Treatment outcomes and prognostic factors of esthesioneuroblastoma—a retrospective study from South India
Lekha Madhavan Nair1, John Mohan Mathew1, Malu Rafi1, Kainickal Cessal Thommachan1, Jagathnath Krishna KM2, Bipin T Varghese3 and Rejnish Ravikumar1
1Department of Radiation Oncology, Regional Cancer Centre, Thiruvananthapuram 695011, India
2Department of Epidemiology and Biostatistics, Regional Cancer Centre, Thiruvananthapuram 695011, India
3Department of Surgical Oncology, Regional Cancer Centre, Thiruvananthapuram 695011, India
Abstract
Esthesioneuroblastoma (ENB) or olfactory neuroblastoma is a rare malignant neoplasm arising from the neural crest cells of the olfactory epithelium. The optimum treatment for this rare disease is still unclear. Most of the available literature on this rare head and neck tumour is limited to small retrospective series and single institutional reports. We conducted a retrospective study to investigate the clinical profile, treatment outcomes and prognostic factors of patients with ENB treated at a tertiary cancer centre in south India. Patients with a histopathological diagnosis of ENB treated from 2000 to 2019 were included. Patient demographics, tumour characteristics, stage, treatment details and outcome data were identified from medical records. Overall survival (OS) and disease-free survival (DFS) were estimated using the Kaplan–Meier method and the log-rank test was used for comparison. The prognostic factors were identified using Cox regression analysis. Forty-two patients underwent treatment for ENB from 2000 to 2019. Twenty-six patients underwent surgery. Twelve patients received radical radiotherapy (RT) while 24 patients underwent adjuvant radiation. After a median follow-up of 71 months, the estimated OS and DFS at 4 years were 64.4% and 54%, respectively. The estimated 4-year OS for modified Kadish A, B, C and D stages was 75.0%, 90.9%, 56.4% and 0%, respectively. Modified Kadish stage, nodal involvement, orbital invasion, intracranial extension, surgery, RT treatment and use of chemotherapy were significant predictors of OS and DFS in univariate Cox regression analysis. Orbital invasion and RT treatment were significant predictors of DFS in the multivariate analysis as well. However, only RT treatment came out to be a significant predictor for OS in multivariate Cox regression analysis. Surgery is the mainstay of treatment. Adjuvant RT may improve local control and survival in advanced cases. Advanced modified Kadish stage, lymph node involvement and orbital invasion are associated with poor outcomes.
Keywords: esthesioneuroblastoma, survival, surgery, radiotherapy
Correspondence to: Rejnish Ravikumar
Email: rejnish@gmail.com
Published: 27/07/2023
Received: 25/04/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Esthesioneuroblastoma (ENB) or olfactory neuroblastoma is a rare malignant neoplasm arising from the neural crest cells of the olfactory epithelium within the nasal cavity [1, 2]. It accounts for 3% of all nasal cavity tumours [3]. The aetiology of this rare round cell tumour is unclear, but it is postulated that viral, genetic and environmental factors play a role in its development [4]. ENB has a varying clinical course, ranging from indolent disease to a highly aggressive neoplasm with local infiltration and distant metastasis.
The optimum treatment for this rare disease is still unclear, with surgery being the mainstay in managing these tumours. The complex anatomical location and the difficulty in achieving a clear surgical margin due to the infiltrative nature of the lesion often pose challenges in treatment [5]. Radiotherapy (RT) is an integral part of the multidisciplinary management of this disease and several retrospective studies have reported improvement in local control with adjuvant RT [3, 6]. The role of chemotherapy is not completely understood in the multimodal setting, relating to tumour stage and pathology. Even though there are reports suggesting good response rates with neoadjuvant chemotherapy in locoregionally advanced disease [7], there are retrospective series that point towards poor outcomes with the use of chemotherapy [1, 8].
Most of the available literature on this rare head and neck tumour is limited to small retrospective series and single institution reports and only limited information is available about the prognostic factors and treatment outcomes. We aimed to investigate the clinical profile, treatment outcomes and prognostic factors of patients with ENB treated at a tertiary cancer centre in south India.
