Nasopharyngeal, tongue and laryngeal cancer in Southern Ethiopia: a seven-year retrospective cross-sectional review
Achamyelesh Gebretsadik1a, Netsanet Bogale2, Dereje Geleta1, Nebiyu Melaku3 and Dubale Dulla4b
1School of Public Health, College of Medicine and Health Sciences, Hawassa University, 1560 Hawassa, Ethiopia
2Faculty of Medicine, College of Medicine and Health Sciences, Hawassa University, 1560 Hawassa, Ethiopia
3Maternal and Child Health Core Process, Southern Nation Nationalities and People Regional Health Bureau, 1560 Hawassa, Ethiopia
4Department of Midwifery, College of Medicine and Health Sciences, Hawassa University, 1560 Hawassa, Ethiopia
ahttps://orcid.org/0000-0002-0060-2103
bhttps://orcid.org/ 0000-0003-0202-763X
Abstract
Background: The burden of cancer is increasing globally and is having a negative impact on people’s physical, mental and financial health. On the other hand, developing countries are not progressing to prevent the disease at the same rate as the disease burden increases. The development of strategies for cancer prevention, control and treatment that contribute to the community’s improved health requires knowledge of cancer epidemiologic data. There is relatively little epidemiologic evidence of nasopharyngeal, tongue and laryngeal cancer in southern Ethiopia. This study aimed to assess the epidemiological burden of nasopharyngeal, tongue and laryngeal cancer among patients treated at Hawassa University Comprehensive and Specialised Hospital (HUCSH) between 2013 and 2019.
Methods: A cross-sectional retrospective review was conducted among 3,002 patients who attended the oncologic care at HUCSH. Data were retrieved between February and May 2020. Data were entered using Epi-data version 3.1 and the data were then exported to IBM SPSS version 22 (IBM Corporation, Armonk, NY, USA) for further processing and analysis. A descriptive analysis was done.
Result: A total of 280 (9.3%) new head and neck cancer (HNC) patients were identified over a period of 7 years. Nasopharyngeal cancer accounts for more than one-fourth (26.4%) of all HNCs, followed by tongue 15% and laryngeal 14.6% cancers. Males constituted nearly two-thirds of the cases. The overall caseloads doubled over the retrieved years.
Conclusion: According to this study, nasopharyngeal, tongue and laryngeal cancer is a more prominent cause of morbidity. According to place, person and time, the frequency of nasopharyngeal, tongue and laryngeal cancer steadily rose in both sexes and across all age categories. Therefore, immediate intervention is needed nationwide to monitor the disease’s explosive growth.
Keywords: nasopharyngeal, tongue and laryngeal cancer, epidemiology, Southern Ethiopia
Correspondence to: Achamyelesh Gebretsadik
Email: agtsadik@gmail.com
Published: 08/10/2024
Received: 29/04/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Cancer has a significant financial impact on society in both economically developed and poor nations; the least resource-rich nations still carry the bulk of the load. Population increase and aging, the rising incidence of established risk factors, and changing reproductive patterns related to urbanisation and economic growth have an impact on the likelihood of developing cancer [1, 2]. Between 2012 and 2018, studies on cancer incidence and mortality showed that there were roughly 8.2 to 9.6 million cancer-related deaths and 11 to 18.7 million new cancer cases worldwide [1, 3–5].
Globally and in Sub-Saharan Africa, which includes Ethiopia, head and neck cancers (HNCs) are ranked sixth and third, respectively [6, 7]. The prevalence of head and neck malignancies has increased in the United States of America; notwithstanding some fluctuations [6–8]. Pharyngeal cancer was found to be the most common cancer in most studies of HNCs conducted around the world [9–11], besides laryngeal cancer was shown to be the most common cancer in other studies [12–15]. In several studies, also oral cancers were the most commonly reported cancers [16–23].
However, staging of HNC based on each anatomical site, the American Joint Committee on Cancer (AJCC) system generally classify as early stages (I and II) and later stages (III and IV), according to a study conducted in the Netherlands, the incidence of oral, oro-pharyngeal and hypo-pharyngeal carcinoma has increased significantly over time [24–26]. Oral and oropharyngeal cancers accounted for the highest grade of HNC, according to a study conducted by Addis Ababa University [27]. The exact burden of HNCs in Africa is unknown due to a variety of factors, including poor case recording, unclear nomenclature and a lack of functional cancer registries [6, 28, 29]. All of these factors could be linked to similar challenges, such as a lack of human, technical and financial resources [19, 25].
