DEHyART trial: Study protocol for phase 2 randomised controlled study assessing the role of dose escalation using [18F] fluoromisonidazole positron emission tomography/computed tomography in head and neck cancers
Sarthak Tandon1,a, Manoj Gupta2, Parveen Ahlawat1, Madhur Verma3, Apoorva Nayak1, Akash Bellige1, Kundan S Chufal1, Jaskaran S Sethi1, Anjali Pahuja1, Shreya Rai4, Abhishek Singh4, Vikas Arora4, Vishal Yadav4, David K Simson1, Irfan Ahmad1, Sandeep Singh5, Dipesh Vashisht5, Azhar Ansari1, Rashmi Bansal6, Abhishek Bhadri1, Harsh Vyas1, Manindra Mishra4, Rajat Saha6, Mudit Agarwal3, Partha S Chowdhary2, Ajay K Dewan4 and Munish Gairola1
1Department of Radiation Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Delhi 110085, India
2Department of Nuclear Medicine, Rajiv Gandhi Cancer Institute and Research Centre, Delhi 110085, India
3Department of Community and Family Medicine, AIIMS, Bhatinda 110029, India
4Department of Surgical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Delhi 110085, India
5Division of Medical Physics, Department of Radiation Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Delhi 110085, India
6Department of Medical Oncology, Rajiv Gandhi Cancer Institute and Research Centre, Delhi 110085, India
ahttps://orcid.org/0000-0002-2319-0391
Abstract
Background: Head and neck squamous cell carcinoma is often treated with radiotherapy, frequently combined with chemotherapy, to improve overall survival (OS). Despite advancements, locoregional control (LRC) remains a significant challenge, with 15%–50% of patients experiencing locoregional recurrence, negatively impacting OS and quality of life. Hypoxia within tumor cells is a critical factor contributing to treatment failure, necessitating higher radiation doses to achieve similar therapeutic effects as in normoxic cells. This study aims to investigate the role of dose escalation using [18F] fluoromisonidazole (FMISO) positron emission tomography/computed tomography (PET CT) to target hypoxic sub-volumes in head and neck cancer (HNC) to improve LRC.
Methods: The dose-escalated hypoxia-adjusted radiotherapy trial is an open-label, parallel, randomised, single-centre, phase II study. Patients with HNC will undergo [18F]. FMISO PET CT to identify hypoxic regions. Normoxic patients will be labeled as Arm 1 and will not be part of the primary assessment. Patients with hypoxia will be stratified into two arms (2 and 3). Arm 2 will receive standard radiotherapy of 70 Gy in 2 Gy fractions, while Arm 3 will receive an additional boost to the hypoxic sub-volumes, delivering a total of 80 Gy (Phase 2). All patients in Arms 2 and 3 will also receive concurrent chemotherapy with cisplatin. Patients will be monitored weekly for treatment tolerance, with acute adverse events recorded according to National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. The primary endpoint is LRC, defined as the time from randomisation to the first histopathologically confirmed relapse of locoregional disease. Secondary endpoints include OS, locoregional relapse-free survival, acute and late toxicity and patient-reported outcomes assessed using the European Organisation for Research and Treatment of Cancer QLQ-C30 and QLQ-H&N35 questionnaires.
Discussion: This study addresses a critical gap in the management of HNC by targeting hypoxic regions within tumours, potentially improving LRC and, consequently, OS. The use of [18F] FMISO PET CT for identifying hypoxic sub-volumes allows for tailored radiation dose escalation, which could overcome the radioresistance associated with hypoxia. By comparing outcomes among standard radiotherapy (Arm 2) and dose-escalated treatment (Arm 3), this trial aims to establish a more effective therapeutic strategy for HNC patients.
Trial registration: This trial is registered with the Clinical Trials Registry of India (CTRI/2024/04/065373), registered on 08th April 2024 on ctri.nic.in and clinicaltrials.gov (NCT06087614) registered on 18th September 2023 on clinicaltrials.gov.
