Prevalence of bacterial vaginosis, sexually transmitted infections and their association with HPV infections in asymptomatic women attending antenatal care in Ethiopia
Johanna M. A. Klein1,2, Isabel Runge1,2, Ann-Katrin Pannen1,2, Tariku Wakuma2,3, Semaw Ferede Abera4,5,6, Adamu Adissie2,7, Susanne Unverzagt8, Markus Schmitt9, Tim Waterboer9, Daniela Höfler9, Christoph Thomssen1,2 and Eva Johanna Kantelhardt1,2
1Department of Gynaecology, Martin Luther University Halle-Wittenberg, Halle (Saale) 06097, Germany
2Global Health Working Group, Martin Luther University Halle-Wittenberg, Halle (Saale) 06097, Germany
3Aira Hospital, Aira, Ethiopia
4Department of Radiation Oncology, Faculty of Medicine, Martin Luther University Halle-Wittenberg, Halle (Saale) 06097, Germany
5School of Public Health, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
6Kilte Awlaelo - Health and Demographic Surveillance Site, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
7School of Public Health, College of Health Sciences, Addis Ababa University, Addis Ababa 1000, Ethiopia
8Institute of General Practice and Family Medicine, Center of Health Sciences, Martin Luther University Halle-Wittenberg, Halle (Saale) 06097, Germany
9Infections and Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg 69120, Germany
Abstract
Sexually transmitted infections (STIs) and human papillomavirus (HPV) infections are common among women of reproductive age and can lead to infertility, adverse pregnancy outcomes, neonatal infections and cervical cancer. In countries with limited medical coverage, untreated infections contribute to high morbidity. This study aimed to expand the current knowledge on the prevalence of bacterial vaginosis (BV) and STIs in pregnant Ethiopian women and assess the association of these conditions with HPV infections. Socio-demographic data and vaginal lavage samples were collected from 779 asymptomatic women aged 18 to 45 years (median age, 25.9 years) attending antenatal care in seven centres across Ethiopia. Multiplex polymerase chain reaction was used to test for BV, Chlamydia trachomatis, Trichomonas vaginalis, Neisseria gonorrhoeae, herpes simplex virus types 1 and 2 (HSV-1/2), Mycoplasma, Ureaplasma, Candida species and HPV. Overall, 26.8% (95% confidence interval (CI): 23.7–29.9) of women tested positive for BV or one of the following STIs: C. trachomatis, T. vaginalis, N. gonorrhoeae, Mycoplasma genitalium, HSV-1/2 or Ureaplasma urealyticum. Additionally, 22.1% tested positive for at least one high-risk HPV type. Chlamydia trachomatis and HSV-2 were significantly more common among women who were positive for HPV and high-risk HPV. This study reveals a high prevalence of asymptomatic pregnant women who are positive for BV, STIs or HPV, putting them at risk of adverse pregnancy outcomes, secondary infertility or cervical cancer in a country with limited medical coverage. Screening and treating these women could be crucial in reducing morbidity.
Keywords: sexually transmitted diseases, bacterial vaginosis, Ethiopia, pregnancy, vaginal lavage, human papillomavirus
Correspondence to: Eva Johanna Kantelhardt
Email: eva.kantelhardt@uk-halle.de
Published: 30/09/2024
Received: 05/03/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Bacterial vaginosis (BV) and sexually transmitted infections (STIs) are common among women of reproductive age. The association between BV and adverse pregnancy outcomes is a subject of ongoing debate [1]. Infections with Chlamydia trachomatis, Neisseria gonorrhoeae, Treponema pallidum, herpes simplex virus type 2 (HSV-2) and Ureaplasma urealyticum are strongly linked to adverse pregnancy outcomes, including miscarriages, premature rupture of membranes, preterm labour and delivery, neonatal infections, low birth weight, puerperal endomyometritis and secondary infertility [2–8]. Delayed diagnosis and treatment, especially in countries with limited medical resources, can lead to serious complications.
BV is characterised by a reduction in lactobacilli and an overgrowth of anaerobic bacteria such as Gardnerella vaginalis, Atopobium vaginae and Mycoplasma hominis, and it is a known risk factor for preterm labour [1]. While these bacteria may not be highly pathogenic on their own, they may facilitate infection by more pathogenic bacteria because the protective role of the physiological lactobacilli vaginal flora is compromised in BV. Consequently, it has been suggested that BV may also facilitate infections with high-risk human papillomavirus (HPV) [9]. High-risk HPV is a necessary, but not sufficient, cause of cervical cancer; however, most infections are transient and cause no or only mild cervical changes [10]. Associations between infections with C. trachomatis or HSV-2 and the development of HPV or cervical cancer suggest that these infections may act as cofactors in the development of cervical cancer and precancerous lesions [11–13].
