delays but also results in unwarranted use of systemic steroids . This case report highlights the importance of maintaining a high index of suspicion for atypical presentations of leukaemia, demonstrating that low back pain in an adolescent can be an ominous symptom of an underlying malignancy. Although paediatric cancers arise de-novo in most cases, a small proportion can have underlying cancer predisposition syndromes. The current case underscores the importance of family history in identifying such syndromes.
Case report
A 17-year-old adolescent male, presented with persistent low back pain, of 2 months duration . The pain was insidious in onset, dull aching and persistent throughout the day with minimal relief with oral analgesics. The pain was more in the sitting posture, with the patient reporting difficulty in bending forwards . This was accompanied by non-migratory, non-erosive , additive joint pain predominantly involving the knee joints, which was more pronounced at night and did not respond to conventional anti-inflammatory medications. The pain was not associated with stiffness but was severe enough to disrupt his routine daily activities. There was a history of intermittent, episodic low-grade fever since the onset of back pain, which responded to antipyretics . The patient also had accompanying malaise and anorexia with significant weight loss.
He was born at term to third-degree consanguineous parents with a normal birth weight. The antenatal and postnatal periods were uneventful. There was history of delayed attainment of developmental milestones and epilepsy for which he was on antiseizure medications . He was completely immunised, and his scholastic performance was average, with poor social and communication skills.
There was no history suggestive of tuberculosis among household contacts or autoimmune disease among first-degree relatives. A positive family history noted was the presence of breast cancer in the patient’s mother and uterine cancer in the maternal grandmother. The first cousin was recently diagnosed with acute leukaemia a few months prior (Figure 1).
At presentation, he was pale and emaciated. He appeared dysmorphic with triangular facies, small-sized head, prominent ears and hypertelorism . His vital parameters were within normal limits. Anthropometry revealed severe thinness , stunting and microcephaly. There was a café au lait macule measuring 3 × 1 cm over the abdomen . There was no icterus, pedal oedema or significant lymphadenopathy . There were no skin bleeds. Oral cavity and dentition appeared normal. There were no limb anomalies and spine examination were normal.
MSK system examination revealed a normal gait and posture. He was unable to bend forward and touch his toes and spine movements were restricted only in forward flexion. Joint examination was normal with no features of inflammation. There was no joint line tenderness or limb length discrepancy. Systemic examination revealed no organomegaly. Neurological examination was normal and there were no focal deficits. Differentials considered at this point included chronic infections such as tuberculosis and retroviral infection affecting bone and joints, inflammatory conditions such as ankylosing spondylitis, juvenile rheumatoid arthritis and systemic lupus erythematosus and neoplastic conditions considering the strong family history.
Initial investigations (Table 1) showed moderate anaemia, with normal total leucocyte counts, normal platelet counts and elevated inflammatory markers. His peripheral smear was normal with no atypical cells. The chest radiograph was normal. Ultrasonogram (USG) of the abdomen revealed mild splenomegaly and knee joint USG showed no evidence of synovitis or effusion. Work up for rheumatological conditions (Table 1) was negative. Due to persistent lowback pain and elevated inflammatory parameters, Magnetic resonance imaging (MRI) of the spine and pelvis was performed, which showed ill-defined T2 hyperintense lesions involving multiple cervical and thoracic vertebrae with Short Tau Inversion Recovery (STIR) hyperintense lesions in bilateral iliac bones and sacrum. There was an osteolytic lesion noticed in the spinous process of C2 with periosteal reaction . There was no leptomeningeal enhancement (Figure 2a and b).
