Factors associated with completeness in documentation of diagnostic work-up and treatment in patients with breast cancer in Sudan
Noon I Eltoum1,2,a, Nicole E Caston2, Lily Gutnik2, Mahmoud A Alfardous Alazm1, Feras O Mohamed1, Lama M Abdalkarem1, Saad A S Ali1, Abrar Z Badawi1, Nicole L Henderson2, Andres Azuero2 and Gabrielle Rocque2
1Faculty of Medicine, University of Medical Sciences and Technology, PO Box 12810, Khartoum, Sudan
2Department of Hematology and Oncology, University of Alabama at Birmingham, 1808 7th Avenue South, Birmingham, AL 35233, USA
ahttps://orcid.org/0000-0003-4210-8012
Abstract
Purpose: This study evaluates the relationship between geography and ethnicity on the completeness of documentation of diagnostic work-up and treatment modalities in Sudan for patients with breast cancer.
Methods: This retrospective study used data abstracted from patients with breast cancer receiving cancer care at Sudan’s largest cancer centre (Radiation and Isotopes Center Khartoum) in 2017. Patient demographic and clinical characteristics were abstracted from paper medical records. Odds ratios and 95% confidence intervals were estimated to evaluate complete diagnostic work-up on ethnic group, origin and residence using binomial logistic regression models.
Results: Of 237 patients, the median age was 52 (interquartile range 43–61). Most often patients identified as Arab (68%), originated from Central, Northeastern and Khartoum regions (all 28%) and lived in the Khartoum region (52%). Overall, 49% had incomplete diagnostic work-up, with modest differences by ethnicity and geography. In adjusted analyses, non-statistical differences were found between the ethnic group, geographic origin and residence and having complete diagnostic work-up. For treatment modality, significant differences were observed between receptor status and receiving hormone therapy (p = 0.004). Only 28% of patients with HR+ breast cancer received hormonal therapy. For those with HR− or undocumented breast cancer subtype, 36% and 17% received hormone therapy, respectively.
Conclusion: Approximately half of Sudanese patients with breast cancer had incomplete diagnostic work-up, irrespective of ethnicity and geography. Moreover, a high proportion of patients received inappropriate treatment. This underlines a considerable systems-based quality gap in care delivery, demanding efforts to improve diagnostic work-up for all patients with breast cancer in Sudan.
Keywords: breast cancer, sub-Saharan Africa, receptor status, incomplete documentation
Correspondence to: Noon I Eltoum
Email: neltoum@uab.edu
Published: 17/11/2023
Received: 17/08/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In many low-resource countries, breast cancer staging and testing for receptors status is inconsistent, resulting in treatment below the standard of care [1–4]. Most radiologic equipment that is needed for staging cancers is only available in major institutions across sub-Saharan Africa (SSA) and others, like bone scintigraphy, are fairly uncommon across the board [4]. A pilot survey conducted in SSA of cancer facilities that were members of the African Organisation for Research and Training in Cancer (AORTIC) found that computed tomography scans and magnetic resonance imaging were only available in 79% and 58%, respectively [4]. Furthermore, previous work in SSA documented that testing for the key biomarkers needed for selecting treatment, oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor-2 (HER2) receptor status is expensive and often inaccessible for many patients and providers [2, 3, 5, 6]. A study conducted in Sudan in 2010, reported that ER and PR status results were only available in 4% of patients [3]. These issues can be compounded by a lack of high-quality medical documentation in many hospitals due to the absence of electronic documentation systems, unreliable handwritten information and poor storage of files. This lack of access to and documentation of appropriate diagnostic tests may lead to suboptimal staging and overall management of breast cancer in under-resourced countries [4].