Materials and methods
This was a retrospective study conducted at a tertiary cancer centre in India. Patients with a histopathological diagnosis of ENB treated from 2000 to 2019 were included in the study. Patients aged less than 13 years were excluded from the study. Patient demographics, tumour characteristics, stage, treatment details and outcome data were identified from medical records. Modified Kadish staging and Dulguerov staging were done for all patients. The information on Hyams grade was not available for all patients and hence its prognostic significance was not assessed. Cross-sectional imaging of the head and neck (contrast-enhanced magnetic resonance imaging or contrast-enhanced computed tomography) was used for staging. Surgery was done for all resectable tumours. Patients with Kadish stages B, C and D received adjuvant RT. Induction chemotherapy was given for unresectable tumours and concurrent cisplatin was given as a part of radical chemoradiation. The common induction regimens were cisplatin-etoposide and vincristine, adriamycin and cyclophosphamide (VAC).
Patients were followed up with clinical examinations every 3 months during the first 2 years and every 6 months thereafter. Cross-sectional imaging was done as and when indicated. Follow-up was updated using clinical and telephonic information. The study was approved by the Institutional Review Board (SRC no: 8/2022/03).
Statistical analysis
The categorical variables were presented using values and percentages. Continuous variables were summarised using mean, median and standard deviation. Overall survival (OS) and disease-free survival (DFS) were estimated using Kaplan–Meier method and the log-rank test was used for comparison. The prognostic factors were identified using Cox regression analysis. OS was calculated from the date of diagnosis to the date of death or last follow-up. DFS was computed from the date of diagnosis to the date of relapse, progression of disease or death. The Cox regression model was used to identify the statistical prognostic factors. Statistical analysis was done using Statistical Package for the Social Sciences software version 11.0.
Results
Forty-two patients underwent treatment for ENB from 2000 to 2019. There were 24 males (57.1%) and 18 females (42.9%). The median age was 47 years (13–78). Table 1 shows the patient and tumour characteristics. No patient had metastatic disease at presentation. Twenty-six (61.9%) patients underwent surgery. Endoscopic surgery was done on 16 patients and 10 patients underwent open surgery. Sixteen patients underwent a biopsy only. Out of the 20 patients with intracranial extension, 7 underwent surgery. Open craniofacial resection was done for four patients, whereas endoscopic surgery was done for three patients. Six patients underwent adjuvant RT after surgery. Out of the 12 patients with orbital invasion, two underwent surgery, which amounted to orbital exenteration. Thirty-six patients (85.7%) underwent RT. Twelve patients received radical RT while 24 patients underwent RT as adjuvant treatment. Seventeen patients received intensity-modulated RT, seven patients received conformal RT and others received conventional treatment. RT doses ranged from 50 to 70 Gy as conventional fractionation. Chemotherapy was given to 18 patients (42.9%), as neo-adjuvant for 15 patients and concurrent (cisplatin) for 2 patients. One patient received adjuvant chemotherapy with cisplatin and 5-fluorouracil. Cisplatin-etoposide was the most common neo-adjuvant regimen used, followed by the VAC regimen. Two patients underwent surgery plus adjuvant RT and eight patients received radical RT after neoadjuvant chemotherapy. Five patients developed disease progression after neoadjuvant chemotherapy (before the local treatment) and they received palliative treatment. Table 2 shows the details of treatment received according to the modified Kadish stage.
Table 1. Baseline patient characteristics.

Table 2. Treatment received according to the modified Kadish stage.

Treatment
Among the 12 patients who underwent RT alone, 8 attained a complete response, 1 attained a partial response, 2 had stable disease and 1 developed disease progression. Out of the 26 patients who underwent surgery as their treatment (surgery + RT or surgery alone), 4 developed local recurrences, 3 developed regional recurrences and 1 developed distant relapse. Five out of 16 patients had disease progression after neoadjuvant chemotherapy. Distant metastases developed in 3 out of the total 42 patients, with the sites of metastases being the liver and lungs.