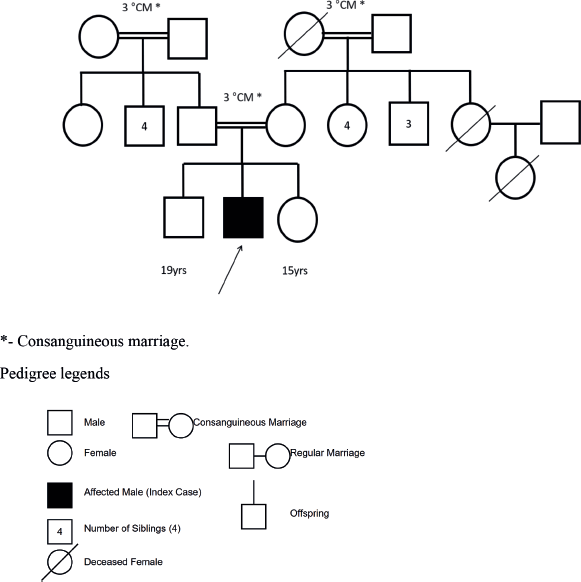
Figure 1. Three-generation family tree of the index patient (indicated by arrow).
Suspecting a possible neoplastic aetiology, bone marrow aspiration and biopsy were done which confirmed acute leukaemia (Figure 3a).
Extended flow cytometry was positive for B-cell precursor markers (CD34, TdT, HLA) and B cell markers (CD 19, 20, 10). Fluorescent in situ hybridisation (FISH ) showed interphase cells and BCR/ABL-1 gene positivity in 15% of cells (Figure 3b).
Based on the above findings, the patient was classified as high-risk B cell ALL and treated as per Indian Childhood Collaborative Leukaemia 2014 regime . He is currently on the maintenance phase of chemotherapy [3]. He was also started on Imatinib Mesylate due to his Philadelphia chromosome positivity. Assessment of minimal residual disease activity at the end of induction was negative. A clinical exome sequencing performed in the child after achieving remission with chemotherapy revealed a pathogenic heterozygous missense variant in exon 8 of the TP53 gene resulting in amino acid substitution of leucine for valine at codon 272, leading to the diagnosis of Li-Fraumeni syndrome (LFS), a rare cancer predisposition syndrome. On subsequent follow-up 9 months later, there has been a significant and sustained improvement in the clinical profile.
Table 1. Investigations of the patient in the hospital.
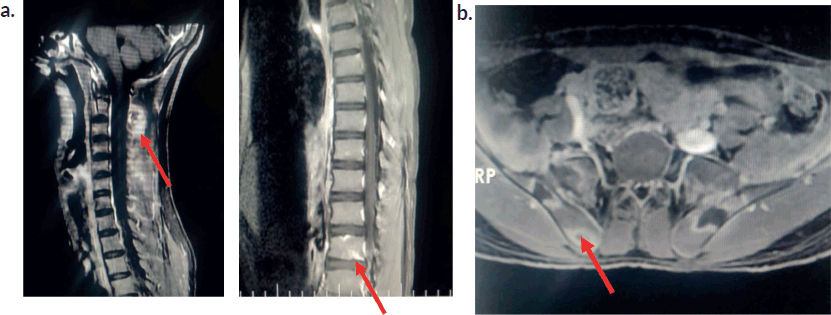
Figure 2. Magnetic resonance imaging of the spine . (a): MRI spine-post contrast enhancement noted in C2 and C6 vertebral bodies.Osteolytic lesion noted at C2. Ill-defined enhancement noted in the paraspinal muscles posterior to C2 and thoracic vertebra. (b): MRI Hip -Ill-defined STIR hyperintense foci noted in bilateral iliac bones and sacrum (ala) with peripheral enhancement.
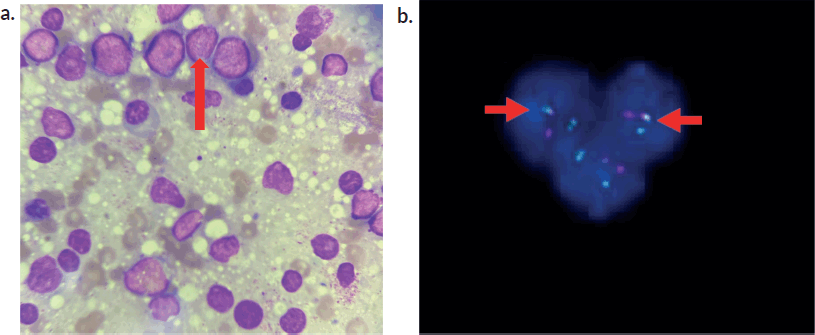
Figure 3. (a): Bone marrow smear cytology. Bone Marrow smear revealing prominent lymphoblasts (arrow) and myeloid cells in various stages of maturation. (b): depicting FISH analysis. BCR/ABL1 gene with fusion positivity in 15 % of cells (in interphase).