Access issues are often aggravated by other country-specific challenges in under-resourced countries, such as Sudan. Since its independence in 1956, Sudan has faced decades of harsh climates, famine, war and political and economic instability [7]. These unstable conditions have had the most impact on healthcare quality and access, ultimately resulting in limited resources, inadequate workforce, unreliable health record system and poor implementation of health policies and programs [7]. Lack of access to health services can also be attributed to the centralisation of healthcare in the capital of Khartoum and the inequitable distribution of health facilities and health workforce between other states. Furthermore, the history of conflict between regions and ethnic subgroups of Sudan has manifested into the systemic marginalisation of certain groups of people. Ethnicity in Sudan is complex and incorporates overlapping factors related to place of origin, language, tribe, genealogy and shared history [8]. There are approximately 600 tribes that speak over 115 languages; however, nearly 70% of Sudanese people identify as being ethnically Arab, with the other 30% distributed between the Beja, the Nubian, the Fur, the Zaghawa and the Nuba [8–11]. Little is known about how language barriers and cultural differences impact receipt of breast cancer diagnostic testing and treatment. We hypothesise that differences in diagnostic work-up and appropriate treatment would be observed for different geographical and ethnic groups. Thus, this study aimed to understand the relationship between geography and ethnic group and the documentation of breast cancer receptor testing and staging in patients in Sudan and the appropriateness of treatment based on receptor status and staging.
Methods
Study design
This retrospective study included patients with breast cancer receiving services at Radiation and Isotopes Center Khartoum (RICK) in 2017. RICK is one of two cancer centres in Sudan, where 80% of patients with cancer receive their medical care [12]. The study population included all patients with breast cancer admitted to RICK in 2017. Data from patient records were abstracted from handwritten patient medical records. For the analysis, ethnic groups (Arab, Hausa/Fulani, Beja, Nubian, Nuba, Darfurians and other) were categorised as Arab and non-Arab, while non-Sudanese patients, male patients and those with missing demographics were excluded. Institutional Review Board approval was obtained from the management of RICK. Ethical considerations were sought from the research technical and ethical committee of the Faculty of Medicine, University of Medical Sciences and Technology (UMST) in Khartoum, Sudan.
Patient demographics and clinical characteristics
Patients’ age at diagnosis and breast cancer stage were obtained from the patient’s medical record (paper-based records).
Outcomes
Availability of breast cancer receptor status and staging
Pathology reports and patient medical records were used to determine documentation of staging and immunohistochemical tests for ER, PR and HER2 receptor status. If available, tumour-node-metastasis classification of breast cancer was obtained from the patient’s file and then grouped into the overall anatomic stage using the American Joint Committee on Cancer (AJCC) staging reference [13]. For breast cancer subtypes, immunohistochemical tests were used to group patients into having HR+/HER2−, HR−/HER2−, HR+/HER2+ or triple-negative breast cancer. Patients were grouped into two groups: having complete diagnostic work-up (i.e., having available receptor testing and staging in their medical record) or having incomplete diagnostic work-up (i.e., missing receptor testing, staging or both).
Breast cancer treatment
Treatment was extracted from patient records and classified as surgery (i.e., mastectomy or lumpectomy with or without axillary lymph node dissection), chemotherapy, hormone therapy, radiation therapy and treatment not available (i.e., not administered or not documented).
Exposures
Regional and ethnic distribution
State of residence, state of origin, and tribe were abstracted from medical record data. States were clustered into groups based on their geographical region in Sudan (Northeastern, Central, Western and Khartoum regions) (Figure 1). Khartoum, the capital of Sudan which lies in the heart of the country, was not included in the Central region and was grouped as an independent region due to its large population and major role in the health system in Sudan; it is also where RICK is located. Patient’s tribe was self-declared, and for descriptive purposes, these tribes were classified into ethnic groups. Due to the complexity of ethnicity in Sudan and a lack of clear ethnic categories, we used ethnic categories used in previous studies that studied the genetics of Sudanese ethnic groups [10, 11]. For our study, we adopted the ethnic classification used by Dobon et al [10] and Babiker et al [11], researchers who focused on the genetic variation and population structure of Sudanese people. Patients were grouped into broader ethnic categories, including Arab, Beja, Hausa/Fulani, Nubian, Nuba, Darfurians, and other. Hausa and Fulani groups were grouped together due to small sample sizes and ethnic similarity. Additionally, although we recognise ‘Darfurian’ is not an ethnic group, Dobon et al [10] categorised non-Arab tribes of West Sudan into one ethnic category called ‘Darfurian’ due to their shared socio-economic activity, geographic region, and linguistic family [10].