The difficulties and complications of treating HNC in low-resource environments have been brought to light by more recent research. Early detection and community awareness are crucial for enhancing the prognosis of Ethiopian HNC patients. The researchers reported that late-stage presentation continues to be a major obstacle to receiving an appropriate course of therapy. This is frequently because of poor public health education regarding cancer symptoms and restricted access to specialised healthcare services. Furthermore, noted that there was an urgent need for improved training for healthcare professionals in the area, and they suggested that putting an emphasis on capacity-building could increase treatment efficacy and diagnostic accuracy. These results highlight the significance of addressing HNC from multiple angles, combining systemic changes in healthcare infrastructure and education with medical developments [26, 27].
This study intends to close the knowledge gaps about the epidemiology of tongue, laryngeal and nasopharyngeal cancers (NPCs) in the southern parts of Ethiopia, particularly at Hawassa University Comprehensive and Specialised Hospital (HUCSH). By doing this, it intends to provide vital information that can guide the distribution of resources and healthcare policies, ultimately enhancing the quality of cancer care and outcomes in the area. Additionally, knowing the local epidemiology of these malignancies can aid in the creation of focused prevention and treatment plans that are adapted to the particular environmental and demographic characteristics of the region. This is especially crucial considering the high incidence of head and neck malignancies worldwide and the particular difficulties in treating these illnesses that low-resource environments experience [28, 29].
Methods
Study setting and period
This study is part of a larger project conducted on the economic burden of cancers in HUCSH, southern Ethiopia, and the method used to conduct it was published in the Journal of Cancer Control in 2021 [30]. The HUCSH in Hawassa is where the study was conducted. The only cancer treatment and diagnosis facility for residents of southern Ethiopia, encompassing Sidama, Southern Nation Nationalities People (SNNP), Oromia, and other nearby regions, is this government-owned hospital. The hospital today provides a variety of treatment options, including surgery, chemotherapy and hormonal treatments, for more than 18 million patients.
Data collection, management and analysis
A cross-sectional study design was used. Data were extracted from the patient’s card and registration book retrospectively using a structured checklist. All data between 2013 and 2019 were included. Records of all patients with a cancer diagnosis in registration books were retrieved for examination by obtaining the card number of each patient registered in an oncology unit throughout the study period. Then, HNCs were searched for in all cancer patient cards. The research team followed a standard checklist to gather data from February through May 2020. Data were entered using Epi-data version 3.1 and then exported for further processing and analysis to IBM SPSS version 22 (IBM Corporation, Armonk, NY, USA). AJCC, eighth edition was used for the Classification of cancer stages. Both descriptive and trend analyses were carried out.
Result
Epidemiologic distributions of cancer
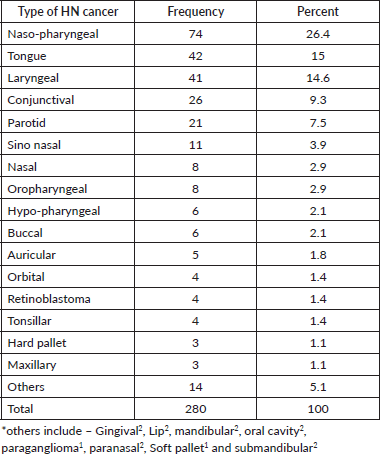
Over the course of seven years, 280 HNC cases with ages ranging from 10 to 90 were extracted from the full records of all new cancer cases. Of these, 26.4% are NPCs, with tongue and laryngeal cancers accounting for 15% and 14.6% of all HNCs, respectively. A few others were accounted for by the gingival, lip, mandibular, oral cavity, paranasal sinus, submandibular, soft palate and paraganglioma Table 1.
Table 1. Nasopharyngeal, tongue, laryngeal and others HNC patients distribution by body site 2013–2019 at HUCSH.