Screening and treating pregnant women is challenging but crucial. Many women are asymptomatic and, therefore, remain untreated, resulting in serious medical complications for both mother and child. Early detection and treatment of BV and STIs may prevent preterm labour and vertical transmission of infections, while in women with high-risk HPV, it can reduce morbidity and mortality due to cervical cancer [12, 14].
A feasible screening method for BV and STIs in low-resource countries is STI profiling (STIP). STIP is a multiplex polymerase chain reaction (PCR) technique that can detect multiple infectious agents simultaneously, requiring only a single vaginal or cervical swab or a lavage sample for testing [15].
Limited data are available on BV, STIs and HPV infection in Ethiopia. The aim of this study was to expand the current knowledge on the prevalence of BV and STIs among asymptomatic young pregnant women in Ethiopia and to evaluate the association of these conditions with HPV infections. Such information will help estimate the number of women at risk for adverse pregnancy outcomes.
Methods
Study population
In 2013 and 2014, a cross-sectional study was conducted to test asymptomatic pregnant women for STIs and HPV across seven centres in Ethiopia [16]. To ensure comprehensive data collection, samples were obtained from antenatal care and family planning units at university hospitals, state and private hospitals, and regional health centres throughout the country.
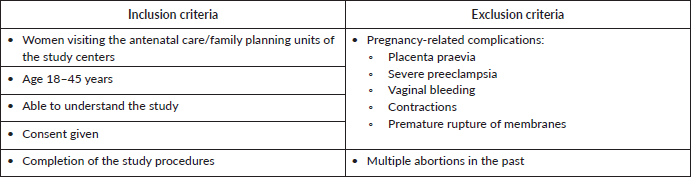
Women aged 18 to 45 years were eligible to participate if they had no signs of placenta praevia, severe pre-eclampsia or other pregnancy-related complications. Women visiting the facility because of vaginal bleeding or discharge were also excluded, although some women showed signs of discharge upon examination (Supplementary Table 1). Of the 1,239 women attending antenatal and family planning units on the enrolment days, 1,041 agreed to participate and completed all study procedures. Due to missing samples and invalid analyses, 262 samples were excluded from the final analysis, leaving 779 valid samples from antenatal care (n = 747) and family planning (n = 32). Women from seven geographical areas were included in the study: Addis Ababa (n = 209), Mekelle (n = 74), Bahir Dar (n = 215), Harar (n = 29), Ginir (n = 38), Wukro (n = 65) and Aira (n = 149).
Patient involvement
The patients were involved in the design and conduct of this research. During the feasibility stage, the research question, choice of outcome measures and recruitment methods were informed by discussions with the patients through a rapid ethnographic assessment session conducted by the research team.
Sample size
To detect a minimum 10% difference between the proportion of STI-positive women in rural areas (assumed 14% prevalence and 20% of participants) and urban areas (assumed 24% prevalence and 80% of participants), 845 women were required for analysis (845 total = 169 rural + 676 urban), with an alpha of 5% and a power of 80%, using the chi-squared test. The ratio of rural to urban participants was derived from a preliminary inquiry at the sites.
Questionnaire
To obtain socio-demographic and reproductive data, local nurses administered a questionnaire that had been previously used in other studies [17]. The questionnaire was slightly modified to suit the study population. It was translated into Amharic, Oromo and Tigrinya and validated by retranslation conducted by native speakers to ensure accuracy and avoid any loss or misinterpretation of information.
Sample collection
Samples were collected during routine antenatal care and family planning visits. Local nurses provided a brief introduction to STIs, their transmission and their symptoms. The study procedures were explained, informed consent was obtained and codes were assigned to ensure confidentiality.
Vaginal samples were collected using a syringe-like self-sampling device (Delphi Bioscience, Scherpenzeel, Netherlands). The device was inserted into the vagina, releasing 3 mL of buffered saline by plunging the handle. The fluid was then retracted back into the device by releasing the handle. The lavage fluid was transferred into transportation tubes containing 3 mL of buffered methanol solution, where it was preserved for up to 3 months at room temperature (20°C–30°C). Medical staff assisted with the sample collection. The samples were analysed at the German Cancer Research Centre (DKFZ) in Heidelberg, Germany.
HPV DNA testing
For the detection of HPV DNA in the vaginal lavage samples, a multiplex papillomavirus genotyping assay was performed as previously described [18]. This method analyses 51 HPV types, of which 14 are considered high-risk: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68a/b [19]. Results were expressed as median fluorescence intensity values. Samples were considered positive if they contained β-globin as a human DNA marker and/or HPV DNA.