Statistical analysis
Descriptive statistics were calculated using frequencies and percentages for categorical variables and median and interquartile range (IQR) for continuous variables. Percentages of odds ratios (OR) and 95% confidence intervals (CI) were estimated to evaluate complete diagnostic work-up on ethnic group, origin and residence using binomial logistic regression models. Models were adjusted for age. The alpha level was set at 0.05 and differences of at least 0.1 in proportions were considered clinically important. Analyses were performed using SAS© software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
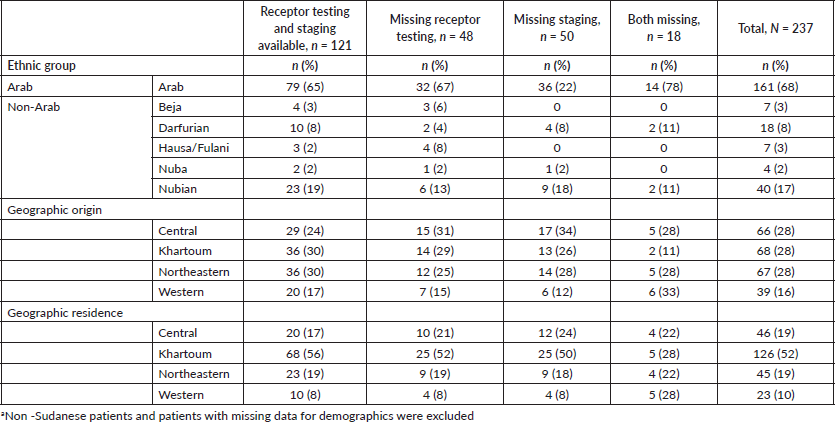
Of 237 female patients with breast cancer included, the median age was 52 years (IQR 42–61). Patients were most often Arab (68%); 17% of patients were Nubian, 8% were Darfurian, 3% were Beja, 3% were Hausa/Fulani and 2% were Nuba. The majority of patients originated from the Central and Northeastern regions (28%), followed by Khartoum (27%) and 16% of patients originated from the Western region. Most patients lived in the Khartoum region (52%); 19% of patients lived in the Central and Northeastern regions; and 10% of patients lived in the Western region (Table 1).
Diagnostic work-up
Approximately half of patients had complete diagnostic work-up (51%); 20% of patients were missing receptor testing and 21% were missing staging; 8% of patients were missing both receptor testing and staging. The mean age of patients with complete diagnostic work-up (52) was similar to the mean age of patients with incomplete diagnostic work-up (52) (t = −0.05, p = 0.958).
After adjusting for age, non-Arab patients had 28% higher odds of having a complete diagnostic work-up when compared to Arab patients, however, this was not statistically significant (OR 1.28; 95% CI 0.74–2.22; Table 2). In adjusted analyses, when compared to patients originating from Khartoum, patients originating from the Central region had 37% lower odds of having a complete diagnostic work-up (OR 0.63; 95% CI 0.47–1.86). Also when compared to patients originating from Khartoum, patients originating from the Western region and the Northeastern region had 15% and 6% lower odds of having complete diagnostic work-up, respectively (OR 0.85; 95% CI 0.38–1.88) (OR 0.94; 95% CI 0.47–1.86). However, these observed differences are not statistically significant. When compared to patients residing in Khartoum, patients residing in the Central and Western regions had 38% lower odds of having complete diagnostic work-up which was not statistically significant (OR 0.62; 95% CI 0.31–1.23) (OR 0. 62; 95% CI 0.25–1.53). Patients living in the Northeastern region had 15% lower odds of having complete diagnostic work-up when compared to patients living in Khartoum (OR 0.85; 95% CI 0.43–1.68). These observed differences are not statistically significant.