The mean age for these patients was 41.9 years. Nearly 44% of all nasopharyngeal, tongue and laryngeal cancer patients were married. The majority of the cases came from Oromia region 121 (43.2%) and SNNPR 157(56.1%). While laryngeal and NPCs were frequently seen in the Oromia region, conjunctiva and tongue cancers were common in SNNPR (Table 2).
The majority of patients had no previous history of cancer. Six percent of cases had a history of non-communicable diseases. Nearly 70% of cases referred themselves to this hospital (Table 3).
Nasopharyngeal, tongue and laryngeal cancer distribution by age and sex
NPC is one of the common types of cancer categorised as HNCs and it is common in early ages less than 20 years up to 5th decades, tongue cancer was found common at the age between 3rd and 5th decades and laryngeal cancer was common at the age of 4th decades Figure 1.
Laryngeal cancer was more common among males than females with a ratio of 12.6:1 followed by nasopharyngeal of 2.2:1 and tongue of 2:1 (Table 4).
Cancer diagnosis and characteristics
Nasopharyngeal cancer
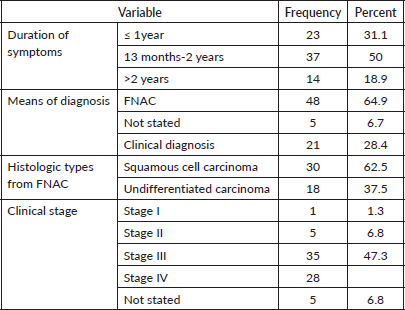
The average length of time between the onset of symptoms and the NPC diagnosis was 14 months. The majority of patients with NPC (48, 64.9%) were diagnosed with fine needle aspiration cytology (FNAC) from neck lymphadenopathy. Squamous cell carcinoma was the most prevalent histologic type, accounting for 30 (62.5%) of all FNAC diagnosed cases. Of this, 63 or 85.1% of the patients had advanced cancer (stage III and IV) (Table 5).
Tongue cancer
An average of 17 months passed between the onset of symptoms and the tongue cancer diagnosis. The majority of tongue cancer patients [31] were diagnosed using FNAC (73.8%) from tongue lesions. Squamous cell carcinoma was the most common histologic type, accounting for 28 (90.3%) of all FNAC diagnosed cases. Of this 28 patients, or 66.7%, of the total patients, had advanced cancer (Stage III and IV) (Table 6).
Table 2. Socio-demographic characteristics of nasopharyngeal, tongue, laryngeal and others HNC patients at HUCSH between 2013 and 2019. N = 280.
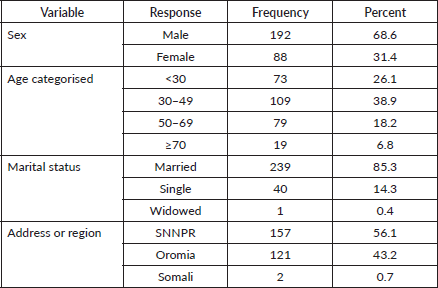
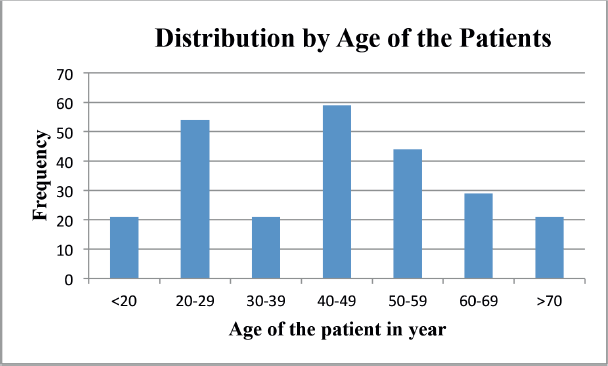
Figure 1. Distribution of cancer by age of nasopharyngeal, tongue, laryngeal and others HNC patients between 2013 and 2019 at HUCSH.
Table 3. Personal and previous medical history of the nasopharyngeal, tongue and laryngeal cancer patients at HUCSH between 2013 and 2019. N = 280.
