Acute and long-term effects of chemotherapy on cognitive function among Indian breast cancer patients
Priti Singh1, Chaithanya Leon1, Simran Kaur1, Atul Batra2, Prashant Tayade1, Muthukrishnan Suriya Prakash1 and Ratna Sharma1
1Stress and Cognitive Electroimaging Laboratory, Department of Physiology, All India Institute of Medical Sciences, New Delhi 110029, India
2Department of Medical Oncology, All India Institute of Medical Sciences, New Delhi 110029, India
Abstract
Introduction: Breast cancer (BC) is the leading cause of morbidity and mortality worldwide. Owing to early diagnosis and better therapeutic care, survivorship in these patients have improved tremendously. Chemotherapy, cornerstone in BC management have been associated with debilitating side effects including the effect on cognitive function, which significantly impairs the quality of life in these patients. Thus, it is imperative to understand the timeline and magnitude of the effects of chemotherapy on cognition to develop better management strategies. This is even more relevant in developing country like India, where there is inconspicuous absence of data in this regard.
Aim: To study the acute and long-term effects of chemotherapy on the cognitive function in BC patients compared to chemotherapy naïve (Cx naïve) BC patients (disease controls) and matched healthy controls (HC) using subjective, objective questionnaires and neuropsychological tests (NPTs).
Methods: The current cross-sectional study involved 120 participants, 30 each of Cx naïve BC patients, during chemotherapy BC patients (during Cx), post-chemotherapy BC patients (post Cx) and HC; all matched for age and education levels. Both subjective and objective assessments of cognitive functions were done in all the groups. Hindi Mental State Examination (HMSE) and FACT Cog questionnaire V3 were used for subjective assessment while Addenbrooke Cognitive Examination-III (ACE-III) questionnaire and domain specific computer based NPT (Wisconsin card sorting task (WCST) (learning), Flanker’s (attention and interference) and n back task (working memory) were done for objective assessment. The data were analysed for descriptive and inferential statistics, as appropriate using GraphPad Prism V9.
Results: The subjective assessment using HMSE questionnaire revealed a significantly lower score in post Cx group as compared to HC (p < 0.001); however, it was comparable in other groups. FACT Cog V3 questionnaire revealed significantly higher cognitive impairment among those during Cx compared to Cx naïve patients (p < 0.001), post Cx BC patients (p < 0.001) and HC (p < 0.0001). Meanwhile, the objective assessment using ACE-III examination revealed significantly lesser scores among during Cx patients (p < 0.001), and post Cx BC patients (p < 0.0001) compared to HC group. In NPTs, WCST and N back working memory task revealed significantly lower accuracy in Cx naïve versus post Cx (p = 0.0054, p = 0.0068, respectively) and HC versus post Cx (p = 0.0054, p = 0.0045, respectively), while no significant difference was found in Flanker’s task. Furthermore, in WCST there were significantly higher scores present in total reaction time in post Cx compared to Cx naïve: p = 0.00444 and HC: p = 0.0003). In Flanker’s task reaction time was higher in all the groups (Cx naïve: p = 0.002, during Cx: p = 0.0007 and Post Cx: p < 0.0001) compared to HC. In addition negative correlation was found between the duration of chemotherapy with Perceived Cognitive Abilities (r = −0.482; p = 0.006), between the total number of cycles with Fact Cog Total (r = −0.373, p = 0.04) and Perceived Cognitive abilities (r = 0.39, p = 0.03), and between total dose and perceived cognitive abilities (r = −0.42, p = 0.014) also a positive correlation was seen between dose of epirubicin with reaction time of n back (r = 0.373, p = 0.04).
Conclusion: Chemotherapy can have a negative impact on the cognitive function in BC patients, manifested as both acute and long-term effects, based on patient reported/subjective and laboratory based/objective cognitive function tests. The deficits were seen mostly seen in domains of attention and working memory across groups compared to matched HC.
Keywords: chemotherapy, cognitive function, breast cancer, Chemobrain
Correspondence to: Simran Kaur
Email: drsimrankaur@aiims.edu
Published: 25/02/2025
Received: 14/06/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer (BC) is a leading cause of morbidity and mortality among women, constituting 11.7% of all reported cancers [1]. According to the global BC statistics report, it is now the most common malignant tumour worldwide, with an estimated 2.261 million new cases and 685,000 deaths worldwide in 2020 [1]. As per the Globocan data 2020, in India, BC accounted for 13.5% of all cancer cases and 10.6% of all deaths with a cumulative risk of 2.81% [2]. The prognosis of BC has significantly improved over time, with current 5-year survival rates of around 90% and 10-year survival at 80%, which makes it crucial to focus on enhancing survivorship in these patients.