STIP
STIP, is a validated multiplex PCR that simultaneously analyses Atopobium vaginae, Candida albicans, Candida glabrata, Candida krusei, Chlamydia trachomatis, Gardnerella vaginalis, HSV-1/2, Mycoplasma genitalium, Mycoplasma hominis, Mycoplasma pneumoniae, Mycoplasma spermatophilum, Neisseria gonorrhoeae, Treponema pallidum, Trichomonas vaginalis, Ureaplasma urealyticum, Ureaplasma parvum and Lactobacillus iners [15]. All other Lactobacillus and Candida species were detected using universal probes. To confirm the presence of human DNA, the PolA sequence was analysed. Samples positive for STIs and/or PolA were considered valid. Results are reported in median fluorescence intensity values.
A score for BV (BV-score) was calculated as previously described, based on the ratios of G. vaginalis and A. vaginae to Lactobacillus [15]. The presence of Mycoplasma hominis was also taken into account.
Statistical analysis
All descriptive and statistical analyses were conducted using SPSS software version 23 (IBM Corp., Armonk, NY, USA). The prevalences of STIs and HPV, along with the corresponding 95% CIs, were calculated. Fisher’s exact test was used to determine statistical significance, with p-values of <0.05 considered statistically significant.
Ethical clearance
Ethical clearance was obtained from Addis Ababa University and Martin Luther University Halle in February 2011 and April 2013, respectively.
Results
Study population
The participants ranged in age from 18 to 45 years, with a mean age of 25.9 years. All major ethnic and religious groups were represented. Table 1 provides an overview of the demographic and reproductive characteristics of the participants.
One fifth of the women had never lived in a city or town and were classified as rural for this study. The remaining women had lived in urban areas or under urban influence and were therefore classified as urban.
More than half of the women (59.7%) identified as housewives, while 15.2% were government employees and 13.2% were merchants. The remaining women were students, employed in other professions or unemployed.
Of the 747 pregnant women, 10.6% were in the first trimester, 35.3% in the second trimester and 54.1% in the third trimester of pregnancy. Most women (94.9%) were married, and 13.6% reported that their husbands had up to three other wives (polygamy). Before becoming pregnant, many women had used hormonal contraceptive methods (70.6%), but condom use was low (1.5%).
One quarter of the women had previously given birth to between one and eight children. Up to four past spontaneous abortions were reported by 18.5% of the women, while 7.8% had undergone up to three induced abortions. No data on the number of sexual partners were obtained to avoid the risk of response bias due to cultural and confidentiality reasons.
Prevalence of STIs and HPV
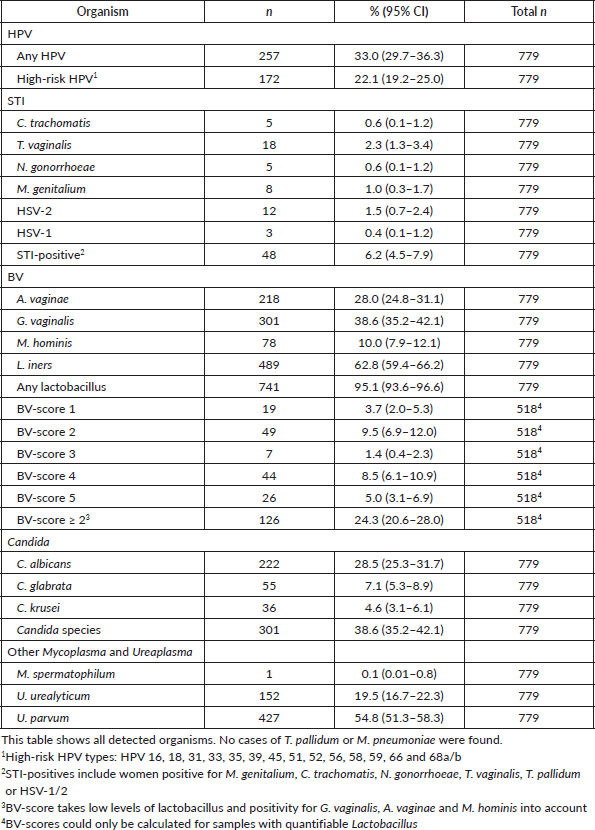
One third of the women (33.0%) tested positive for at least one mucosal HPV type, and 22.1% were positive for at least one high-risk HPV type. Table 2 presents the prevalence of STIs, organisms of the vaginal flora and HPV.
Infections with C. trachomatis, T. vaginalis, N. gonorrhoeae, M. genitalium and HSV-1/2 were categorised as STIs. The overall prevalence of these STIs in this population was low.
Only a small number of women tested positive for cervical infections with C. trachomatis (0.6%) and N. gonorrhoeae (0.6%). Infections with T. vaginalis (2.3%) and HSV-2 (1.5%) were detected more frequently but remained relatively low. A total of 6.2% of the women were diagnosed with at least one STI. However, only three women had two concurrent STIs: one woman had C. trachomatis and T. vaginalis, one had C. trachomatis and M. genitalium, and one had M. genitalium and HSV-2. No woman was positive for more than two STIs.