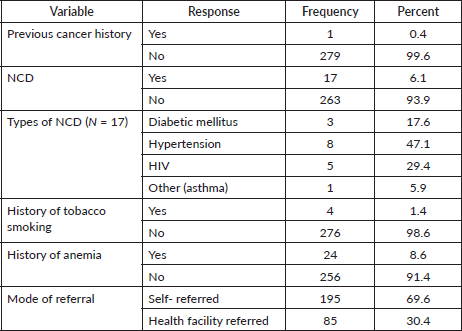
Table 4. Male and female ratios of nasopharyngeal, tongue, laryngeal and other HNCs between 2013 and 2019 at HUCSH.
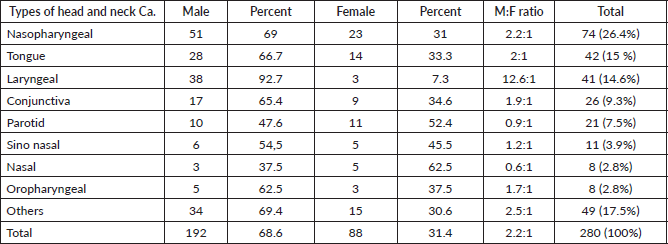
Table 5. Characteristics, stage and histological of NPC among study participants N = 74.
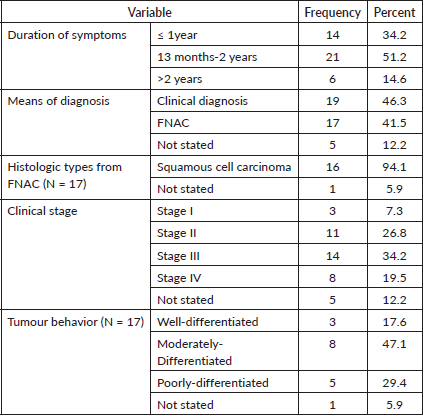
The average time between the onset of symptoms and the laryngeal cancer diagnosis was 15 months. The majority of laryngeal cancer cases [19] (46.3%) were identified clinically by computerised tomography (CT) scan. Squamous cell carcinoma was the most common histologic type, accounting for 16 (94.1%) of all FNAC diagnosed cases. More than half of the laryngeal cancer patients (22 or 53.7%) had advanced cancer (Stage III and IV), and more than a quarter of the cases (11 or 26.8%) had stage II (Table 7).
Treatment
Because radiotherapy is not offered at HUCSH, early-stage NPC cases (stage I and II) were referred to Tikur Anbessa Specialised Hospital (TASH) for concomitant chemo radiotherapy.
Patients with locally advanced NPC (stage III) received six rounds of neoadjuvant chemotherapy consisting of cisplatin 70 mg/m2 IV on day 1 and gemcitabine 1,000 mg/m2 IV on days 1 and 8 every 21 days for six cycles. These patients were then referred for radiotherapy to TASH for radiotherapy. Stage IVNPC patients were treated with systemic chemotherapy, cisplatin 70 mg/m2 IV day 1 and Gemcitabine 1,000 mg/m2 IV day 1 and day 8 every 21 days for six cycles.
Likewise, patients with stage I and II early stage tongue and laryngeal cancer were referred to TASH for concurrent chemoradiotherapy. Patients with locally advanced tongue and laryngeal cancer (stage III) received six cycles of neoadjuvant chemotherapy consisting of cisplatin 70 mg/m2 IV day 1 and paclitaxel 175 mg/m2 IV day 1 every 21 days, followed by a referral to TASH for radiotherapy. Patients with stage IV tongue and laryngeal cancer received systemic chemotherapy, consisting of six cycles of cisplatin 70 mg/m2 IV day 1 and paclitaxel 175 mg/m2 IV day 1 every 21 days.
Trends of nasopharyngeal, tongue and laryngeal cancers from 2013 to 2019
According to this study, the number of nasopharyngeal, tongue and laryngeal cancer cases increased from 2013 to 2019 (2.9%–32.5%). In addition, the study found that the number of cases of nasopharyngeal, tongue and laryngeal cancer had increased more than a fold since 2016. For instance, 27 (9.5%) in 2016, 67 (23.9%) in 2017 and 91 (32.5%) in 2019 Figure 2.
Table 6. Characteristics, stage and histological of tongue cancer among study participants N = 42.