Keywords: dose escalation, IMRT, HNSCC, radiotherapy, hypoxia, FMISO
Correspondence to: Sarthak Tandon
Email: drsarthaktandon@yahoo.in
Published: 02/07/2025
Received: 26/02/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Background and rationale
Radiotherapy is the mainstay in treating head and neck squamous cell carcinoma (HNSCC). The concurrent chemotherapy with radiotherapy gives an additional absolute survival benefit of 6.5% at 5 years [1]. Despite advancements in radiation and chemotherapy, the overall survival (OS) in HNSCC has been dismal. Locoregional control (LRC) drives the overall survival in most head and neck squamous cell carcinoma, however, it is reported that about 15%–50% of patients fail locoregionally [2,3,4].Locoregional recurrence not only causes a drop in OS but is also a major concern for adding overall morbidity and deteriorating patients’ quality of life. Therefore, further improving on LRC would be a prudent therapeutic strategy for increasing OS and enhancing the quality-of-life indices. The authors assessed the pattern of failure for HNSCC being treated with intensity-modulated radiotherapy (IMRT). They found that with a 3-year LRC of 48.9%, most failed in the high-dose region (69.2%), suggesting inherent biological resistance to treatment [5,6]. One hypothesised reason for this treatment failure is the presence of hypoxia in tumour cell lines, which may necessitate three times the radiation dose to achieve an isotherapeutic effect comparable to that in normoxic cells [7].
Hypoxia in head and neck patients
Tumour hypoxia is a major risk factor for local and distant failure after radiotherapy [8]. It is reported that up to 80% of HNSCC have baseline hypoxia, with sites like hypopharyngeal and oropharyngeal having a higher incidence of hypoxia than the other sites of the head and neck [9]. Non-invasive hypoxia assessment in head and neck cancer (HNC) has been extensively conducted using various radioactive tracers such as [18F] fluoromisonidazole (FMISO), [18F] fluoroazomycin-arabinoside (FAZA) and [18F] HX4. These have been individually studied in various studies [10–14] and have been correlated with other hypoxia biomarkers in numerous studies as well [15–17]. A recent meta-analysis showed that positron emission tomography (PET)-measured hypoxia with FMISO or FAZA strongly impacted OS and LRC in HNSCC. Most trials have used FAZA and FMISO, which have shown equivalence in multicentre trials [18]. The meta-analysis by Zschaeck et al [18]. showed that the hypoxia PET parameter was an independent risk factor in multivariate analysis, irrespective of any established clinical parameter. Studies have investigated whether subsequent recurrences overlap with the initial hypoxic volumes, yielding discordant results. Boeke et al [19]. reported a high median overlap of 42%, while two other studies [20, 21] found no significant overlap. Nishikawa et al [22] demonstrated that an initial tissue-to-muscle ratio (TMR) greater than 2.42 could predict failure in terms of spatial location. These findings underscore the need for a second FMISO scan during the course of radiotherapy, which may better predict chronic hypoxia. Considering the significant influence of tumour-associated hypoxia on the response of HNSCCs to radiotherapy, different treatment strategies have been proposed and tested in exploratory analyses, trying to identify the stability of these sub-volumes over multiple fractions and the possibility of escalating treatment doses to high-risk hypoxic sub-volumes (HSVs) [23–25].
Rationale for dose escalation in HNC
Theoretical planning studies have been conducted to assess the dose escalation to the biology-selected FMISO volumes [26–28]. Thorwarth et al [27] predicted the increase in tumour control probability from 55.9% to 70.9% by increasing the dose up to 84 Gy to the FMISO-selected hypoxic volumes. In a recent Phase II randomised study by Welz et al [29], escalated doses to the hypoxic volumes determined by FMISO. The authors escalated doses to this volume to 77 Gy (2.2 Gy per fraction) by the simultaneous integrated boost technique and compared it to standard fractionation and doses. There was a non-significant increase in 5-year local control favouring dose dose-escalated arm. However, the study was prematurely closed due to poor accrual. Another flaw in the study design could be a marginal increase in dose escalation, with a 2% mean dose escalation against a planned 10% dose escalation. The mean hypoxic volume subjected to dose escalation was 2.9 ml, which might be too small to make any significant difference in outcomes. An ongoing phase III trial, ESCALOX [30], assesses dose escalation to the hypoxic volumes to a dose of 80.5 Gy and is currently accruing patients.
Objectives
There exists a critical need to increase LRC in HNC while maintaining the patients’ quality of life. Numerous studies [31–33] have investigated dose and volume modifications in p16-positive oropharyngeal cancers. However, there is a paucity of literature addressing these modifications in p16-negative oropharyngeal and non-oropharyngeal tumours, underscoring a significant research gap. The primary objective of this study – DEHyART is to assess the role of dose escalation using [18F]. FMISO positron emission tomography/computed tomography (PET CT) in HNC to improve LRC by targeting HSV. Specifically, the study aims to evaluate whether increasing the radiation dose to hypoxic areas within tumours, identified through FMISO PET CT, can enhance treatment outcomes in terms of LRC compared to standard treatment.
The secondary objectives include evaluating the impact of this treatment on overall survival, acute and late toxicity, and patient-reported outcomes such as quality of life. The study also aims to compare the outcomes between the dose-escalated group and the standard treatment group in a randomised controlled trial setting.