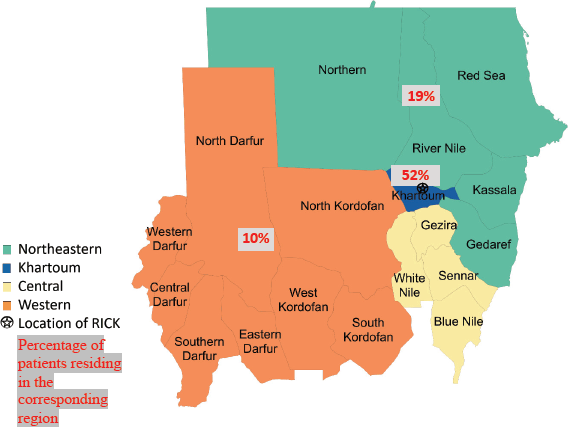
Figure 1. Map of Sudan showing the 18 states and the different geographic regions with the distribution of patient residence.
Table 1. Demographic and clinical characteristics of sample population (N = 237).
Staging
Of 237 patients, 29% had missing documentation of staging. Among all patients, stage III breast cancer was the most common (37%), followed by stage II and stage IV (14% each). Stage I was the least common (6%).
Receptor status
Overall, 29% of patients did not have documentation of receptor status. Amongst those with receptor status available, HR+/HER2− (30%) breast cancer was the most common subtype. Triple-negative breast cancer is the second most frequent subtype at 19%; 12% of patients had HR+/HER2+ and 11% had HR−/HER2+ breast cancer (Table 3).
Treatment modality
Majority of patients (90%) had documentation of treatments prescribed or received. The most common treatment modality was chemotherapy alone (41%), followed by the combination of surgery and chemotherapy (11%), the combination of chemotherapy and hormone therapy (9%), hormone therapy alone (7%), the combination of chemotherapy and radiation therapy (5%), surgery alone (4%) and the combination of surgery, chemotherapy and hormone therapy (4%).
Significant differences were observed between receptor status and receiving surgery (p = 0.013), but not between stage and receiving surgery (p = 0.166) (Table 4). When compared to patients who didn’t receive surgery, 40% of patients with TNBC, 30% of patients with HR+/HER2− disease, 29% of patients with HR+/HER2+ disease, 16% of patients with HR−/HER2+ disease and 13% of patients without receptor status documentation received surgery.
Table 2. Model-estimated odds ratios, predicted probabilities, and 95% CIs evaluating complete diagnostic workup (N = 237).
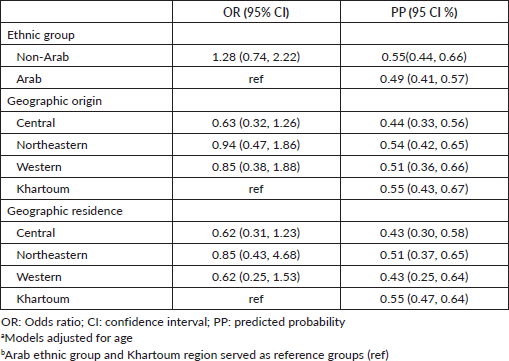
Table 3. Frequencies of treatment modalities offered or administered (N = 237).
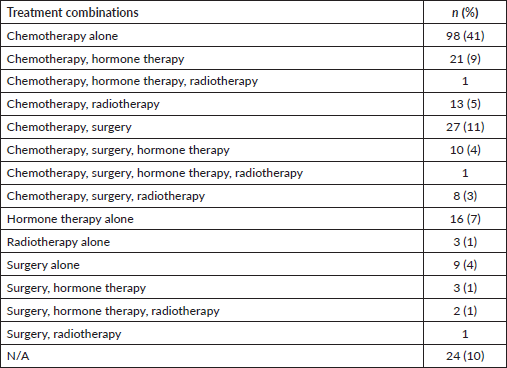
Table 4. Association between breast receptor status and stage and treatment modality received.