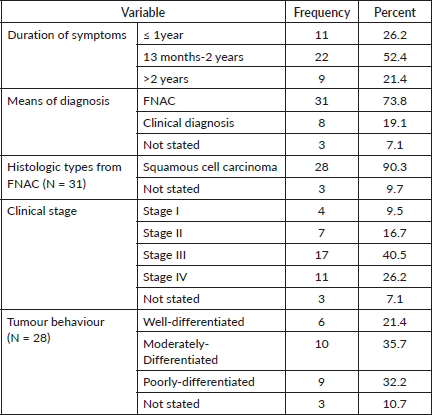
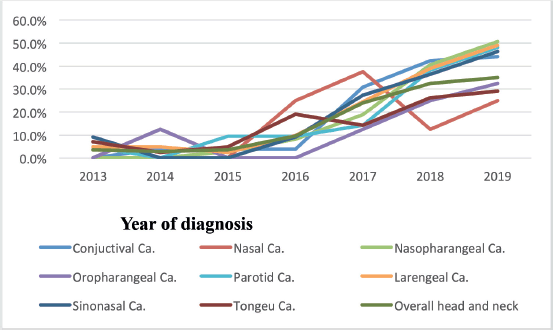
Figure 2. Trends of nasopharyngeal, tongue, laryngeal and others cancers patients at HUCSH from 2013 to 2019.
Table 7. Characteristics, stage and histological of laryngeal cancer among study participants N = 41.
Discussion
The case of nasopharyngeal, tongue and laryngeal cancer was steadily increasing from year to year, with a lot of variation (2.9%–32.5%). It is almost identical to research conducted at TASH in Addis Ababa (9.72%) [31] and in Africa [32]. The similarities in living habits and personal living standards of the study population in both Tikur Anbesa and all the African communities are probable reasons for the consistency of the findings.
NPC accounts for more than one-quarter of all HNCs (26.4%), followed by tongue (15%) and laryngeal (14.6%) malignancies. The findings of a study done in TASH were similarly congruent with this study, with a nasopharyngeal prevalence of 27% [31]. A Pakistani study conducted on the Epidemiological Review of HNCs in Karachi indicated that oral cancer cases accounted for 30% of all HNCs, followed by nasopharyngeal and laryngeal cancers in those under the age of 40 years [23] which is consistent with the recent study However, according to a study conducted in Nigeria, NPC accounts for only 12% of all HNC cases [33]. Further explanation of this difference might need a large setting study.
NPC (2.2:1), which was found common in both populations in earlier studies [34], Similarly, other studies found that the male-to-female ratio for NPC was the same (2.3–2.5:1) [3, 26]. The large number of cases and the short time of the evaluation resulted in a wide range of findings.
The majority of nasopharyngeal, tongue and laryngeal cancers were found in adults aged 20 to 40 in this study. While nasopharyngeal and tongue cancers were more likely among people over the age of twenty. NPC is more likely in those under 30 who are active and productive [21, 26].
The most prevalent cancer histology of nasopharyngeal, tongue and laryngeal cancer in this study was squamous cell carcinoma. This is in line with research conducted in Nigeria and Niger [27, 33].
Most nasopharyngeal, tongue and laryngeal cancer patients visited the hospital once their diseases had progressed to advanced stage. This outcome is consistent with research from Nigeria and South Africa [10, 11]. This may be due to the nature of the disease, which causes no discomfort until it has progressed, or it may be because the community does not have a regular annual or biannual medical exam routine or it may be a lack of cancer care services and community awareness about these cancers.
The number of nasopharyngeal, tongue and laryngeal cancer cases was rising year over year. Perhaps further research is required to determine the root cause and contributing elements. The majority of studies agreed with this conclusion [11, 14, 24, 32].
The rising rate of tongue, laryngeal and NPCs underscores serious public health issues, especially in areas like Oromia and SNNPR. For many individuals, the advanced stages of presentation and delayed diagnosis highlight the need for enhanced screening initiatives and increased public awareness. Research has demonstrated that treatment outcomes and survival rates are much enhanced by early diagnosis [35]. Furthermore, although its efficacy may vary between serum and saliva samples, the discovery of biomarkers such as Epstein-Barr virus nuclear antigen-1 (EBNA1) in patients with NPC offers interesting avenues for monitoring therapy efficacy [36].