Study/Trial design
DE-HyART is an open-label, parallel, randomised, single-centre, phase II study designed to evaluate the effectiveness and feasibility of dose escalation using FMISO PET scans for HNC. This superiority trial aims to assess the impact of integrating IMRT with simultaneous integrated boost (SIB) and dose escalation to hypoxic sub-volumes, as delineated by [18-F] FMISO, compared to standard-dose radiotherapy. The treatment protocol also incorporates concurrent chemotherapy with standard dosing of the physician’s choice and patients’ fitness. The protocol has been developed in accordance with SPIRIT guidelines [34, 35].
Participants, interventions and outcomes
DE-HyART is an investigator-initiated study coordinated by the Department of Radiation Oncology at Rajiv Gandhi Cancer Institute and Research Centre, Delhi. The department will oversee the entire study, including registration, data management and radio-oncological quality assurance. The study has received approval from the Scientific Review Board and Institutional Review Board (IRB) for scientific and ethical compliance. All participants will provide written informed consent prior to enrollment, which will outline the randomised nature, scope and potential consequences of the study taken by the principal investigator or research coordinator. The flow of the study will be as mentioned in Table 1 and Figure 1.
Eligibility criteria
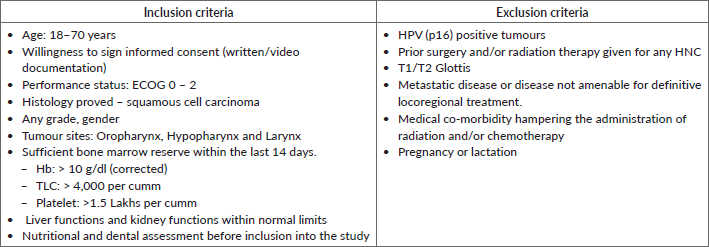
All HNC patients planned for radical radiotherapy (± concurrent chemotherapy) in the department of radiation oncology were screened as per the inclusion and exclusion criteria mentioned in Table 2.
Participant screening, recruitment and consent [18F] FMISO PET CT and hypoxia identification
All eligible patients will be subjected to screening [18F] FMISO scan, labelled as baseline FMISO. The primary challenge with using FMISO is its lipophilic nature, which slows its clearance from normal tissues such as blood and muscle, resulting in a low signal-to-background ratio. Numerous studies [12, 36–39] have evaluated FMISO parameters with clinical endpoints, demonstrating that a TMR ranging from 1.2 to 1.6 significantly correlates with the reduction of hypoxic volumes during the course of radiotherapy.
Therefore, a TMR of 1.4 will be employed to differentiate hypoxic from normoxic patients (TMR ≥ 1.4 and < 1.4 will be labelled as hypoxic and normoxic, respectively). Standardised assessment of SUVmax and SUVmean will be utilised. Tumour SUVmax will be determined by generating a region of interest (ROI) of 1 cm² at the most metabolically active region of the tumour. For background reference, the contralateral sternocleidomastoid muscle at the caudal edge of the hyoid bone will be used, with a similar ROI to calculate the TMR.
Timing of [18F] FMISO PET CT
All enrolled patients will undergo an [18F] FMISO PET CT scan. Patients exhibiting hypoxia, as determined by the PET results, will be randomised into either Arm 2 or Arm 3. A second [18F] FMISO PET CT scan will be performed between the fourth and fifth weeks of radiation therapy to evaluate the temporal variability of HSV.
Table 1. Schedule of enrolment, interventions, and assessments for the DEHyART study as per SPIRIT guidelines.