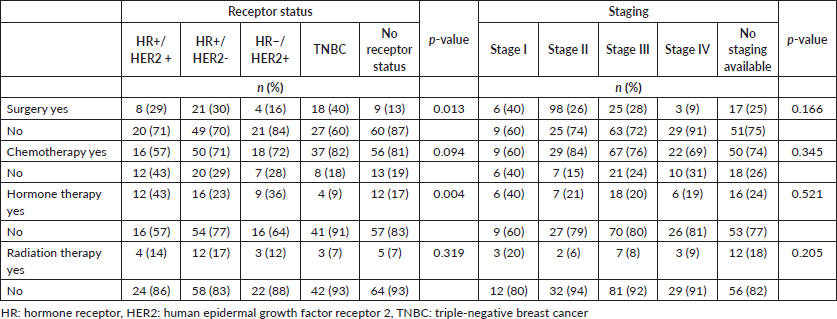
Additionally, significant differences were observed between receptor status and receiving hormone therapy (p = 0.004). Among patients with HR+ breast cancer, only 28% received hormonal therapy. For those with HR− or undocumented breast cancer breast cancer subtype, 36% and 17% received hormone therapy, respectively. Differences observed between other chemotherapy and radiotherapy and receptor status and staging were also not significant.
Discussion
Our study suggests that there are widespread gaps in the completion of diagnostic work-up (i.e., both receptor testing and staging) among all patients with breast cancer in Sudan, irrespective of geography and ethnicity. Overall, 49% of patients had incomplete breast cancer diagnostic work-up; 20% of patients were missing receptor status; 21% of patients were missing staging, and 8% were missing both. While considerably lower than the standard of care, these findings closely align with research on other cancers from the African region. It is important to highlight that there is a limited body of research addressing unstaged breast cancer in SSA. Nevertheless, a population-based study by Seraphin et al [14] within the same geographical context revealed that 47% of patients with prostate cancer were unstaged, and most patients with incomplete documentation did not receive diagnostic workup or treatment. In contrast, a study that assessed the New Zealand Cancer registry, found that 12.3% of patients with breast cancer in New Zealand are unstaged [15]. In the US, there has been a sharp decline in unstaged cancers, however, several studies have suggested that increased levels of unstaged breast cancer are seen with increasing age, patients of racial/ethnic minorities [16, 17], and patients from rural areas [18]. In contrast to our hypothesis that ethnicity and geographic residence and origin would impact diagnostic work-up among Sudanese patients, non-statistical differences were observed, suggesting the role these factors play on the availability of receptor testing and staging are minor compared to overall quality gaps within the populations.
A possible significant contributing factor to the gap in recommended diagnostic work-up and lack of access is poverty secondary to the political and economic instability that has plagued Sudan since its independence. Approximately half of the Sudanese population lives below the national poverty line [19]. High out-of-pocket expenses for health services have impacted their access and utilisation. Additionally, a limited number of healthcare workers, poor communication between clinicians and labs impact cancer care in the region [20]. The absence of a well-established electronic health system and inadequate health facilities also further complicate quality healthcare delivery [20].
Our study found that hormone receptor status did not fully guide the delivery of hormone therapy. In fact, only 28% of patients who had HR+ disease received hormone therapy. On the other hand, 36% of patients who had HR disease received hormone therapy, which has been proven to be ineffective since HR− breast cancer does not respond to hormone therapy [21, 22]. One possible explanation for the considerable number of patients with HR – disease who received hormone therapy could be a delay in diagnosis. It is conceivable that these patients may have been treated blindly initially due to barriers to pathological receptor testing and alternative affordable therapies, and then subsequently found to have hormone negative disease. However, due to the nature of the incomplete handwritten medical records this information along with any treatment changes may have not been documented. Follow-up and regular monitoring of treatment effectiveness is an additional challenge, especially for patients with low income and those traveling from rural parts of Sudan. This may lead to the continuation of hormone therapy despite it being ineffective. Additionally, 17% of patients who did not have documentation of receptor testing received hormone therapy, suggesting either a lack of documentation of pathology results or inappropriate treatment administration. These findings were consistent with prior studies showing that deficiencies in diagnostic work-up have impelled clinicians in Sudan to blindly administer therapy irrespective of a patient’s receptor status and staging, leading to a decrease in the quality of cancer care [1, 3–5, 23]. Studies in other SSA countries have also demonstrated similar findings. In a hospital-based study conducted in Zimbabwe, 67% of patients were treated without knowledge of their hormone receptor status [5]. Ultimately, adequate receptor testing is needed to maximise both patients receiving appropriate therapy and avoidance of ineffective therapy for those unlikely to benefit.