Most of the BC patients receive treatment in the form of chemotherapy, wherein they are often plagued with numerous debilitating side effects. Chemobrain is also one of the consequences of chemotherapy-induced cognitive dysfunction/impairment seen in these patients. After treatment for BC, many patients complain of impaired memory, attention, speed of processing and other basic cognitive functions reflecting neurotoxicity due to chemotherapy [3]. The prevalence of chemotherapy-induced cognitive dysfunction is estimated to be 17%–75% of patients receiving chemotherapy [4–6]. Out of the affected patients, 17% to 30% appear to sustain long- 3 term cognitive impairment after Cx, even after Cx is over more than a year ago [7].
According to International Cancer Cognition and Cancer Task Force (ICCTF) most affected cognitive domains are learning, memory, processing speed and executive function by Wefel et al [8]. The committee developed recommendations for a core set of neuropsychological tests (NPTs), common criterion for defining cognitive impairment and cognitive changes and common approaches to improve the homogeneity of study methods in the patients of cancer receiving chemotherapy.
Among the regimens given, the routinely administered drug that is used for the treatment of BC are anthracyclines, particularly doxorubicin. Doxorubicin is a leading chemotherapeutic drug that can halt cellular replication and induce p53-dependent apoptosis in cancerous tissue. Although doxorubicin cannot cross the blood-brain barrier, but it potentially causes indirect neurotoxicity through neuroinflammatory mechanisms, possibly leading to cognitive deficits [9]. Furthermore, in a recent study by Vayyat et al [10] in Sarcoma patients, a positive correlation was found between of dose of doxorubicin and impairment in cognitive function.
The current study was designed to assess the acute and long-term effect of chemotherapy on cognitive function in BC patients on chemotherapy compared to chemotherapy naïve (Cx naïve) BC, Post Cx BC patients and healthy controls (HC) using subjective and objective questionnaires. To achieve this aim, we performed the subjective cognitive assessment using Hindi mental state examination (HMSE) [11] and Functional assessment of cancer therapy cognitive questionnaire version III (FACT Cog-V3) [12], and objective assessment using the Addenbrookes cognitive examination version III (ACE-III) [13] and computer-based NPT for various cognitive domains Wisconsin Card Sorting Test (WCST: learning, Flanker’s: attention, n back: working memory).
Furthermore, comparisons were made between the groups to see the effect of chemotherapy across both subjeive and objective cognitive assessments.
We hypothesised that there would be cognitive impairment in the patients during chemotherapy compared to Cx naïve, post chemotherapy BC patients and HC.
Materials and methods
Study design
This cross-sectional and observational study was conducted at the Stress and Cognitive Electroimaging Lab, Department of Physiology, All India Institute of Medical Sciences, New Delhi. After obtaining the ethical approval (Ref. No.-IECPG-203/24.03.2022), BC patients were recruited from the Department of Medical Oncology of Dr. B. R. Ambedkar Institute Rotary Cancer Hospital (Dr. B.R.A. IRCH), AIIMS, New Delhi, and matched HC were recruited on volunteering basis using advertisement, flyers and announcements in the community. The recruitment was done during the period from April to November 2022 (Figure 1).
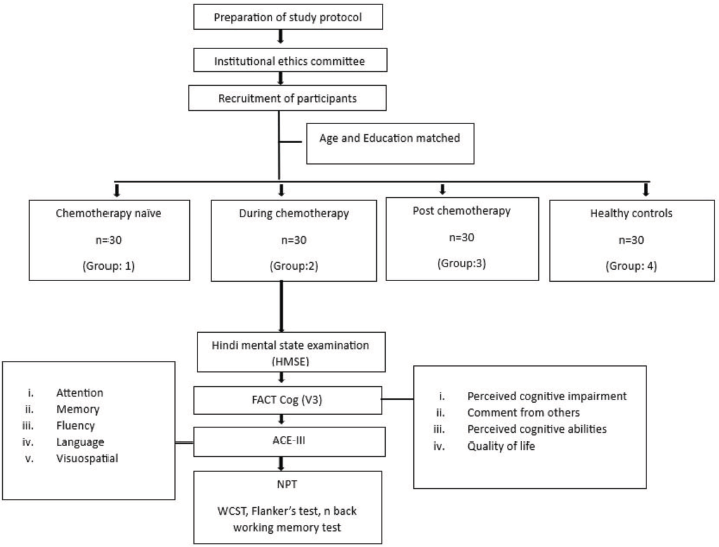
Figure 1. Flowchart describing the study protocol. After ethical approval, women diagnosed with BC and HC, in the age group of 18–65 years were recruited. Based on the chemotherapy status, the BC patients were divided in to three groups: Cx naïve, during chemotherapy- 3 months after the initiation of chemotherapy and post chemotherapy- 3 months after cessation of chemotherapy. Then the study participants were subjected to subjective assessments using HMSE and Functional Assessment of Cancer Therapy- Cognitive Function questionnaire Version 3 (FACT-Cog V3) and objective assessment using ACE-III. Further they performed NPT using WCST, Flanker’s test and n back working memory test.