Among the 145 women who tested positive for BV, 126 had a BV-score of ≥2, indicating an unbalanced vaginal flora.
In total, 209 (26.8%) women were positive for BV (score of ≥2), any STI or U. urealyticum.
Women from rural backgrounds were more likely to have a BV-score of >2 (36.5% [95% CI: 26.2–46.7]) than women from urban backgrounds (22.0% [95% CI: 18.1–25.9]). No differences were found in the prevalence of STIs between rural and urban areas. Additionally, no significant differences were observed in the BV or STI prevalence based on age, trimester of pregnancy, educational level, age at sexual debut or circumcision status.
Table 1. Socio-demographic and reproductive data of the study population.
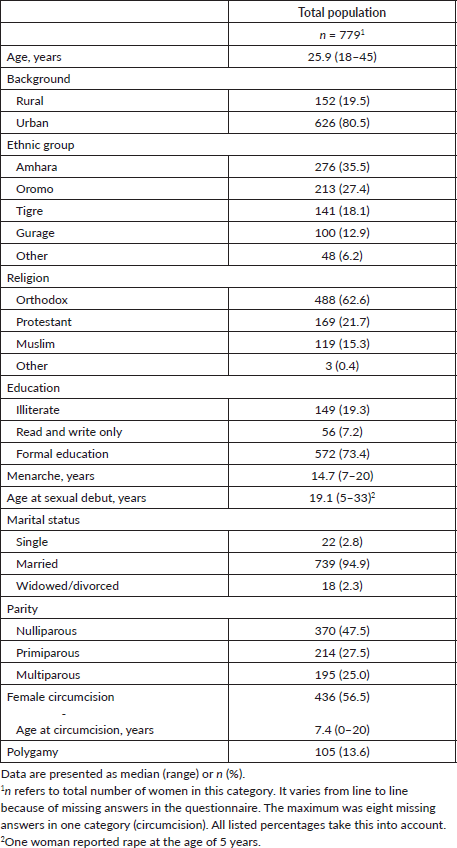
Table 2. Prevalence of STIs and HPV.
Correlation between STIs and HPV
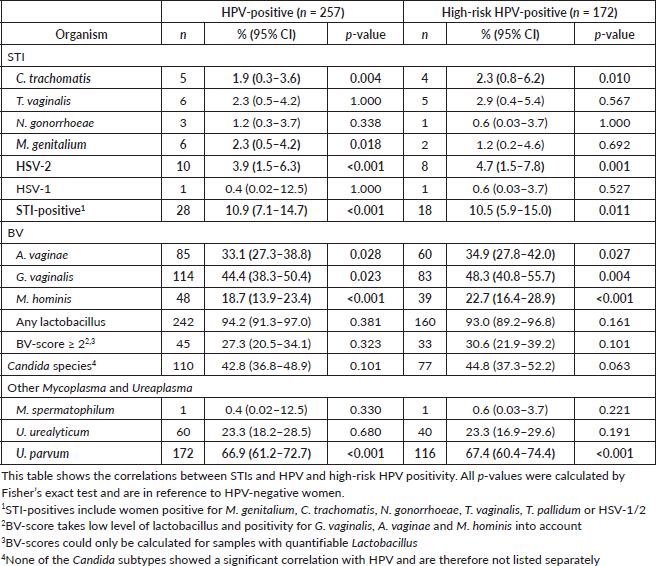
The observed correlations between STIs and HPV are shown in Table 3.
Chlamydia trachomatis and HSV-2 were significantly more common among women who tested positive for HPV and high-risk HPV, while M. genitalium was more frequent in women with HPV infections but not in those with high-risk HPV infections.
Although all three bacteria associated with BV (A. vaginae, G. vaginalis and M. hominis) were significantly associated with both HPV and high-risk HPV, having a positive BV-score (BV-score ≥ 2) was not associated with these infections. Candidiasis was also not associated with HPV. Among other bacteria, only U. parvum was found to be associated with HPV infection.
Table 3. Correlations between HPV and STI.
Discussion
Summary
This is the first multicentre survey to provide data on a broad spectrum of STIs in pregnant Ethiopian women attending antenatal care. This study included only women who considered themselves asymptomatic and were therefore not included in the syndromic approach to STI treatment, as recommended by the Ethiopian national plan. Among these asymptomatic women, 26.8% tested positive for BV, STIs or U. urealyticum, and 33.0% tested positive for HPV.
Background
According to Molla et al. [20], health-seeking behaviour regarding STIs is poor among young Ethiopians because of a lack of knowledge about STIs and the stigma associated with premarital sexual activities. Poor medical coverage and limited knowledge of treatment options among physicians further complicate the situation [21]. Apart from HIV and haemoglobin testing, no routine tests are performed on pregnant women in Ethiopia.