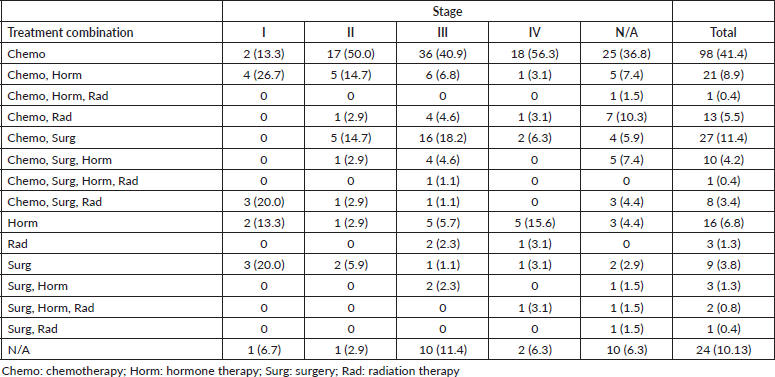
Furthermore, our study found low rates of radiotherapy (11%), which is consistent with a different study that found only ten radiation therapy machines across the four comprehensive cancer centres in Sudan [24]. Breast cancer is often treated with more than one treatment modality, however, in our study, the main treatment modality physicians resorted to was chemotherapy alone (41%), followed by the combination of surgery and chemotherapy (11%) and the combination of chemotherapy and hormone therapy (9%) (Table 3). Although chemotherapy is the most prescribed treatment for Sudanese patients with breast cancer, our study found a lack of statistical differences across stages, suggesting a lack of tailoring to the risk of disease recurrence (Tables 4 and 5). It is imperative to highlight the importance of tailored, targeted therapy in enhancing overall outcomes and survival rates of patients with breast cancer, especially in those with HR + disease. Furthermore, it is crucial to recognise the potential benefits of radiotherapy in local control, decreasing recurrence and improving survival.
Table 5. Association between staging and treatment combinations received.
To address these issues, we suggest interventions that aim to establish sufficient cancer centres with appropriate and high-quality services in Sudan and SSA. However, sustaining expensive radiologic equipment in complex environments with inadequate electricity supply and poor maintenance is a huge challenge [25]. Thus, global support and governmental funding allocations must help with running costs and long-term maintenance of radiologic equipment to allow patients with breast cancer to have access to affordable radiologic imaging. This could expand diagnostic capabilities and therefore, improve cancer outcomes among patients in SSA. Additionally, since pathology testing in Sudan is inaccessible and often suboptimal, funding allocations for instituting reliable labs for pathologic testing is essential. There is also a need for educating and training physicians and healthcare workers on appropriate medical documentation. Finally, transitioning from paper-based medical records to an electronic health system will also bridge gaps in medical documentation and interdisciplinary communication.