Survival
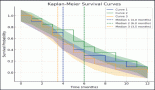
The median follow-up was 71 months (2–230 months). The estimated 4-year OS was 64.4% (Figure 1). The estimated 4-year OS for modified Kadish A, B, C and D stages is 75.0%, 90.9%, 56.4% and 0%, respectively (Figure 2). Age is evaluated as a continuous variable in the present study and a cutoff value of 40 years was obtained using Receiver Operating Characteristic (ROC) curve analysis. No significant difference in OS or DFS was noticed in patients aged <40 years versus ≥40 years. A significant difference in OS was noticed for the modified Kadish stage, T stage, nodal stage, presence of orbital invasion, presence of intracranial extension, surgical treatment, use of adjuvant or radical RT and chemotherapy treatment (Table 3). The estimated 4-year DFS was 54% (Figure 3). There was a significant difference in DFS probability according to the modified Kadish stage, presence of orbital invasion, presence of intracranial extension, surgical treatment and use of RT and chemotherapy (Table 4). Advanced modified Kadish stage [hazard ratio (HR) 9.419, 95% confidence interval (CI) 0.917–96.785, p-0.036], presence of nodal involvement (HR 5.820, 95% CI 1.512–22.406, p-0.010), presence of orbital invasion (HR 4.131, 95% CI 1.476–11.567, p-0.007), presence of intracranial extension (HR 3.340, 95% CI 1.135–9.826, p-0.029) and use of chemotherapy (HR 4.184, 95% CI 1.419–12.337, p-0.009) were associated with decreased survival in univariate Cox regression analysis (Table 5). Patients who underwent surgery (HR 0.173, 95% CI 0.059–0.512, p-0.002) and those who received RT as adjuvant or radical treatment (HR 0.220, 95% CI 0.067–0.729, p-0.013) were significantly associated with better OS. The use of radical or adjuvant RT was associated with better survival in multivariate analysis as well (HR 0.105, 95% CI 0.025–0.433, p-0.002). Univariate Cox regression analysis for DFS (Table 5) showed significant association with nodal involvement (HR 3.892, 95% CI 1.065–14.227, p-0.040), presence of orbital invasion (HR 3.175, 95% CI 1.267–7.960, p-0.014), presence of intracranial extension (HR 2.909, 95% CI 1.134–7.463, p-0.026) and the use of chemotherapy (HR 3.326, 95% CI 1.304–8.483, p-0.012). Patients who underwent surgical treatment (HR 0.293, 95% CI 0.117–0.730, p-0.008), and those who received adjuvant or radical RT (HR 0.153, 95% CI 0.053–0.440, p-0.001) were associated with significantly better DFS (Table 4). The presence of orbital invasion (HR 3.051, 95% CI 1.197–7.773, p-0.019) and use of RT treatment (HR 0.158, 95% CI 0.054–0.463, p-0.001) were significant predictors of DFS in the multivariate analysis also.
Discussion
ENB is a malignant neoplasm of the nasal cavity with varying understanding of this rare tumour in published literature. We are reporting on the treatment outcomes of 42 patients treated over a span of 20 years at a tertiary cancer centre in South India.

Figure 1. Kaplan–Meier curve showing OS probability. OS: Overall survival.

Figure 2. OS probability according to modified Kadish stages (p-value 0.010). OS: Overall survival.
A bimodal age distribution in the second and sixth decades of life has been reported for ENB [9]. About half of the patients in this study presented in the fourth to sixth decades, with a median age at presentation of 47 years (range 13–78 years). A similar distribution of age was reported in other studies by Yin et al [10] and Tural et al [11] also. The incidence among males and females was reported to be equal in some studies [11–13] while some others showed a male predilection [14–16]. The male-female ratio in this study was 1.3.
Table 3. OS probability at 2 and 4 years.

There is no separate American Joint Committee on Cancer/The International Union Against Cancer staging for ENB. Multiple staging systems have been proposed, the most commonly used systems being modified Kadish staging and Dulguerov staging. Most of the patients in this cohort were modified Kadish stage C, similar to other published series [13, 15]. There was a significant difference in DFS and OS according to the modified Kadish stage. The OS for stages A, B, C and D was 75%, 90.9%, 56.4% and 0%, respectively. The 4-year DFS was 75%, 83.3%, 41.3% and 0% for stages A, B, C and D, respectively. There were 4 patients with modified Kadish A stages, of whom 1 developed lung metastasis and died of disease progression, whereas only 2 out of the 13 modified Kadish B patients died of disease. That may be the reason for improved survival in patients with modified Kadish B stage compared to Kadish A. The tumours were staged according to the Dulguerov T staging system as well. But there was no significant difference in OS or DFS according to the T stage. Two large retrospective series reported the Kadish stage as a significant predictor of OS in univariate analysis [3, 17]. A retrospective population-based cohort study by Jethanamest et al [18] also showed the modified Kadish stage as a significant factor affecting OS and disease-specific survival. The modified Kadish stage was significantly correlated with OS in the present study as well. Some studies have concluded that the existing staging systems do not accurately predict survival [19, 20]. A new Tumour, Node, Metastasis (TNM) staging system has been proposed by Sun et al [21] but has to be validated in future studies.