Participants
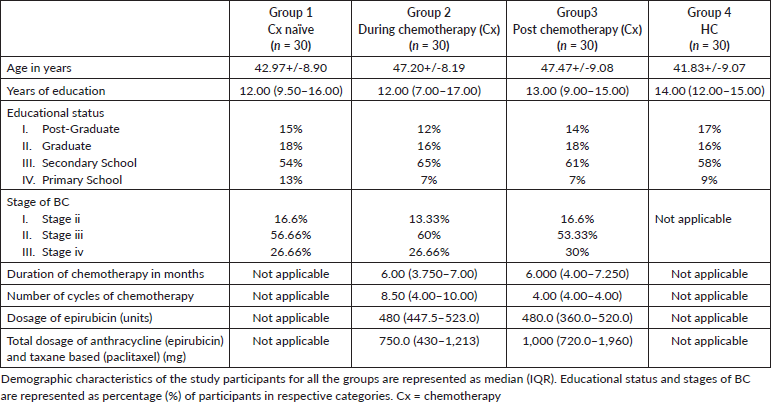
The study was performed on 120 subjects, 30 Cx naïve BC patients (recruited after the diagnosis of BC and before the cancer treatment (Group 1). 30 during Cx BC patients who had received at least 3 months of chemotherapy and come for follow-up within 7 days after the recent chemotherapy (Group 2) post Cx BC patients, who had completed their chemotherapy at least before 1 year (Group 3) (all BC participants, stage II–IV of Groups 2 and 3 received standard care of treatment comprising of anthracycline and Taxane for more than 3 months as mentioned in Table 1) and HC (Group 4). All the participants across groups were age and education matched and no confounding factors contributed to the matching of participants across groups. The participants recruited for Groups 1–3 had been clinically and histologically diagnosed with BC; they were age- and education-matched, had no history of neuropsychiatric or neurodegenerative disorders, and had no known brain metastases or central nervous system (CNS) lesions. Group 2 patients received a standard chemotherapy regimen consisting of anthracycline and taxane for more than 3 months, whereas Group 3 patients had finished the same regimen within the previous year. Group 4 consisted of age and education-matched females with no history of CNS lesions or neuropsychiatric/neurodegenerative disorders. The socio-demographic details are provided in Table 1.
Procedure
After the recruitment of the participants for respective groups, written informed consent was obtained and detailed procedure was explained to them. Subsequently, socio-demographic and clinical history were collected and questionnaires were administered to each participant. For each participant, all the assessments were done on the same day and data were collected.
Table 1. Demographic characteristics of the study participants for all the groups.
Administration of questionnaires
Hindi mental state examination
HMSE was used as a global cognitive measurement questionnaire. There were 23 questions in HMSE and assessed orientation in time and place, memory, attention, concentration, recognition of objects, language function and comprehension and expressive speed. The total score of HMSE is 30, if the scores were above or equal to 24, it was considered as normal and below 24 was taken as cognitive impairment [11]. HMSE is better suited for neurocognitive testing of the largely Hindi-speaking Indian population and has a high sensitivity (0.81) and specificity (0.60).
Functional assessment of cancer therapy cognitive questionnaires (Fact Cog), version-3
It is a self-assessment scale validated in routine clinical practice to assess cognitive function in cancer patients [14]. It consisted of four subscales namely: perceived impairment (score range between 0 and 76), perceived abilities (score range between 0 and 28), comments by others (score range between 0 and 16) and impact on quality of life (score range between 0 and 16). The Cronbach's alpha for the scale for reliability analysis of the total scale and subscales were α ≥ 0.7 and ≥ 0.8, respectively [14].
Addenbrooke’s cognitive examination
It was used for the objective assessment of cognitive function for all the groups. It is composed of five cognitive domains: attention, memory, language, verbal fluency and visuospatial abilities [15]. It takes ~20 minutes to complete and has a maximum score of 100 (Attention = 18 points, Memory = 26 points, fluency = 14 points, language = 26 points, visuospatial skills = 16 points). Higher scores indicate better cognitive functioning. ACE-III also had good sensitivity and specificity for mild cognitive impairment with respect to Indian subpopulation, within a range of 0.83–1.00 at corresponding cut-off values of 84–89 [16]. Both Indian English and Hindi version of the 26 questionnaires are validated and free to use for clinical practice [17].