Discussion
MSK involvement in childhood leukaemia occurs with a frequency ranging from 7.1% to 62.3% [4]. Malignancies causing bone pain in children include leukaemia, lymphoma, neuroblastoma and sarcoma. Postulated mechanisms for articular symptoms include joint infiltration, synovial reaction, periosteal or capsular lesions, intra-articular or peri-articular haemorrhage, neoplastic cytokine secretion or infection [5]. About one third of paediatric patients with ALL initially present with MSK symptoms [6]. These may include limb pain, bone pain, joint pain and gait abnormalities such as limping, as described in a systematic review where limb pain constituted 43% of all pooled symptom frequencies [6]. Vertebral body collapse and back pain in leukaemia in the absence of overt systemic symptoms and signs are not well described in the literature . Leukaemic arthritis may be misdiagnosed as juvenile idiopathic arthritis and steroid initiation in these patients may further contribute to diagnostic delay. Severe pain disproportionate to physical findings, pain in an atypical location (metaphyseal region), nocturnal pain, poor response to anti-inflammatory drugs and development of early osteopenia or lytic lesions are clues in favour of a possible neoplastic process [7]. The severe nocturnal pain, joint findings disproportionate to pain severity and poor response to pain killers were pointers towards a non-rheumatological pathology in this patient .
A retrospective review describing the clinical profile and outcomes of children with MSK presentation of leukaemia (n = 81) observed that rheumatological involvement was more likely in children with ALL (88.9%) as compared to those with acute myeloid leukaemia (11.1%), notably B cell ALL (69.4%). The study also reported that children with MSK involvement were more likely to have fever. However, skin bleeds and splenomegaly were found to occur more frequently in the group with MSK presentation (p < 0.001 and 0.043, respectively ), which was not seen in our patient. Those with MSK presentations had higher haemoglobin and platelet counts with lower WBC count as compared to those without MSK involvement [4]. The absence of peripheral blasts was significantly higher in the group with MSK manifestations (17.3% versus 9.6%, p = 0.04). These laboratory findings corroborate with what was observed in our patient as well.
MRI has become a useful tool to diagnose and stage haematological malignancies and is preferred over other modalities of imaging to evaluate bone pathology . The pattern of marrow signal (i.e., diffuse, variegated or localised) may indicate the underlying disease process. A diffuse involvement throughout the marrow is usually observed in acute leukaemia with a decrease in signal intensity on T1 weighted images. T2 or STIR images help in detecting the abnormal increased marrow signal intensity with fat-suppressed T2W signals relative to the intervertebral discs and paravertebral muscles [8, 9]. Other bony abnormalities detected by imaging modalities in children with haematolymphoid malignancies include lucent metaphyseal bands, smooth to irregular patterns of periosteal reaction, pathological fractures, osteopenia, geographic lytic lesions and sclerosis [10].
Yoshikawa et al [11] in their case series demonstrated the usefulness of MRI in children with leukaemia, especially in situations where MSK manifestations appear prior to haematological abnormalities in the peripheral blood. In this series, the lag period between the onset of MSK symptoms and the diagnosis of ALL varied from 20 days to as long as 6 months. During this period, the patients were misdiagnosed with pyogenic osteomyelitis or arthritis due to high signal intensity on T2 - weighted imaging of the involved bones or joints. The authors concluded that diffuse low signal intensity on T1 - weighted images, especially in regions adjacent to localised sites of MSK symptoms, although not specific, when considered in combination with high signal intensity on T2 weighted images , may provide valuable information to diagnose insidious leukaemia. Similar neuroimaging findings observed in this patient prompted us to proceed with further bone marrow studies [11].