BV
BV during pregnancy can lead to premature rupture of membranes and preterm labour but is often asymptomatic, remaining undetected and untreated [1]. In this study, 24.3% of asymptomatic women tested positive for BV, which is consistent with results from different regions in Ethiopia showing BV prevalences of 19.4%–29.4% [22–25]. Although the benefits of treatment during pregnancy are debated, there is evidence supporting early treatment [26]. Unexpectedly, rural women were more likely to test positive for BV. The influence of hygienic conditions and nutritional factors should be explored in these settings.
HPV
High-risk HPV is a necessary but not sufficient cause of cervical cancer [27]. HPV can be sexually transmitted, and although most people will be infected at some point in their lives, the majority of infections are transient and resolve within months [10]. Young and sexually active individuals are particularly at risk. In this study, 33.0% of the population tested positive for any HPV and 22.1% tested positive for high-risk HPV. These findings are consistent with other studies that reported a 33.6% prevalence of HPV in East Africa and Ethiopia [28, 29].
Chlamydia trachomatis and N. gonorrhoeae
Cervical infections with C. trachomatis and N. gonorrhoeae during pregnancy may lead to miscarriages, preterm labour, neonatal infections, postpartum upper genital tract infection and secondary infertility [2–5, 30]. Adequate antimicrobial therapy can prevent these outcomes [30]. In this study, only 0.6% of participants tested positive for C. trachomatis and N. gonorrhoeae. Studies from around the world have shown higher prevalence rates in the female adult population, ranging from 5.0% to 14.7% for C. trachomatis and 0.4% to 6.7% for N. gonorrhoeae, with prevalences of 7.8% to 9.8% for C. trachomatis and 2.4%–4.3% for N. gonorrhoeae in Eastern Africa and Ethiopia [5, 31, 32, 33–35]. Our asymptomatic, fertile and married population might be expected to have a lower prevalence of cervical STIs in general. However, the high prevalence of other vaginal STIs and HPV suggests that the low detection of cervical infections may have been due to limitations in the detection method used, rather than reflecting the actual prevalence.
Other vaginal infections
The pregnant women in this study also demonstrated various types of vaginal infections, such as T. vaginalis (2.3%) and U. urealyticum (19.5%), which are suspected to cause preterm labour and placental inflammation [14, 36, 8]. However, treatment appears to be beneficial only for U. urealyticum infections, not for trichomoniasis [37, 38]. The prevalence of M. genitalium infections (1.0%) in this population is consistent with other studies [31].
Primary infections with HSV-2 during pregnancy are known to cause severe neonatal infections and continue to be a concern [39]. In this study, 1.5% of the women had active HSV-2 infections, although it is unclear whether these were primary or recurrent infections. Fortunately, no cases of active syphilis were detected in this population.
Candida infections are common among pregnant women and were detected in 38.6% of women in this study. While the impact of Candida infections on pregnancy outcomes is debated, the morbidity associated with recurring Candida infections is undisputed [40].
Correlations of BV and STIs with HPV
Studies have suggested a possible correlation between HPV and BV, which was not observed in this study, although G. vaginalis, A. vaginae and M. hominis were significantly associated with both HPV and high-risk HPV [9]. A significant correlation was found between HPV and C. trachomatis and HSV-2, as noted in previous studies [11, 41]. While this correlation could be partly explained by the number of sexual partners, the lack of association between HPV and other STIs makes this an unlikely sole explanation.
In this study, U. parvum was associated with HPV (odds ratio, 2.12 [1.55–2.89]). This finding has not been reported in the literature, and further studies are needed to determine whether this is a coincidence.
Strengths and limitations
The main strength of this study lies in its large sample size of pregnant women from seven urban and rural centres across Ethiopia, capturing the country’s diversity. Additionally, a broad spectrum of bacterial, viral and parasitic STIs was detected using a single sample analysed by PCR, an effective detection method. This study provides a comprehensive picture of the prevalence of BV, STIs and HPV in this pregnant population.
However, this study has certain limitations. The main limitation is the lack of information on recent antibiotic use, which may have reduced the prevalence of vaginal infections. Because we described a routine cohort, we assume this represents a normal population, including a proportion of women who have recently used antibiotics, which is common in the Ethiopian context. Another limitation is the disproportionate overrepresentation of urban women due to the location of the included health centres and possible misperception of the term ‘urban’ by participants from villages.
Although the vaginal lavage and STIP methods are validated, the combination has not been previously used in pregnant women. We were unable to compare this method with routine clinical tests for STIs in the Ethiopian setting. It is possible that due to technical issues, the lavage may not have reached the cervix as intended, potentially leading to an underestimation of cervical infections such as C. trachomatis and N. gonorrhoeae. Further studies should evaluate this combined method. Additionally, no data on pregnancy outcomes in this population could be obtained because of organisational limitations.