Neuropsychological tests
The NPT were designed using E prime V3, as per the cognitive domains recommended by ICCTF [18]. The following cognitive function tests were employed:
WCST for learning and processing speed
The WCST comprised response cards with four types of geometric figures which differed based on form, number and color. In the WSCT, participants were required to apply sorting principles through trial and error [19]. The participants matched stimulus cards to four response cards every time without instructions from the test examiner. Participants were expected to deduce sorting principles according to feedback on correct or wrong matches and had to follow the sorting principle despite the changing conditions and neglect other unrelated geometric figures. To identify the prevailing category, participants had to rely on feedback that was provided during the task as ‘correct’ (positive feedback) or ‘incorrect’ (negative feedback) on each trial. The total number of trials were 60 and the behavioral parameters collected were reaction time, accuracy and a number of perseverative and non-perseverative errors and subsequently analysed (Figure 2a).
Flanker’s test for attention and processing
An arrow version of the Eriksen Flanker task was used for the current study [20]. During each trial, participants were presented with five black arrows for 200 ms against a white background. They were instructed to respond as quickly and as accurately as possible to indicate the direction of the middle arrow by pressing the respective buttons. A total of 60 trials were administered to the participants, comprising of congruent (Flanker’s pointing in the same direction as central arrow), incongruent (Flanker’s pointing in opposite direction to the central arrow) and neutral trials (Flanker’s were diamond that were not associated with response). A total of 60 trials were administered to all the four groups and responses were recorded through a button press. The behavioral parameters analysed were reaction time (total, correct and incorrect trials) and accuracy (Figure 2b)
n-back task for working memory task
Participants were presented a sequence of trial structures consisting of a blue colour small square, which can be in any of the eight possible locations of the big square. For every trial structure, the participants were required to respond to the current trial structure related to one presented one trial before (i.e., n = 1). The single trial comprised of the fixation cross for 2,500 ms followed by blue coloured trial structure, presented for 2,000 ms. In case the location of the subsequent blue square matches with the one shown just before, it is a match and the subject has to press 1; if not a match, the subject pressed the key 2. Behavioral parameters were ascertained in the form of reaction time and accuracy. A total of 60 trials were administered to all the subjects in the four groups (Figure 2c)
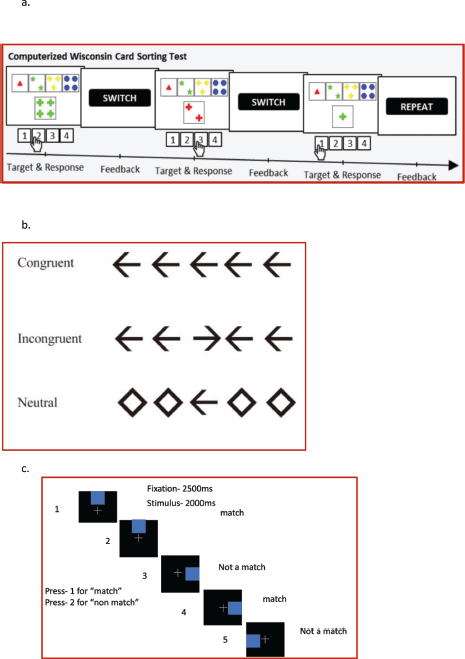
Figure 2. (a): Schematic representation of WCST, (b): Schematic representation of Flanker’s Task, (c): Schematic representation of n back working memory task.
Statistical analysis
GraphPad Prism 9.0 software was used to statistically analyse the demographic data and scores of the HMSE and FACT Cog-III and ACE-III questionnaires and NPT (WCST, Flanke’s test and n back working memory test). Kolmogorov- Smirnov test was applied to test the normality of the age distribution and education in years. Since the distribution was non parametric, Kruskal-Wallis test was used for comparisons between the groups. The correlation was done between the attributes of chemotherapy with questionnaire scores in Cx naïve, during chemotherapy group and post chemotherapy group using Spearman rho correlation analysis.
Results
Demographic and clinical characteristics
The study was performed on 120 subjects, 30 of each group. Table 1 shows the demographic profile of all the groups. Statistical analysis was done using GraphPad Prism 9 software. Kolmogorov-Smirnov test was applied to test the normality of the age distribution and education in years. It was found that there were no significant differences between the groups, i.e., the groups were age and education matched.
Cognitive assessment using HMSE
The total scores of the post chemotherapy participants were significantly lower (p < 0.001) as compared to HC group while no significant difference was found in other group comparisons.