The family history of malignancy prompted the investigators to search for a possible underlying hereditary cancer syndrome. The index case fulfilled the Chompret criteria for LFS [12]. LFS is an autosomal dominant hereditary condition caused by a germline mutation in the tumour protein 53 (TP53 gene), leading to an increased risk of multiple cancers. The five core malignancies described in this condition are adrenocortical carcinoma, breast carcinoma, central nervous system tumors, osteosarcomas and soft-tissue sarcomas [13]. Leukaemias compromise a small percentage among the malignancies in LFS, with maximum predisposition among children less than 15 years of age [14, 15]. The most common leukaemia is hypodiploid ALL, followed by B-cell ALL [15]. Periodic surveillance , including clinical workup and regular imaging on a quarterly basis , is recommended in these patients [16, 17].
The literature review did not show any association between LFS and developmental delay . Kuhlen et al [18] described that some of the oncogenes and tumour suppressor genes causing cancer susceptibility syndromes overlap with those involved in autism , which needs further studies to unveil the association between developmental delay and LFS. Though there is no cure for LFS, regular surveillance can facilitate early diagnosis of cancers. Individual cancers are treated as per standard protocols. However, radiation therapy should be used cautiously due to the increased risk of radiation-induced cancer [15]. This patient has been on regular follow up and is being monitored periodically for early detection of new cancers.
Conclusion
Acute leukaemia is known to mimic MSK disorders. Pain disproportionate to physical examination findings, unexplained systemic symptoms, low normal platelet count and algorithmic approach to changes in signal intensities in MRI spine were the clues in this patient that prompted the search for an alternate diagnosis. A detailed family history is indispensable to the suspicion of underlying hereditary cancer predisposition syndromes in children with cancer.
Abbreviations
ABL: Abelson tyrosine kinase gene at chromosome 9, ALL: Acute lymphoblastic leukaemia, ALP: Alkaline phosphatase, BCR: Break point cluster gene at chromosome 22, CD: Cluster of Differentiation, CHPS: Childhood predisposing syndromes, GGT: Gamma glutamyl transpeptidase, HLA: Human Leukocyte Antigen, LFS: Li Fraumeni Syndrome, MRI: Magnetic resonance imaging, MSK: Musculoskeletal, N:C: Nuclear to cytoplasmic ratio, STIR: Short tau inversion recovery, TdT: Terminal deoxynucleotidyl transferase, USG: Ultrasonography.
Acknowledgments
The authors acknowledge MEDGENOME Laboratories for carrying out Next Generation Sequencing on the patient’s DNA sample. The authors express their gratitude to the departments of clinical pathology and radiology, St. John’s Medical College Hospital for their contributions towards the diagnosis.
Conflicts of interest
All authors have declared no competing interests.
Funding
No funding was obtained for this case report.
Author contributions
MB, SC, PP and RS: wrote the manuscript. RS, ST, SC and PP: Managed the patient in the hospital and have followed up the patient. All authors have read and approved the final manuscript.
Ethical approval
The Institutional Ethics Committee (IEC) of St. John’s Medical College has approved this case report. IEC Study reference number is 315/2024.