Conclusion
This study has demonstrated that many asymptomatic pregnant women aged 18 to 45 years have BV or curable STIs, putting them at risk for adverse pregnancy outcomes and secondary infertility. A relatively high proportion of women with potentially pathogenic infections was detected. In settings with poor medical coverage, preterm labour or infection of the unborn child can result in high morbidity or even mortality. Screening and treatment for BV and STIs may prevent these adverse outcomes.
As previous studies have shown, knowledge about STIs in Ethiopia is low [20]. Efforts should be made to educate young people about the transmission, symptoms, consequences and prevention of STIs. Adolescents should be educated at a younger age, preferably before sexual debut. Antenatal care presents a valuable opportunity to educate, screen and treat women for STIs and HPV and should be included in antenatal care protocols.
The self-sampling method is well accepted and could be suitable for low-resource countries. However, the multiplex methodology requires a well-equipped laboratory with expensive technology and specialised laboratory technicians.
Finding adequate screening methods and funding remains a challenge, but it is essential to protect pregnant women and their unborn children.
Conflicts of interest
The authors declare no conflict of interest.
Funding
This work was supported by a grant from the Else Kröner Foundation (EJK, 2018_HA31SP) and by a grant from the German Ministry for Research and Education through the RHISSA programme to Martin Luther University (01KA2220B). Additional funding was provided by the ESTHER partnership between Martin Luther University Halle, Germany and Addis Ababa University, Ethiopia, supported by the German Ministry for Economic and Development Cooperation (BMZ) through the ESTHER University and Hospital Partnership Initiative of German International Cooperation (GIZ).
Ethical approval
Ethical clearance was obtained from Addis Ababa University, Ethiopia, in February 2011 (124/10/IM;032/2011) and April 2013 (050/2013), and from Martin Luther University Halle-Wittenberg, Germany, on 23 August 2010.
Informed consent
Verbal informed consent for publication was obtained from the patients, as suggested by the ethics committee due to low literacy rates and since a written consent is only provided concerning marriage or business contracts.
Authors’ contributions
IR, JMAK, AA and EJK designed the research. JMAK, IR, DHoe, AA, SU, CT and EJK analysed the data. IR, JMAK, DHoe and EJK drafted the manuscript. JMAK, IR, AKP, TW, SFA, AA, MS, TW and DH provided the original samples, laboratory results, information on the respective populations and advice on study design, analysis and interpretation of the findings. All authors provided critical interpretation of the results and reviewed the first draft. All authors read and approved the final manuscript.
Availability of data and materials
Data are available on reasonable request and can be requested by email.
References
1. Juliana NCA, Suiters MJM, and Al-Nasiry S, et al (2020) The association between vaginal microbiota dysbiosis, bacterial vaginosis, and aerobic vaginitis, and adverse pregnancy outcomes of women living in Sub-Saharan Africa: a systematic review Front Public Health 8 567885 https://doi.org/10.3389/fpubh.2020.567885 PMID: 33363078 PMCID: 7758254
2. den Heijer CDJ, Hoebe CJPA, and Driessen JHM, et al (2019) Chlamydia trachomatis and the risk of pelvic inflammatory disease, ectopic pregnancy, and female infertility: a retrospective cohort study among primary care patients Clin Infect Dis 69(9) 1517–1525 https://doi.org/10.1093/cid/ciz429 PMCID: 6792126
3. Olson-Chen C, Balaram K, and Hackney DN (2018) Chlamydia trachomatis and adverse pregnancy outcomes: meta-analysis of patients with and without infection Matern Child Health J 22(6) 812–821 https://doi.org/10.1007/s10995-018-2451-z PMID: 29417367
4. Tang W, Mao J, and Li KT, et al (2019) Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: a global systematic review and meta-analysis Sex Transm Infect 96(5) 322–329 https://doi.org/10.1136/sextrans-2019-053999 PMID: 31836678 PMCID: 7292777
5. Warr AJ, Pintye J, and Kinuthia J, et al (2019) Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study Sex Transm Infect 95(1) 60–66 https://doi.org/10.1136/sextrans-2018-053597