Patient-reported cognitive assessment using FACT-Cog v3
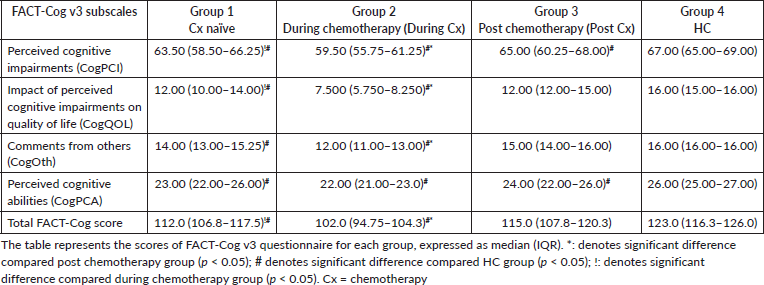
The scores of FACT-Cog v3 questionnaire for each group are shown in Table 2. The total FACT- Cog score of the Cx naïve group was significantly higher than during Cx BC patients (p < 0.001) and significantly lower than HC (p < 0.05). Furthermore, scores in the chemotherapy group were lesser than post Cx (p < 0.5) and HC (p < 0.001).
Table 2. The scores of the subjective cognitive symptoms from FACT-Cog v3 questionnaire.
Objective neurocognitive assessment using ACE-III
The scores of (ACE)-III questionnaires for each group are shown in Table 3. The total ACE-III (p < 0.001) score along with memory and frequency domain scores (p < 0.01 and p < 0.05, respectively) were significantly lower in Cx naïve group as compared to the HC group. Furthermore, the total ACE-III scores of the during Cx group (p < 0.001) and post Cx (p < 0.0001) were significantly lower as compared to the HC group. We also observed the scores for language and frequency domain to be significantly lower in during Cx group compared to post Cx (p < 0.0001) and HC group (p < 0.0001).
Objective neurocognitive assessment using NPT
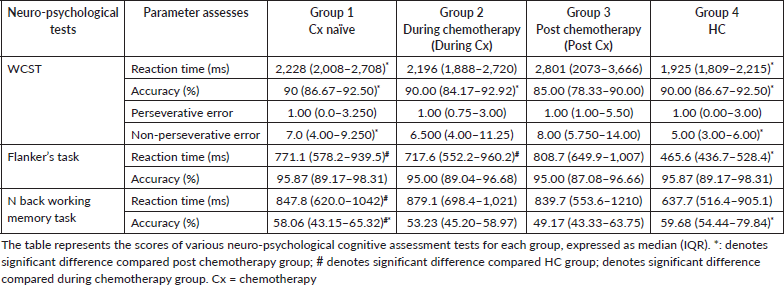
The scores of WCST, Flankers task and n-back working memory task for each group are shown in Table 4.
WCST: The reaction time and non-preservative error of post Cx group was found to be higher than Cx naïve and HC (p = 0.0444 and p = 0.0003; p = 0.0288 and p < 0.0001, respectively). While, the accuracy score of post Cx was lower than Cx naïve, during Cx and HC groups (p = 0.0054, p = 0.0174 and p = 0.0054, respectively).
Flanker’s task: The reaction time was found to be higher in Cx naïve, during Cx, post Cx as compared to HC (p = 0.0002, p = 0.0007 and p < 0.0001, respectively), and while no significant difference was found in accuracy scores on between-group comparison.
n-back working memory task: The reaction time of Cx naïve group was found to be higher than HC group (p = 0.0151) while, accuracy was found to be lower in Cx naïve as compared to HC and post Cx (p = 0.0123 and p = 0.0068, respectively).
Correlation analysis
Correlation analysis was done between the total no. of the cycle of chemotherapy, total duration and total dosage of chemotherapy with cognitive function tests and questionnaire scores of during the chemotherapy group. Since the data were non parametric, Spearman rho correlation analysis was performed. A negative correlation was found between the duration of chemotherapy with perceived cognitive abilities (r = −0.482; p = 0.006) as well as between the total number of cycles with a total score of Fact Cog questionnaire (r = −0.373, p = 0.04) and perceived cognitive abilities (r = 0.39, p = 0.03). Also, between total dose and perceived cognitive abilities (r = −0.42, p = 0.014). While, a positive correlation was seen between the dose of epirubicin and with reaction time of N back task (r = 0.373, p = 0.04).
Table 3. The scores of the objective cognitive assessment from the ACE-III.
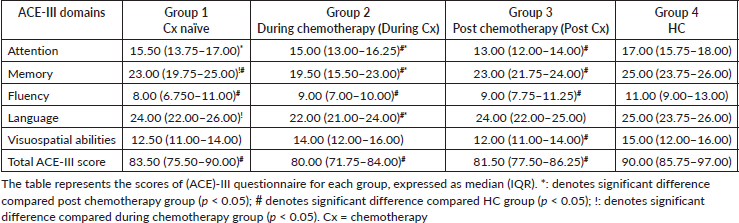
Table 4. Behavioural scores (reaction time and accuracy) as assessed using objective neuro-psychological cognitive assessment tests.