Figure 3. Kaplan–Meier curve showing DFS probability. DFS: Disease free survival.
Table 4. DFS probability at 2 and 4 years.

Table 5. Univariate Cox regression for OS and DFS.

Orbital invasion, which is staged as C as per the modified Kadish system, is found to be associated with poor prognosis and this was seen in 41.7% of patients in this series. A similar rate of orbital involvement was reported by Song et al [17]. The 4-year OS probability for patients with and without orbital invasion was 41.7% and 73.35%, respectively. These figures are lower than the large single institution report by Song et al [17]. Only 16% of patients with orbital involvement underwent surgery in the present study. That may be the reason for inferior outcomes compared to the previously published series. Li et al [22] categorised patients with orbital invasion into three grades and there was a significant difference in OS and PFS between grades I, II and III lesions in univariate analysis. There was no difference in survival among patients treated with and without surgery and they concluded that radical RT is a viable option for patients with advanced and unresectable tumours extending into the orbit. Surgery followed by adjuvant RT is the recommended treatment for operable tumours. Inoperable lesions may be treated by radical RT.
Olfactory neuroblastoma has a pathway of direct spread to the brain through the cribriform plate. Surgery plays a crucial role in the management of these tumours and adjuvant RT may be beneficial for improved local control. Although 20 patients in this series had an intracranial extension, only 7 underwent
surgical treatment, 4 open Cranio-Facial resections and 3 endoscopic surgeries. A systematic review and meta-analysis by Fu et al [23] showed that endoscopic approaches have comparable control rates to open craniofacial resection. Patients with intracranial involvement had inferior OS and DFS (45.6% versus 35.1%) and this was reflected in the univariate analysis although no significance was found in the multivariate analysis. The intracranial extension did not influence survival in two previously reported retrospective studies [17, 24].
Cervical lymph nodes are the most frequent sites of regional spread of the disease with level II nodes being the most common [25]. The disease can also spread to levels I, III and retropharyngeal nodes [26, 27]. The incidence of lymph node metastasis was 9.5% which is similar to other published series [3, 12, 14]. Several retrospective studies have reported lymph node metastasis as a significant predictor of outcome [17]. Lymph node involvement was associated with decreased OS and DFS in this study as well. In univariate Cox regression, lymph node involvement was a significant predictor of OS and DFS. Patients underwent neck dissection and nodal irradiation in cases of clinical, radiological or pathological evidence of lymph node involvement. There is no strong evidence for prophylactic neck irradiation [28]. There were three isolated nodal recurrences in this study and all of them were successfully salvaged by surgery and radiation.
There is documented evidence to support the difficulties in achieving negative surgical margins due to the anatomical location and infiltrative nature of this disease and this theoretically opens avenues for the use of adjuvant RT for reducing local recurrence [5]. Even though reports from the SEER (Surveillance, Epidemiology, and End Results) database showed no benefit for adjuvant RT [4], a large population-based database of 931 patients from the University of Utah reported a 47% decrease in risk of death with the addition of RT after surgery [29]. We found that RT, given in either a radical or adjuvant setting, reduced the risk of death and recurrence.
Although chemotherapy is widely used in the neoadjuvant, concurrent and palliative setting, its exact role in the management of these tumours is debatable. The use of neoadjuvant chemo often helps in shrinking the tumour prior to definitive treatment, thereby enabling better resections or safer RT delivery. We found that patients who received chemotherapy had a four times higher risk of death and a 3.3 times higher risk of recurrence compared to patients without chemotherapy treatment in the univariate analysis. This difference was significant in multivariate analysis as well. These findings are in concurrence with the findings of two large population database studies which brought out the detrimental effects of chemotherapy [8, 30]. The poor outcomes for patients who received chemotherapy could be a reflection of the fact that the majority of the patients who were given chemotherapy had either Kadish C or D disease and most had unresectable disease or had difficult RT planning.