This disparity implies that even while EBNA1 might be a useful tool in clinical settings, more investigation is required to maximise its use and comprehend the underlying mechanisms influencing its presence in various body fluids. Furthermore, the correlation between HNCs and alcohol, tobacco use and HPV suggests that public health interventions aimed at these risk factors may play a critical role in lowering incidence rates [37, 38]. Therefore, the management and prognosis of head and neck malignancies may be greatly impacted by comprehensive methods that integrate biomarker utilisation, early detection and preventive interventions.
Due to a shortage of data, we were unable to report on the effectiveness of the treatment and illness progression in this study. This study also lacks genetic test results as a result of a lack of genetic test results.
Conclusion
The study found that the burden of morbidity related to nasopharyngeal, tongue and laryngeal cancer is increasing. The distributions of nasopharyngeal, tongue and laryngeal cancer were frighteningly surging in both sexes and throughout all age categories, increasing from year to year by place, person and time. Therefore, quick reactions are crucial everywhere to track the disease’s rapid spread. A crucial step in monitoring progress is the establishment of a population-based and institutional cancer registry, and expanding cancer centers and awareness creation activities throughout the country.
List of abbreviations
AJCC, American Joint Committee on Cancer; FNAC, Fine needle aspiration cytology; HNC, Head and neck cancer; HPV, Human papilloma virus; HUCSH, Hawassa University Comprehensive Specialised Hospital; SNNPR, Southern Nation Nationalities People and Regional State; SPSS, Statistical Package for the Social Science; WHO, World Health Organisation.
Acknowledgment
The authors would like to express our heartfelt thanks to Hawassa University College of Health Science, School of Public Health for allowing the conduct of this study. Our deepest gratitude also extends to data collectors and record office staff for their immense support during data collection time.
Conflicts of interests
The authors declare that they have no competing interests.
Funding
The authors received no specific funding for this study.
Consent to publish
Not applicable.
Ethical consideration
Approval of this study was given by the research and the ethics committee of the School of Public Health and the Institutional Review Board of the College of Medical and Health Sciences at Hawassa University with an official letter of the reference number IRB/027/11. Permission to undertake this study was also obtained from the Hawassa University comprehensive specialised referral hospital administrative director then an official letter was sent to the medical record department from the chief clinical service officer of the hospital. Since then, all data has been derived from secondary sources, such as medical records, with no direct interaction with patients. To protect the privacy and confidentiality of sensitive information, each participant’s name was replaced with a code.
Availability of data and materials
The datasets used/or analysed during the current study is available from the corresponding author upon reasonable request.
Author contributions
AG, NB, DG, NM and DD participated in planning the study, writing a proposal, monitoring the data collection process and analysing the data, and writing the result and the manuscript. All authors agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
References
1. Fitzmaurice C, Allen C, and Barber RM, et al (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study JAMA Oncol 3(4) 524–548 https://doi.org/10.1001/jamaoncol.2016.5688
2. Fitzmaurice C, Abate D, and Abbasi N, et al (2019) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study JAMA Oncol 5(12) 1749–1768 https://doi.org/10.1001/jamaoncol.2019.2996 PMID: 31560378 PMCID: 6777271
3. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Int J Cancer 136(5) E359–E386 https://doi.org/10.1002/ijc.29210
4. Ferlay J, Colombet M, and Soerjomataram I, et al (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods Int J Cancer 144(8) 1941–1953 https://doi.org/10.1002/ijc.31937
5. Kamangar F, Dores GM, and William FA (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world J Clin Oncol 24(14) 2137–2150 https://doi.org/10.1200/JCO.2005.05.2308 PMID: 16682732
6. Mourad M, Jetmore T, and Jategaonkar AA, et al (2017) Epidemiological trends of head and neck cancer in the United States: a SEER population study J Oral Maxillofac Surg 75(12) 2562–2572 https://doi.org/10.1016/j.joms.2017.05.008 PMID: 28618252 PMCID: 6053274
7. Guo K, Xiao W, and Chen X, et al (2021) Epidemiological trends of head and neck cancer: a population-based study BioMed Res Int 2021 1738932 PMID: 34337000 PMCID: 8294963
8. Cohen N, Fedewa S, and Chen AY (2018) Epidemiology and demographics of the head and neck cancer population Oral Maxillofac Surg Clin 30(4) 381–395 https://doi.org/10.1016/j.coms.2018.06.001
9. Jakobsen KK, Grønhøj C, and Jensen DH, et al (2018) Increasing incidence and survival of head and neck cancers in Denmark: a nation-wide study from 1980 to 2014 Acta Oncol 57(9) 1143–1151 https://doi.org/10.1080/0284186X.2018.1438657 PMID: 29447088
10. da Lilly-Tariah OB, Somefun AO, and Adeyemo WL (2009) Current evidence on the burden of head and neck cancers in Nigeria Head Neck Oncol 1(1) 18 https://doi.org/10.1186/1758-3284-1-14
11. Naidoo K, Simonds H, and Ebrahim AK, et al (2020) A descriptive epidemiological study of head and neck cancers at a Major Referral Center in Southern Africa Authorea [Preprints]
12. Siddiqui MS, Chandra R, and Aziz A, et al (2012) Epidemiology and histopathological spectrum of head and neck cancers in Bihar, a state of Eastern India Asian Pac J Cancer Prev 13(8) 3949–3953 https://doi.org/10.7314/APJCP.2012.13.8.3949 PMID: 23098498
13. Onyango J, Awange D, and Njiru A, et al (2006) Pattern of occurrence of head and neck cancer presenting at Kenyatta National Hospital, Nairobi East Afr Med J 83(5) 288 PMID: 16866224
14. Nocini R, Molteni G, and Mattiuzzi C, et al (2020) Updates on larynx cancer epidemiology Chin J Cancer Res 32(1) 18–25 https://doi.org/10.21147/j.issn.1000-9604.2020.01.03 PMID: 32194301 PMCID: 7072014
15. Tataru D, Mak V, and Simo R, et al (2017) Trends in the epidemiology of head and neck cancer in London Clin Otolaryngol 42(1) 104–114 https://doi.org/10.1111/coa.12673
16. Tangjaturonrasme N, Vatanasapt P, and Bychkov A (2018) Epidemiology of head and neck cancer in Thailand Asia Pac J Clin Oncol 14(1) 16–22 https://doi.org/10.1111/ajco.12757
17. Boscolo-Rizzo P, Zorzi M, and Del Mistro A, et al (2018) The evolution of the epidemiological landscape of head and neck cancer in Italy: is there evidence for an increase in the incidence of potentially HPV-related carcinomas? PLoS One 13(2) e0192621 https://doi.org/10.1371/journal.pone.0192621 PMID: 29415020 PMCID: 5802923
18. Semprini J, Pagedar NA, and Boakye EA, et al (2024) Head and neck cancer incidence in the United States before and during the COVID-19 pandemic JAMA Otolaryngol Head Neck Surg 150(3) 193–200 https://doi.org/10.1001/jamaoto.2023.4322 PMID: 38206603 PMCID: 10784997
19. Joshi P, Dutta S, and Chaturvedi P, et al (2014) Head and neck cancers in developing countries Rambam Maimonides Med J 5(2) e0009 https://doi.org/10.5041/RMMJ.10143 PMID: 24808947 PMCID: 4011474
20. Barsouk A, Aluru JS, and Rawla P, et al (2023) Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma Med Sci (Basel, Switzerland) 11(2) 42 https://doi.org/10.3390/medsci11020042