References
1. Calvo-Muñoz I, Gómez-Conesa A, and Sánchez-Meca J (2013) Physical therapy treatments for low back pain in children and adolescents: a meta-analysis BMC Musculoskeletal Disord 14 1 https://doi.org/10.1186/1471-2474-14-55
2. Kesarapu S, Vangala N, and Uppin SG, et al (2020) Acute leukemia presenting with musculoskeletal manifestations: a case series Indian J Med Paediatric Oncol 41(01) 29–33 https://doi.org/10.4103/ijmpo.ijmpo_113_19
3. Das N, Banavali S, and Bakhshi S, et al (2022) Protocol for ICiCLe-ALL-14 (InPOG-ALL-15-01): a prospective, risk stratified, randomised, multicentre, open label, controlled therapeutic trial for newly diagnosed childhood acute lymphoblastic leukaemia in India Trials 23(1) 102 https://doi.org/10.1186/s13063-022-06033-1 PMID: 35101099 PMCID: 8805436
4. Kittivisuit S, Sripornsawan P, and Songthawee N, et al (2022) Musculoskeletal involvement in childhood leukemia: characteristics and survival outcomes Pediatric Rheumatol 20(1) 34 https://doi.org/10.1186/s12969-022-00692-9
5. Fonseca MB, Gomes FHR, and Valera ET, et al (2017) Signs and symptoms of rheumatic diseases as first manifestation of pediatric cancer: diagnosis and prognosis implications Revista Brasileira De Reumatologia 57 330–337 https://doi.org/10.1016/j.rbr.2016.11.001 PMID: 28743360
6. Clarke RT, Van Den Bruel A, and Bankhead C, et al (2016) Clinical presentation of childhood leukaemia: a systematic review and meta-analysis Arch Dis Childhood 101(10) 894–901 https://doi.org/10.1136/archdischild-2016-311251
7. Morais SA, Du Preez HE, and Akhtar MR, et al (2016) Musculoskeletal complications of haematological disease Rheumatology 55(6) 968–981 https://doi.org/10.1093/rheumatology/kev360
8. Moulopoulos LA and Dimopoulos MA (1997) Magnetic resonance imaging of the bone marrow in hematologic malignancies Blood J Am Soc Hematology 90(6) 2127–2147
9. Hanrahan CJ and Shah LM (2011) MRI of spinal bone marrow: part 2, T1-weighted imaging-based differential diagnosis Am J Roentgenology 197(6) 1309–1321 https://doi.org/10.2214/AJR.11.7420
10. Gallagher DJ, Phillips DJ, and Heinrich SD (1996) Orthopedic manifestations of acute pediatric leukemia Orthopedic Clinics North Amer 27(3) 635–644 https://doi.org/10.1016/S0030-5898(20)32108-8
11. Yoshikawa T, Tanizawa A, and Suzuki K, et al (2016) The usefulness of T1‐weighted magnetic resonance images for diagnosis of acute leukemia manifesting musculoskeletal symptoms prior to appearance of peripheral blood abnormalities Case Rep Pediatrics 1(1) 2802596
12. Tinat J, Bougeard G, and Baert-Desurmont S, et al (2009) 2009 version of the Chompret criteria for Li Fraumeni syndrome J Clin Oncol 27(26) e108–e109 https://doi.org/10.1200/JCO.2009.22.7967 PMID: 19652052
13. Evans DG and Woodward ER (2021) New surveillance guidelines for Li-Fraumeni and hereditary TP53 related cancer syndrome: implications for germline TP53 testing in breast cancer Familial Cancer 20 1–7 https://doi.org/10.1007/s10689-020-00207-z
14. Bougeard G, Renaux-Petel M, and Flaman JM, et al (2015) Revisiting Li-Fraumeni syndrome from TP53 mutation carriers J Clin Oncol 33(21) 2345–2352 https://doi.org/10.1200/JCO.2014.59.5728 PMID: 26014290
15. Schneider K, Zelley K, and Nichols KE, et al (2025) Li-Fraumeni Syndrome GeneReviews®[Internet]
16. Kumamoto T, Yamazaki F, and Nakano Y, et al (2021) Medical guidelines for Li–Fraumeni syndrome 2019, version 1.1 Int J Clin Oncol 26 2161–2178 https://doi.org/10.1007/s10147-021-02011-w PMID: 34633580 PMCID: 8595164
17. Kratz CP, Achatz MI, and Brugieres L, et al (2017) Cancer screening recommendations for individuals with Li-Fraumeni syndrome Clin Cancer Res 23(11) e38–e45 https://doi.org/10.1158/1078-0432.CCR-17-0408 PMID: 28572266
18. Kuhlen M, Taeubner J, and Wieczorek D, et al (2017) Autism spectrum disorder and Li-Fraumeni syndrome: purely coincidental or mechanistically associated? Mol Cellular Pediatrics 4 1–2 https://doi.org/10.1186/s40348-017-0075-9