Discussion
BC is one of the prevalent cancers with millions of women diagnosed each year across developed and developing countries. Due to the advent of newer treatments and better screening, the survivorship of these patients has improved. This calls for the need to focus on enhancing their quality of life by exploring the impact of chemotherapy [21]. One of the potential effects of chemotherapy is chemotherapy-induced cognitive deficits, also called Chemobrain [22]. The current study aimed to address the cognitive effects during chemotherapy in BC patients, across both subjective and objective domains. Concurring to the ICCTF [8], the core cognitive domains that were investigated in patients of BC (Cx naive), during Cx, post Cx as compared to matched HC were executive function, processing speed, attention and memory and learning.
Subjective assessment using HMSE in BC patients revealed comparable scores with HC. This is in line with Shen et al [23] but contrary to Liu et al [24] wherein BC patients who received chemotherapy had lower scores in MMSE compared to matched healthy subjects. Meanwhile, FACT Cog v3 questionnaire showed lower scores in during the chemotherapy (during Cx) group compared to post chemotherapy (post Cx), Cx naïve and HC. We also observed a decline in cognitive function domains of perceived cognitive impairment, comment from others, perceived cognitive abilities and quality of life in during the chemotherapy group compared to Cx naïve, post Cx and HC. Similar observations demonstrating the cancer-related perceived cognitive challenges were reported in BC patients, after three cycles of chemotherapy compared to baseline [25] and poor FACT cog scores within the chemotherapy group compared to Cx naïve patients [26]. Contrary to this, Lange et al [27] detailed no significant difference in subdomains of FACT Cog scale in Cx naïve versus post Cx except for perceived cognitive abilities.
Objective cognitive evaluation utilising ACE-III revealed lower scores found in during Cx and post Cx as compared to HC. The deficits were seen only in sub-domains of memory and visuospatial skills, language and frequency. This suggests that cognitive changes related to chemotherapy may not consistently affect all cognitive domains measured by the ACE-III. This underscores the significance of considering specific cognitive functions and their vulnerability to chemotherapy-related effects when assessing cognitive performance in cancer patients [11].
Objective assessment was also done using computer-based cognitive function tests. Post Cx displayed significantly higher reaction times, lower accuracy and increased non-perseverative errors compared to Cx naïve and HC utilising WCST. In line with our study, Cerulla et al [28] reported higher perseverative errors compared to pre-chemotherapy and 6-month chemotherapy groups. The performance using Flanker’s task showed no significant differences in accuracy, but Cx naïve, during Cx, and post-Cx displayed higher reaction time compared to HC. Contrary to what Swainston et al [29] reported, higher error rates in a chemotherapy group compared to a non-cancer group and no significant differences were reported in reaction time between the two groups. These findings highlight the complex nature of chemotherapy-related cognitive changes and emphasise the significance of assessing multiple cognitive domains to fully understand their impacts [29]. To assess, the effect on memory, N-back working memory task was used, wherein higher reaction time was seen in Cx-naïve individuals compared to HC, with lower accuracy in all BC groups compared to HC. McDonald et al [30] also reported no significant difference in reaction time and accuracy of groups between those who had completed 1 month of chemotherapy, completion 1 year after 1 month of chemotherapy and baseline (BC patient without chemotherapy utilising n back working memory task.
In addition, literature also reports chemotherapy patients display longer reaction times compared to HC, indicating potential delays in processing information among individuals undergoing chemotherapy. However, no significant differences were observed in accuracy between the chemotherapy group and the HC in our study. These discoveries further highlight the multifaceted nature of cognitive changes related to chemotherapy and emphasise the importance of examining various cognitive domains in understanding its impact [31]. A prospective study of patients with BC found significant cognitive decline soon after chemotherapy, which in some patients partially recuperated 1 year after treatment [32].
Furthermore, we found negative correlations between the duration of chemotherapy and perceived cognitive abilities, as well as between the total number of chemotherapy cycles and scores on the FACT Cog v3 total scale and perceived cognitive abilities. Additionally, a negative correlation was observed between the entire dosage of chemotherapy and perceived cognitive abilities. Also, a positive correlation emerged between the dosage of epirubicin and reaction time of n back task which is in line with a study by Vayyat et al [10] wherein correlation was found in cumulative dose of chemotherapy and cognitive functions.
The study's findings emphasise the need to create a care plan for these patients that allows for the monitoring of chemotherapy's side effects. This could support a potential modification to the BC treatment plan, specifically with respect to the total dosage and/or length of chemotherapy, thereby improving the quality of life in these patients.
Strength and limitations
The present study describes the chemotherapy-related cognitive deficits in BC patients recruited from a tertiary health care centre. One of the strengths of the present study is the comprehensive evaluation of cognitive functions using both patients reported, subjective and objective assessment tools. Also, the participants in the study were recruited across 4 groups, matched in age, education and socio-economic status, especially in Indian settings, where there is sparse data in the domain of chemotherapy-related cognitive deficits.