Adjuvant or definitive RT is challenging in ENB due to the complex anatomical relations of these tumours which includes vital areas like the optic apparatus, brain stem and the brain. Even though long-term follow-up data is lacking, proton beam therapy and carbon ion therapy are being established as effective and safe treatment alternatives to conventional external beam RT, due to their ability to attain sharp dose falloff beyond the depth of Bragg peak [31–34]. But long-term follow-up data is needed to establish the role of particle beam therapy.
Multiple studies have identified different genetic and cytogenetic alterations in ENB. The most common chromosomal alterations include deletion on chromosome 11 and gain on chromosome 1p, which is associated with poor prognosis [35]. Alterations in p53, EGFR, KIT, RET, APC, FGFR2, PDGFRA, SMAD4 and CTNNB1 genes are also associated with ENB [36–38]. This might provide potential therapeutic pathways by targeting these genetic alterations.
The retrospective nature of the study could have resulted in selection bias. Due to small patient numbers, some statistical evaluations could not be fully done. The study population was sourced over a 19-year period, treatment approaches may have changed during this period.
Conclusion
The majority of patients with ENB present in advanced stages. Surgery is the mainstay of treatment. Adjuvant RT may improve local control and survival in advanced cases. Advanced modified Kadish stage, lymph node involvement and orbital invasion have prognostic importance.
Acknowledgments
Nil.
List of abbreviations
CI: Confidence interval; DFS: Disease free survival; ECOG: Eastern Co-operative Oncology Group; ENB: Esthesioneuroblastoma; OS: Overall survival; RT: radiotherapy; UICC: The International Union Against Cancer.
Conflicts of interest
None.
Funding
None.
Author contributions
Dr Rejnish Ravikumar, Dr Lekha Madhavan Nair and Dr John Mohan Mathew contributed to study conception and design. Material preparation, data collection and analysis were performed by Dr Lekha M Nair, Dr John Mohan Mathew and Dr Jagathnath Krishna K M. The first draft of the manuscript was written by Dr Lekha M Nair and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
References
1. Alotaibi HA, Priola SM, and Bernat AL, et al (2019) Esthesioneuroblastoma: summary of single-center experiences with focus on adjuvant therapy and overall survival Cureus 11(6) e4897 PMID: 31423376 PMCID: 6689473
2. Bao C, Hu W, and Hu J, et al (2020) Intensity-modulated radiation therapy for esthesioneuroblastoma: 10-year experience of a single institute Front Oncol 10 1158 https://doi.org/10.3389/fonc.2020.01158 PMID: 32766154 PMCID: 7379860
3. Xiong L, Zeng XL, and Guo CK, et al (2017) Optimal treatment and prognostic factors for esthesioneuroblastoma: retrospective analysis of 187 Chinese patients BMC Cancer 17 254 https://doi.org/10.1186/s12885-017-3247-z PMID: 28399835 PMCID: 5387340
4. Platek ME, Merzianu M, and Mashtare TL, et al (2011) Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database Radiat Oncol Lond Engl 6 41 https://doi.org/10.1186/1748-717X-6-41
5. Kumar R (2015) Esthesioneuroblastoma: multimodal management and review of literature World J Clin Cases 3(9) 774–778 https://doi.org/10.12998/wjcc.v3.i9.774 PMID: 26380824 PMCID: 4568526
6. Yin ZZ, Gao L, and Luo JW, et al (2016) Long-term outcomes of patients with esthesioneuroblastomas: a cohort from a single institution Oral Oncol 53 48–53 https://doi.org/10.1016/j.oraloncology.2015.11.021