This study has limitations. One limitation of this study is the use of data from 2017. Nonetheless, due to factors like the 2019 Revolution and the COVID-19 pandemic which have both adversely affected healthcare in Sudan, there is not anticipated to be substantial improvement in medical documentation or cancer care delivery. Additionally, many patients could have never received a diagnosis of cancer and died without seeking medical care. Another limitation is that ethnicity in Sudan is not well studied, and the usage of broader groups to categorise patients may have blunted the results of the study. Our study is also subject to information bias since data was abstracted from handwritten paper-based medical records. Due to insufficiency of medical documentation, there was no way to confirm if more pathologic testing and radiologic imaging were completed. Also, reasons for not getting diagnostic work-up were not documented in patient files and were therefore not evident. Finally, the reasons that physicians selected treatments, and the available resources for individual patients were not available for this analysis. Further work is needed to understand if practice patterns contradictory to guidelines (e.g., hormone therapy for HR breast cancer) are due to a lack of available systemic therapies or to other potential intervening factors.
Conclusion
A high proportion of Sudanese patients with breast cancer have deficient diagnostic work-up including missing receptor testing and staging, which limits cancer care delivery, and ultimately contributes to inadequate or inappropriate treatment, which may result in poor survival. Gaps are country-wide and not isolated to specific geographical or ethnic groups. Further work is needed to understand the reasons for these deficiencies and to develop interventions to improve diagnostic work-up and treatment in low-income countries such as Sudan.
Acknowledgments
We would like to acknowledge the late Dr Girgis Ramsi Gelda, Professor of Surgery at the University of Medical Sciences and Technology for his guidance and support. Part of this project was a presented as a poster presentation at the American Society of Clinical Oncology (ASCO) Quality Care Symposium 2022.
Conflicts of interest
Dr Rocque received research funding from Genentech, Pfizer and Carevive and consulting fees for Genentech and Pfizer. All other authors declare they have no conflicts of interest.
Funding
All other authors declare that no funds, grants or other support was received during the preparation of this manuscript.
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by MA, LA, SA, AB and NE. Analysis was performed by NC and NE and revised by AA. The first draft of the manuscript was written by NE. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The data generated during and analysed during the study are not publicly available due to possible breach in patient confidentiality but are available from the corresponding author on reasonable request.
References
1. Mariani -Costantini R, Elhassan MMA, and Aceto GM, et al (2017) Epidemiology, pathology, management and open challenges of breast cancer in central Sudan: a prototypical limited resource African setting Breast Cancer-From Biology to Medicine (Pescara: Università degli Studi “G. d'Annunzio” Chieti) https://doi.org/10.5772/67175
2. Sayed S, Moloo Z, and Wasike R, et al (2014) Is breast cancer from Sub Saharan Africa truly receptor poor? Prevalence of ER/PR/HER2 in breast cancer from Kenya Breast 23 591–596 https://doi.org/10.1016/j.breast.2014.06.006 PMID: 25012047
3. Elgaili EM, Abuidris DO, and Rahman M, et al (2010) Breast cancer burden in central Sudan Int J Women's Health 2 77
4. Vanderpuye VDNK, Olopade OI, and Huo D (2016) Pilot survey of breast cancer management in Sub-Saharan Africa J Glob Oncol 3 194–200 https://doi.org/10.1200/JGO.2016.004945
5. Mushonga M, Ndlovu N, and Nyakabau AM, et al (2020) Biomarkers in breast cancer: quantifying discordance with best practice when hormone receptor status is an extravagance S Afr J Oncol 4 1–8 https://doi.org/10.4102/sajo.v4i0.134