Though the present study provides an important vision regarding the neurocognitive impairments caused by chemotherapy and cancer per se but it is limited by its sample size and further, a prospective longitudinal study would shed more light on the effects of chemotherapy on cognitive function, which authors plan to do in the future.
Conclusion
The current study demonstrates the presence of cognitive impairment both on subjective and objective cognitive functions, in Cx naïve, during chemotherapy and post chemotherapy BC patients as compared to HC. These impairments persist even after the cessation of chemotherapy, thereby calling for a regular neuro-rehabilitative follow-up of these patients, to address the long-term effects of chemotherapy, support recovery and improve the overall quality of life in these patients.
Acknowledgments
The authors are thankful to the participants who volunteered for the current study.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding
The authors received no funding for this study.
Author contributions
Priti Singh: Material preparation, data collection, analysis, manuscript writing
Chaithanya Leon: Data collection, analysis and manuscript writing.
Simran Kaur: Study conception, supervised data collection, analysis and manuscript revision.
Atul Batra: Contributed to the study conception and research design.
Prashant Tayade: Contributed to the study conception and research design.
Suriya Prakash M: Contributed to the study conception and research design.
Ratna Sharma: Contributed to the study conception and research design.
The first draft of the article was written by Priti Singh, and all authors reviewed and approved the final version.
Data statement
The data which are in support of this study are available on request from the corresponding author. These data are not publicly available due to privacy and ethical restrictions.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Mehrotra R and Yadav K (2022) Breast cancer in India: present scenario and the challenges ahead World J Clin Oncol 13(3) 209–218 https://doi.org/10.5306/wjco.v13.i3.209 PMID: 35433294 PMCID: 8966510
3. Cascella M, Di Napoli R, and Carbone D, et al (2018) Chemotherapy-related cognitive impairment: mechanisms, clinical features and research perspectives Recenti Prog Med 109(11) 523–530 PMID: 30565571
4. Janelsins MC, Kesler SR, and Ahles TA, et al (2014) Prevalence, mechanisms, and management of cancer-related cognitive impairment Int Rev Psychiatry 26(1) 102–113 https://doi.org/10.3109/09540261.2013.864260 PMID: 24716504 PMCID: 4084673
5. Janelsins MC, Heckler CE, and Peppone LJ, et al (2017) Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study J Clin Oncol 35(5) 506–514 https://doi.org/10.1200/JCO.2016.68.5826 PMCID: 5455314
6. Wang XM, Walitt B, and Saligan L, et al (2015) Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy Cytokine 72(1) 86–96 https://doi.org/10.1016/j.cyto.2014.12.006 PMID: 25573802 PMCID: 4750385
7. Ahles TA and Saykin AJ (2002) Breast cancer chemotherapy-related cognitive dysfunction Clin Breast Cancer 3 S84–S90 https://doi.org/10.3816/CBC.2002.s.018
8. Wefel JS, Vardy J, and Ahles T, et al (2011) International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer Lancet Oncol 12(7) 703–708 https://doi.org/10.1016/S1470-2045(10)70294-1 PMID: 21354373
9. Eide S and Feng ZP (2020) Doxorubicin chemotherapy-induced “chemo-brain”: meta-analysis Eur J Pharmacol 881 173078 https://doi.org/10.1016/j.ejphar.2020.173078
10. Vayyat S, Revand R, and Rastogi S, et al (2024) Patient-reported cognitive impairments and objective neuropsychological deficits in young sarcoma patients undergoing chemotherapy and its comparison to healthy controls: a tertiary health care study from India Int J Cancer 154(8) 1413–1422 https://doi.org/10.1002/ijc.34818
11. Ganguli M, Ratcliff G, and Chandra V, et al (1995) A hindi version of the MMSE: the development of a cognitive screening instrument for a largely illiterate rural elderly population in India Int J Geriatr Psychiatry 10(5) 367–377 https://doi.org/10.1002/gps.930100505
12. Wagner LI, Lai JS, and Cella D, et al (2004) Chemotherapy-related cognitive deficits: development of the FACT-Cog instrument Ann Behav Med 27
13. Bruno D and Schurmann Vignaga S (2019) Addenbrooke’s cognitive examination III in the diagnosis of dementia: a critical review Neuropsychiatr Dis Treat 15 441–447 https://doi.org/10.2147/NDT.S151253 PMCID: 6387595