7. Su SY, Bell D, and Ferrarotto R, et al (2017) Outcomes for olfactory neuroblastoma treated with induction chemotherapy Head Neck 39(8) 1671–1679 https://doi.org/10.1002/hed.24822 PMID: 28561956
8. Cranmer LD and Chau B (2019) Chemotherapy in the management of olfactory neuroblastoma/esthesioneuroblastoma: an analysis of the surveillance, epidemiology, and end results (SEER) 1973-2015 database J Clin Oncol 37(15_suppl) e17573–e17573 https://doi.org/10.1200/JCO.2019.37.15_suppl.e17573
9. Thompson LDR (2009) Olfactory neuroblastoma Head Neck Pathol 3(3) 252–259 https://doi.org/10.1007/s12105-009-0125-2
10. Yin Z, Wang Y, and Wu Y, et al (2018) Age distribution and age-related outcomes of olfactory neuroblastoma: a population-based analysis Cancer Manag Res 10 1359–1364 https://doi.org/10.2147/CMAR.S151945 PMID: 29881306 PMCID: 5985786
11. Tural D, Yildiz O, and Selcukbiricik F, et al (2011) Olfactory neuroblastomas: an experience of 24 years ISRN Oncol 2011 e451086
12. Mantsopoulos K, Koch M, and Iro H, et al (2022) Olfactory neuroblastomas: what actually happens in the long-term? J Clin Med 11(9) 2288 https://doi.org/10.3390/jcm11092288 PMID: 35566413 PMCID: 9105484
13. Tajudeen BA, Arshi A, and Suh JD, et al (2015) Esthesioneuroblastoma: an update on the UCLA experience, 2002–2013 J Neurol Surg Part B Skull Base 76(1) 43–49 https://doi.org/10.1055/s-0034-1390011
14. Zeng Q, Tian Y, and He Y, et al (2021) Long-term survival outcomes and treatment experience of 64 patients with esthesioneuroblastoma Front Oncol 11 624960 https://doi.org/10.3389/fonc.2021.624960 PMID: 33747939 PMCID: 7969639
15. Sun M, Wang K, and Qu Y, et al (2020) Long-term analysis of multimodality treatment outcomes and prognosis of esthesioneuroblastomas: a single center results of 138 patients Radiat Oncol Lond Engl 15 219 https://doi.org/10.1186/s13014-020-01667-4
16. Konuthula N, Iloreta AM, and Miles B, et al (2017) Prognostic significance of Kadish staging in esthesioneuroblastoma: an analysis of the national cancer database Head Neck 39(10) 1962–1968 https://doi.org/10.1002/hed.24770 PMID: 28815831 PMCID: 5993196
17. Song X, Wang J, and Wang S, et al (2020) Prognostic factors and outcomes of multimodality treatment in olfactory neuroblastoma Oral Oncol 103 104618 https://doi.org/10.1016/j.oraloncology.2020.104618 PMID: 32126517
18. Jethanamest D, Morris LG, and Sikora AG, et al (2007) Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors Arch Otolaryngol Head Neck Surg [Internet] 133(3) 276–280 Date accessed: 22/02/23 https://doi.org/10.1001/archotol.133.3.276 PMID: 17372086
19. Joshi RR, Husain Q, and Roman BR, et al (2019) Comparing Kadish, TNM, and the modified Dulguerov staging systems for esthesioneuroblastoma J Surg Oncol 119(1) 130–142 https://doi.org/10.1002/jso.25293 PMCID: 6352736
20. Bell D, Saade R, and Roberts D, et al (2014) Prognostic utility of hyams histological grading and Kadish-Morita staging systems for esthesioneuroblastoma outcomes Head Neck Pathol 9(1) 51–59 https://doi.org/10.1007/s12105-014-0547-3 PMID: 24806334 PMCID: 4382491
21. Sun M, Wang K, and Qu Y, et al (2021) Proposal of a TNM classification-based staging system for esthesioneuroblastoma: more precise prediction of prognosis Head Neck 43(4) 1097–1104 https://doi.org/10.1002/hed.26559
22. Li R, Tian S, and Zhu Y, et al (2019) Management of orbital invasion in esthesioneuroblastoma: 14 years’ experience Radiat Oncol 14(1) 107 https://doi.org/10.1186/s13014-019-1313-1 PMID: 31196122 PMCID: 6567903
23. Fu TS, Monteiro E, and Muhanna N, et al (2016) Comparison of outcomes for open versus endoscopic approaches for olfactory neuroblastoma: a systematic review and individual participant data meta-analysis Head Neck 38(Suppl 1) E2306–E2316 https://doi.org/10.1002/hed.24233