21. Larsen-Reindorf R, Owusu-Afriyie O, and Acheampong AO, et al (2014) A six-year review of head and neck cancers at the Komfo Anokye Teaching Hospital, Kumasi, Ghana Int J Otolaryngol Head Neck Surg 3(05) 271 https://doi.org/10.4236/ijohns.2014.35050
22. Komeela N, Hannah S, and Abdul-Kader E, et al (2020) A descriptive epidemiological study of head and neck cancers at a Major Referral Center in Southern Africa Authorea https://doi.org/10.22541/au.158921627.77274854
23. Bhurgri Y, Bhurgri A, and Usman A, et al (2006) Epidemiological review of head and neck cancers in Karachi Asian Pac J Cancer Prev 7(2) 195–200 PMID: 16839210
24. Braakhuis BJ, Leemans CR, and Visser O (2014) Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011 Oral Oncol 50(7) 670–675 https://doi.org/10.1016/j.oraloncology.2014.03.008] PMID: 24735546
25. Duncan K, Cira MK, and Barango P, et al (2019) Challenges and opportunities in the creation and implementation of cancer-control plans in Africa ecancermedicalscience 13 938 https://doi.org/10.3332/ecancer.2019.938 PMID: 31552111 PMCID: 6722107
26. Beyene ET, Ketema SG, and Alebachew AN, et al (2021) Descriptive epidemiology of nasopharyngeal carcinoma at Tikur Anbessa Hospital, Ethiopia BMC Cancer 21(1) 1–5 https://doi.org/10.1186/s12885-021-08311-8
27. Garba SM, Hami H, and Zaki HM, et al (2020) 283P descriptive epidemiology of head and neck cancer in Niger: first results from the National Cancer Registry Ann Oncol 31 S1352 https://doi.org/10.1016/j.annonc.2020.10.277
28. Fekadu A, Rick TJ, and Tigeneh W, et al (2022) Clinicopathology and treatment patterns of head and neck cancers in Ethiopia JCO Glob Oncol 8 e2200073 https://doi.org/10.1200/GO.22.00073 PMID: 35939776 PMCID: 9470133
29. Arigawu AS and Enqusilase F (2015) Factors influencing the completion of head and neck cancer treatment: a case study of Tikur Anbessa Referral Hospital, Addis Ababa, Ethiopia J Sci Sustain Dev 3(2) 57–72 https://doi.org/10.20372/au.jssd.3.2.2015.048
30. Gebretsadik A, Bogale N, and Negera DG (2021) Epidemiological trends of breast cancer in Southern Ethiopia: a seven-year retrospective review Cancer Control 28 10732748211055262 https://doi.org/10.1177/10732748211055262 PMID: 34931549 PMCID: 8728771
31. Tefera AT, Bekele BG, and Dejene D, et al (2019) The epidemiology of primary head and neck cancer in Black Lion Specialized Hospital Oncology Center, Ethiopia: a hospital based retrospective study Res Sq https://doi.org/10.21203/rs.2.16439/v1
32. Adeola HA, Afrogheh AH, and Hille JJ (2018) The burden of head and neck cancer in Africa: the status quo and research prospects S Afr Dent J 73(8) 477–488 https://doi.org/10.17159/2519-0105/2018/v73no8a1
33. Kodiya AM, Adamu AI, and Nggada HA, et al (2015) Epidemiology of head and neck cancers in Maiduguri-Northeastern Nigeria J Adv Med Med Res 11(5) 1–7 https://doi.org/10.9734/BJMMR/2016/20344
34. Ngwa W, Addai BW, and Adewole I, et al (2022) Cancer in sub-Saharan Africa: a Lancet Oncology Commission Lancet Oncol 23(6) e251–e312 https://doi.org/10.1016/S1470-2045(21)00720-8 PMID: 35550267 PMCID: 9393090
35. Wu L, Li C, and Pan C (2018) Nasopharyngeal carcinoma: a review of current updates Exp Ther Med 15 3687–3692 https://doi.org/10.3892/etm.2018.5878 PMID: 29556258 PMCID: 5844099
36. Midoen YH, Suryandari DA, and Yunaini L, et al (2021) Epstein-Barr virus nuclear antigen-1 is useful as therapeutic efficacy marker in serum but not in saliva of nasopharyngeal cancer patients who underwent radiotherapy ecancermedicalscience 15 1254 https://doi.org/10.3332/ecancer.2021.1254 PMID: 34267810 PMCID: 8241448
37. Kreimer AR, Clifford GM, and Boyle P, et al (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review Cancer Epidemiol Biomarkers Prev 14(2) 467–475 https://doi.org/10.1158/1055-9965.EPI-04-0551 PMID: 15734974
38. Shield KD, Ferlay J, and Jemal A, et al (2017) The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012 Cancer J Clin 67(1) 51–64 https://doi.org/10.3322/caac.21384