14. Cheung YT, Shwe M, and Chui WK, et al (2012) Effects of chemotherapy and psychosocial distress on perceived cognitive disturbances in Asian breast cancer patients Ann Pharmacother 46(12) 1645–1655 https://doi.org/10.1345/aph.1R408 PMID: 23249868
15. Mathuranath PS, Nestor PJ, and Berrios GE, et al (2000) A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia Neurology 55(11) 1613–1620 https://doi.org/10.1212/01.wnl.0000434309.85312.19 PMID: 11113213
16. Menon RN, Varghese F, and Paplikar A, et al (2020) Validation of Indian Council of Medical Research neurocognitive tool box in diagnosis of mild cognitive impairment in India: lessons from a harmonization process in a linguistically diverse society Dement Geriatr Cogn Disord 49(4) 355–364 https://doi.org/10.1159/000512393
17. Newman JP (2005) Brief assessment of cognitive mental status in Hebrew: Addenbrooke’s cognitive examination Isr Med Assoc J 7(7) 451–457 PMID: 16011062
18. Deprez S, Kesler SR, and Saykin AJ, et al (2018) International Cognition and Cancer Task Force recommendations for neuroimaging methods in the study of cognitive impairment in non-CNS cancer patients J Natl Cancer Inst 110(3) 223–231 https://doi.org/10.1093/jnci/djx285 PMID: 29365201 PMCID: 6658857
19. Chiu EC and Lee SC (2021) Test–retest reliability of the Wisconsin card sorting test in people with schizophrenia Disabil Rehabil 43(7) 996–1000 https://doi.org/10.1080/09638288.2019.1647295
20. Imburgio MJ, Banica I, and Hill KE, et al (2020) Establishing norms for error-related brain activity during the arrow Flanker task among young adults Neuroimage 213 116694 https://doi.org/10.1016/j.neuroimage.2020.116694 PMID: 32142881 PMCID: 7197955
21. Hermelink K (2015) Chemotherapy and cognitive function in breast cancer patients: the so-called chemo brain JNCI Monogr 2015(51) 67–69 https://doi.org/10.1093/jncimonographs/lgv009
22. Rastogi S, Sharma R, and Kaur S (2018) Cognitive studies for cancer survivors in India: is this the right time or should we cross the bridge only when we will come to it? Indian J Med Paediatr Oncol 39(03) 269–271 https://doi.org/10.4103/ijmpo.ijmpo_173_17
23. Shen CY, Chen VCH, and Yeh DC, et al (2019) Association of functional dorsal attention network alterations with breast cancer and chemotherapy Sci Rep 9(1) 104 https://doi.org/10.1038/s41598-018-36380-6 PMID: 30643203 PMCID: 6331552
24. Liu Z, Han X, and Tian C, et al (2018) A study on the relationship between chemotherapy-induced cognitive impairment and age in patients with breast cancer J BUON 23(6) 1601–1605
25. Keetile NM, Osuch E, and Lentoor AG (2021) Chemotherapy-related subjective cognitive impairment in breast cancer patients in semi-rural South Africa Health SA 26 1605 PMID: 34394969 PMCID: 8335783
26. Tong T, Lu H, and Zong J, et al (2020) Chemotherapy-related cognitive impairment in patients with breast cancer based on MRS and DTI analysis Breast Cancer 27(5) 893–902 https://doi.org/10.1007/s12282-020-01094-z PMID: 32301096
27. Lange M, Joly F, and Vardy J, et al (2019) Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors Ann Oncol 30(12) 1925–1940 https://doi.org/10.1093/annonc/mdz410 PMID: 31617564 PMCID: 8109411
28. Cerulla N, Arcusa À, and Navarro JB, et al (2019) Cognitive impairment following chemotherapy for breast cancer: the impact of practice effect on results J Clin Exp Neuropsychol 41(3) 290–299 https://doi.org/10.1080/13803395.2018.1546381
29. Swainston J, Louis C, and Moser J, et al (2021) Neurocognitive efficiency in breast cancer survivorship: a performance monitoring ERP study Int J Psychophysiol 168 9–20 https://doi.org/10.1016/j.ijpsycho.2021.06.013 PMID: 34242661 PMCID: 9092800
30. McDonald BC, Conroy SK, and Ahles TA, et al (2012) Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study J Clin Oncol 30(20) 2500–2508 https://doi.org/10.1200/JCO.2011.38.5674 PMID: 22665542 PMCID: 3397784
31. Mackenzie MJ, Zuniga KE, and Raine LB, et al (2016) Associations between physical fitness indices and working memory in breast cancer survivors and age-matched controls J Womens Health (Larchmt) 25(1) 99–108 https://doi.org/10.1089/jwh.2015.5246
32. Collins B, Mackenzie J, and Tasca GA, et al (2014) Persistent cognitive changes in breast cancer patients 1 year following completion of chemotherapy J Int Neuropsychol Soc 20(4) 370–379 https://doi.org/10.1017/S1355617713001215