24. Wang J, Wang L, and He H, et al (2021) The treatment outcomes of olfactory neuroblastoma patients with frontal lobe invasion Front Oncol 11 640892 https://doi.org/10.3389/fonc.2021.640892 PMID: 34290975 PMCID: 8289277
25. Howell MC, Branstetter BF, and Snyderman CH (2011) Patterns of regional spread for esthesioneuroblastoma AJNR Am J Neuroradiol 32(5) 929–933 https://doi.org/10.3174/ajnr.A2401 PMID: 21349970 PMCID: 7965541
26. Zollinger LV, Wiggins RH, and Cornelius RS, et al (2008) Retropharyngeal lymph node metastasis from esthesioneuroblastoma: a review of the therapeutic and prognostic implications Am J Neuroradiol 29(8) 1561–1563 https://doi.org/10.3174/ajnr.A1114 PMID: 18499797 PMCID: 8119040
27. Kim HJ, Kim J, and Yoon JH (2006) Retropharyngeal lymph node metastasis from olfactory neuroblastoma: a report of two cases Eur Arch Otorhinolaryngol Head Neck 263(8) 778–782
28. Zanation AM, Ferlito A, and Rinaldo A, et al (2010) When, how and why to treat the neck in patients with esthesioneuroblastoma: a review Eur Arch Otorhinolaryngol 267(11) 1667–1671 https://doi.org/10.1007/s00405-010-1360-6 PMID: 20706843 PMCID: 3005584
29. Orton A, Boothe D, and Evans D, et al (2018) Esthesioneuroblastoma: a patterns-of-care and outcomes analysis of the national cancer database Neurosurgery 83(5) 940–947 https://doi.org/10.1093/neuros/nyx535 PMID: 29481629
30. Burnham AJ, Burnham PA, and Horwitz EM (2021) Survival associations between patient age and treatment modality in olfactory neuroblastoma: a retrospective population-based study J Clin Med 10(12) 2685 https://doi.org/10.3390/jcm10122685 PMID: 34207118 PMCID: 8235675
31. Nakamura N, Zenda S, and Tahara M, et al (2017) Proton beam therapy for olfactory neuroblastoma Radiother Oncol 122(3) 368–372 https://doi.org/10.1016/j.radonc.2016.12.020 PMID: 28062086
32. Nishimura H, Ogino T, and Kawashima M, et al (2007) Proton-beam therapy for olfactory neuroblastoma Int J Radiat Oncol Biol Phys 68(3) 758–762 https://doi.org/10.1016/j.ijrobp.2006.12.071 PMID: 17398027
33. Koto M, Demizu Y, and Saitoh JI, et al (2018) Definitive carbon-ion radiation therapy for locally advanced sinonasal malignant tumors: subgroup analysis of a multicenter study by the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Int J Radiat Oncol 102(2) 353–361 https://doi.org/10.1016/j.ijrobp.2018.05.074
34. Suefuji H, Koto M, and Demizu Y, et al (2018) A retrospective multicenter study of carbon ion radiotherapy for locally advanced olfactory neuroblastomas Anticancer Res 38(3) 1665–1670 PMID: 29491100
35. Bockmühl U, You X, and Pacyna-Gengelbach M, et al (2004) CGH pattern of esthesioneuroblastoma and their metastases Brain Pathol Zurich Switz 14(2) 158–163 https://doi.org/10.1111/j.1750-3639.2004.tb00048.x
36. Topcagic J, Feldman R, and Ghazalpour A, et al (2018) Comprehensive molecular profiling of advanced/metastatic olfactory neuroblastomas PLoS One 13(1) e0191244 https://doi.org/10.1371/journal.pone.0191244 PMID: 29324814 PMCID: 5764485
37. Lazo de la Vega L, McHugh JB, and Cani AK, et al (2017) Comprehensive molecular profiling of olfactory neuroblastoma identifies potentially targetable FGFR3 amplifications Mol Cancer Res 15(11) 1551–1557 https://doi.org/10.1158/1541-7786.MCR-17-0135 PMID: 28775129
38. Czapiewski P, Kunc M, and Haybaeck J (2016) Genetic and molecular alterations in olfactory neuroblastoma: implications for pathogenesis, prognosis and treatment Oncotarget 7(32) 52584 https://doi.org/10.18632/oncotarget.9683 PMID: 27256979 PMCID: 5239575