6. Bezerra MLdMB, Fernandes FECV, and Oliveira Nunes JP de, et al (2019) Congenital syphilis as a measure of maternal and child healthcare, Brazil Emerg Infect Dis 25(8) 1469–1476 https://doi.org/10.3201/eid2508.180298 PMID: 31310223 PMCID: 6649332
7. Shi T-L, Huang L-J, and Xiong Y-Q, et al (2018) The risk of herpes simplex virus and human cytomegalovirus infection during pregnancy upon adverse pregnancy outcomes: a meta-analysis J Clin Virol 104 48–55 https://doi.org/10.1016/j.jcv.2018.04.016 PMID: 29729547
8. Goldenberg RL, Andrews WW, and Goepfert AR, et al (2008) The alabama preterm birth study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants Am J Obstet Gynecol 198(1) 43.e1-5 https://doi.org/10.1016/j.ajog.2007.07.033 PMID: 18166302 PMCID: 2278008
9. Gillet E, Meys JF, and Verstraelen H, et al (2011) Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis BMC Infect Dis 11 10 https://doi.org/10.1186/1471-2334-11-10 PMID: 21223574 PMCID: 3023697
10. Ho GY, Bierman R, and Beardsley L, et al (1998) Natural history of cervicovaginal papillomavirus infection in young women N Engl J Med 338(7) 423–428 https://doi.org/10.1056/NEJM199802123380703 PMID: 9459645
11. Samoff E, Koumans EH, and Markowitz LE, et al (2005) Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents Am J Epidemiol 162(7) 668–675 https://doi.org/10.1093/aje/kwi262 PMID: 16120706
12. Naldini G, Grisci C, and Chiavarini M, et al (2019) Association between human papillomavirus and chlamydia trachomatis infection risk in women: a systematic review and meta-analysis Int J Public Health 64(6) 943–955 https://doi.org/10.1007/s00038-019-01261-w PMID: 31175391
13. Smith JS, Herrero R, and Bosetti C, et al (2002) Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer J Natl Cancer Inst 94(21) 1604–1613 https://doi.org/10.1093/jnci/94.21.1604 PMID: 12419786
14. Mullick S (2005) Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries Sex Transm Infect 81(4) 294–302 https://doi.org/10.1136/sti.2002.004077 PMID: 16061534 PMCID: 1745010
15. Schmitt M, Depuydt C, and Stalpaert M, et al (2014) Bead-based multiplex sexually transmitted infection profiling J Infect 69(2) 123–133 https://doi.org/10.1016/j.jinf.2014.04.006 PMID: 24814157
16. Runge IH (2017) Prävalenz Humaner Papillomaviren und anderer Sexuell Übertragbarer Erreger bei Schwangeren in Äthiopien [Dissertation zur Erlangung des akademischen Grades Doktor der Medizin (Dr Med)] (Halle: Martin-Luther Universität Halle-Wittenberg)
17. Dondog B, Clifford GM, and Vaccarella S, et al (2008) Human papillomavirus infection in Ulaanbaatar, Mongolia: a population-based study Cancer Epidemiol Biomarkers Prev 17(7) 1731–1738 https://doi.org/10.1158/1055-9965.EPI-07-2796 PMID: 18628425
18. Schmitt M, Dondog B, and Waterboer T, et al (2008) Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers J Clin Microbiol 46(3) 1050–1059 https://doi.org/10.1128/JCM.02227-07 PMID: 18199790 PMCID: 2268381
19. Schmitt M, Depuydt C, and Benoy I, et al (2013) Prevalence and viral load of 51 genital human papillomavirus types and three subtypes Int J Cancer 132(10) 2395–2403 https://doi.org/10.1002/ijc.27891
20. Molla M, Emmelin M, and Berhane Y, et al (2009) Readiness of youth in rural Ethiopia to seek health services for sexually transmitted infections Afr J AIDS Res 8(2) 135–146 https://doi.org/10.2989/AJAR.2009.8.2.2.854 PMID: 25875565
21. Alemayehu A and Godana W (2015) Knowledge and practice of clinicians regarding syndromic management of sexually transmitted infections in public health facilities of Gamo Gofa Zone, South Ethiopia J Sex Transm Dis 2015 310409 PMID: 26605102 PMCID: 4641938
22. Mengistie Z, Woldeamanuel Y, and Asrat D, et al (2014) Prevalence of bacterial vaginosis among pregnant women attending antenatal care in Tikur Anbessa University Hospital, Addis Ababa, Ethiopia BMC Res Notes 7 822 https://doi.org/10.1186/1756-0500-7-822 PMID: 25409756 PMCID: 4247656
23. Yalew GT, Muthupandian S, and Hagos K, et al (2022) Prevalence of bacterial vaginosis and aerobic vaginitis and their associated risk factors among pregnant women from northern Ethiopia: a cross-sectional study PLoS One 17(2) e0262692 https://doi.org/10.1371/journal.pone.0262692 PMID: 35213556 PMCID: 8880645
24. Ahmed M, Admassu Ayana D, and Abate D (2022) Bacterial vaginosis and associated factors among pregnant women attending antenatal care in Harar City, Eastern Ethiopia Infect Drug Resist 15 3077–3086 https://doi.org/10.2147/IDR.S364229 PMID: 35754781 PMCID: 9215287