6. Eng A, McCormack V, and dos-Santos-Silva I (2014) Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis PLoS Med 11 e1001720 https://doi.org/10.1371/journal.pmed.1001720 PMID: 25202974 PMCID: 4159229
7. Charani E, Cunnington AJ, and Yousif AH, et al (2019) In transition: current health challenges and priorities in Sudan BMJ Glob Health 4 e001723 https://doi.org/10.1136/bmjgh-2019-001723 PMID: 31543996 PMCID: 6730568
8. el Din Sabr M, Spaulding JL, and Sikainga AA, et al (2022) Sudan Encyclopedia Britannica
9. Adesina MA (2020) The health status and demographics of a conflicting country: the Sudan experience Eur J Environ Public Health 4 em0032
10. Dobon B, Hassan HY, and Laayouni H, et al (2015) The genetics of East African populations: a Nilo-Saharan component in the African genetic landscape Sci Rep 5 1–11 https://doi.org/10.1038/srep09996
11. Babiker H, Schlebusch CM, and Hassan HY, et al (2011) Genetic variation and population structure of Sudanese populations as indicated by 15 identifiler sequence-tagged repeat (STR) loci Investig Genet 2 1–13 https://doi.org/10.1186/2041-2223-2-12
12. Abdalla Elhassan SI (2020) The five-year survival rate of breast cancer at Radiation and Isotopes Centre Khartoum, Sudan Heliyon 6 e04615 https://doi.org/10.1016/j.heliyon.2020.e04615 PMID: 32904288 PMCID: 7452576
13. Giuliano AE, Connolly JL, and Edge SB, et al (2017) Breast cancer – major changes in the American Joint Committee on Cancer eighth edition cancer staging manual CA Cancer J Clin 67 290–303 https://doi.org/10.3322/caac.21393 PMID: 28294295
14. Seraphin TP, Joko-Fru WY, and Hämmerl L, et al (2021) Presentation, patterns of care, and outcomes of patients with prostate cancer in sub-Saharan Africa: a population-based registry study Cancer 127 4221–4232 https://doi.org/10.1002/cncr.33818 PMID: 34328216
15. Seneviratne S, Campbell I, and Scott N, et al (2014) Accuracy and completeness of the New Zealand Cancer Registry for staging of invasive breast cancer Cancer Epidemiol 38 638–644 https://doi.org/10.1016/j.canep.2014.06.008 PMID: 25037979
16. Merrill RM, Sloan A, and Anderson AE, et al (2011) Unstaged cancer in the United States: a population-based study BMC Cancer 11 402 https://doi.org/10.1186/1471-2407-11-402 PMID: 21936934 PMCID: 3188514
17. Yedjou CG, Tchounwou PB, and Payton M, et al (2017) Assessing the racial and ethnic disparities in breast cancer mortality in the United States Int J Environ Res Public Health 14 486 https://doi.org/10.3390/ijerph14050486 PMID: 28475137 PMCID: 5451937
18. Howe HL, Katterhagen JG, and Yates J, et al (1992) Urban-rural differences in the management of breast cancer Cancer Causes Control 3 533–539 https://doi.org/10.1007/BF00052750 PMID: 1420856
19. World Bank Group (2020) Poverty & Equity Brief: Sudan (Washington: World Bank Group)
20. Ebrahim EM, Ghebrehiwot L, and Abdalgfar T, et al (2017) Health care system in Sudan: review and analysis of strength, weakness, opportunity, and threats (SWOT analysis) Sudan J Med Sci 12 133–150 https://doi.org/10.18502/sjms.v12i3.924
21. Uray IP and Brown PH (2010) Chemoprevention of hormone receptor-negative breast cancer: new approaches needed Clin Cancer Prev 188 147–162 https://doi.org/10.1007/978-3-642-10858-7_13
22. Nahleh Z (2008) Androgen receptor as a target for the treatment of hormone receptor-negative breast cancer: an unchartered territory Future Oncol 4 15–21 https://doi.org/10.2217/14796694.4.1.15 PMID: 18240997
23. Vanderpuye V, Grover S, and Hammad N, et al (2017) An update on the management of breast cancer in Africa Infect Agents Cancer 12 13 https://doi.org/10.1186/s13027-017-0124-y
24. Christ SM, Siddig S, and Elbashir F, et al (2021) Radiation oncology in the land of the pyramids: how sudan continues to push the frontiers of cancer care in eastern Africa Int J Radiat Oncol Biol Phys 110 931–939 https://doi.org/10.1016/j.ijrobp.2021.02.013 PMID: 34171244
25. Frija G, Blažić I, and Frush DP, et al (2021) How to improve access to medical imaging in low-and middle-income countries? EClinicalMedicine 38 101034 https://doi.org/10.1016/j.eclinm.2021.101034