25. Aklilu A, Woldemariam M, and Manilal A, et al (2024) Aerobic vaginitis, bacterial vaginosis, and vaginal candidiasis among women of reproductive age in Arba Minch, Southern Ethiopia Sci Rep 14 9813 https://doi.org/10.1038/s41598-024-58654-y PMID: 38684716 PMCID: 11059176
26. Ugwumadu A, Manyonda I, and Reid F, et al (2003) Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial Lancet 361(9362) 983–988 https://doi.org/10.1016/S0140-6736(03)12823-1 PMID: 12660054
27. Bosch FX, Lorincz A, and Muñoz N, et al (2002) The causal relation between human papillomavirus and cervical cancer J Clin Pathol 55(4) 244–265 https://doi.org/10.1136/jcp.55.4.244 PMID: 11919208 PMCID: 1769629
28. Bruni L, Diaz M, and Castellsagué X, et al (2010) Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings J Infect Dis 202(12) 1789–1799 https://doi.org/10.1086/657321 PMID: 21067372
29. Runge I, Klein JMA, and Pannen A-K, et al (2024) Prevalence of human papillomaviruses in self-collected samples among women attending antenatal care in Ethiopia: a cross-sectional study Ecancer 18 1739 https://doi.org/10.3332/ecancer.2024.1739
30. Adachi K, Klausner JD, and Xu J, et al (2016) Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-infected pregnant women and adverse infant outcomes Pediatr Infect Dis J 35(8) 894–900 https://doi.org/10.1097/INF.0000000000001199 PMID: 27164464 PMCID: 4945428
31. Clarivet B, Picot E, and Marchandin H, et al (2014) Prevalence of chlamydia trachomatis, neisseria gonorrhoeae and mycoplasma genitalium in asymptomatic patients under 30 years of age screened in a French sexually transmitted infections clinic Eur J Dermatol 24(5) 611–616 https://doi.org/10.1684/ejd.2014.2413 PMID: 25322708
32. Hokororo A, Kihunrwa A, and Hoekstra P, et al (2015) High prevalence of sexually transmitted infections in pregnant adolescent girls in Tanzania: a multi-community cross-sectional study Sex Transm Infect 91(7) 473–478 https://doi.org/10.1136/sextrans-2014-051952 PMID: 25834122 PMCID: 4591089
33. Nyemba DC, Haddison EC, and Wang C, et al (2022) Prevalence of curable STIs and bacterial vaginosis during pregnancy in sub-Saharan Africa: a systematic review and meta-analysis Sex Transm Infect 98(7) 484–491 https://doi.org/10.1136/sextrans-2021-055057
34. Zenebe MH, Mekonnen Z, and Loha E, et al (2021) Prevalence, risk factors and association with delivery outcome of curable sexually transmitted infections among pregnant women in Southern Ethiopia PLoS One 16(3) e0248958 https://doi.org/10.1371/journal.pone.0248958 PMID: 33760867 PMCID: 7990168
35. Schönfeld A, Feldt T, and Tufa TB, et al (2018) Prevalence and impact of sexually transmitted infections in pregnant women in central Ethiopia Int J STD AIDS 29(3) 251–258 https://doi.org/10.1177/0956462417723545
36. Meites E, Gaydos CA, and Hobbs MM, et al (2015) A review of evidence-based care of symptomatic trichomoniasis and asymptomatic trichomonas vaginalis infections Clin Infect Dis 61(Suppl 8) S837–S848 https://doi.org/10.1093/cid/civ738 PMID: 26602621 PMCID: 4657597
37. Vouga M, Greub G, and Prod’hom G, et al (2014) Treatment of genital mycoplasma in colonized pregnant women in late pregnancy is associated with a lower rate of premature labour and neonatal complications Clin Microbiol Infect 20(10) 1074–1079 https://doi.org/10.1111/1469-0691.12686 PMID: 24849820
38. Klebanoff MA, Carey JC, and Hauth JC, et al (2001) Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection N Engl J Med 345(7) 487–493 https://doi.org/10.1056/NEJMoa003329 PMID: 11519502
39. Sampath A, Maduro G, and Schillinger JA (2016) Infant deaths due to herpes simplex virus, congenital syphilis, and HIV in New York City Pediatrics 137(4) e20152387 https://doi.org/10.1542/peds.2015-2387 PMID: 26933212
40. Farr A, Kiss H, and Holzer I, et al (2015) Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome Acta Obstet Gynecol Scand 94(9) 989–996 https://doi.org/10.1111/aogs.12697 PMID: 26084843
41. Zhao Y, Cao X, and Zheng Y, et al (2012) Relationship between cervical disease and infection with human papillomavirus types 16 and 18, and herpes simplex virus 1 and 2 J Med Virol 84(12) 1920–1927 https://doi.org/10.1002/jmv.23353 PMID: 23080497
Supplementary information
Supplementary Table 1. Inclusion and exclusion criteria.