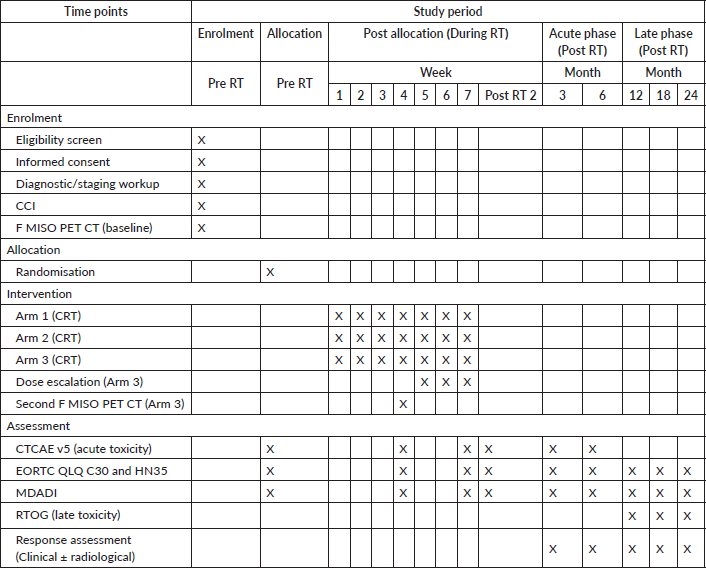
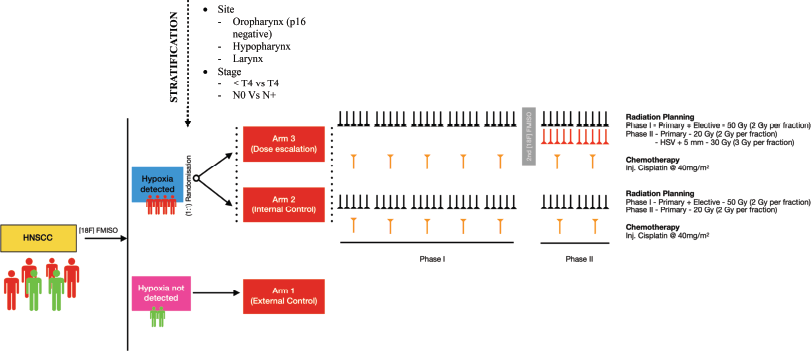
Figure 1. A detailed schematics showing flow of the study.
Table 2: Inclusion and exclusion criteria for enrolment in DEHyART study.
Arm allocation and randomisation
Depending upon the result of the baseline FMISO, the patient will be either hypoxic or normoxic. Patients exhibiting no hypoxia (TMR < 1.4) in their tumour will be labelled as Arm 1 and act as an external cohort. Patients with hypoxia (TMR ≥ 1.4) will be stratified by T stage, N stage and tumour site, then randomly assigned in a 1:1 ratio to either Arm 2 or Arm 3 using a computer-generated randomisation sequence to ensure balanced distribution across these variables. Both arms will be subjected to chemoradiation by IMRT and concurrent chemotherapy with cisplatin at 40 mg/m2. In Arm 3, the trial arm will receive an additional dose of 10 Gy @ 2 Gy per fraction in phase II (total 80 Gy) to the HSV + 5mm isotropic margin.
Intervention
All patients will be treated using IMRT (with pre-specified image guidance protocol as per institutional standards).
Arm 1
Patients will receive standard care and serve as external controls, representing typical clinical practice. Their cases will be reviewed in the head and neck multidisciplinary clinic, following the institutional approach. These patients will undergo treatment analogous to that of ‘Arm 2,’ but with allowances for protocol deviations at the discretion of the treating radiation oncologist.
Arm 2
The prescribed radiotherapy dose will be 70 Gy, delivered in daily fractions of 2 Gy. The elective volume will receive 50 Gy, administered in 2 Gy daily fractions over the first 5 weeks. This will be followed by a boost to the high-risk volume, delivering an additional 20 Gy in 2 Gy daily fractions over the subsequent 2 weeks. The entire treatment will be delivered in a phased approach using sequential planning.
Arm 3
The radiation dose will be similar to ‘Arm 2’. In addition, the HSV identified on baseline FMISO scans will be contoured and an isotropic margin of 3 mm will be given. This volume will be boosted in phase II to a total dose of 80 Gy. (Addition of 30 Gy in 3 Gy daily fraction added in phase II as a simultaneous integrated boost – SIB) (Figure 2). The BED and EQD2 for Arm 3 (to HSV) are 99 Gy10 and 82.5Gy, respectively, in comparison to 84 Gy10 and 70 Gy, respectively, in Arm 2.
Contouring protocol
The patients will be immobilised using a four-clip thermoplastic cast (Orfit Ray™ Cast) as per standard institutional protocol. The treatment planning scans will be performed with IV contrast and 3 mm slice thickness. Daily IGRT is not mandatory but recommended. The daily shift should be at most 5mm of the planning target volume in any axes.
Arm 2 and 3: Gross tumour volume (GTV) will be delineated using MRI and FDG PET CT per standard institutional standards. Clinical target volume (CTV 1) will include GTV based on clinical and radiologic information (including primary tumour and involved lymph nodes), expanded with isotropic 5-mm margins. Clinical target volume (CTV 2) will consist of the high-risk areas harbouring microscopic disease and elective nodal regions. CTV 2 will be created individually for the primary target and the secondary lymphatic. A 5 mm margin will be added to each CTV 1 and 2 to form planning target volume (PTV) 1 and 2, respectively. A model dose of 50 Gy in 25 fractions will be prescribed to PTV 2 in phase I and 20 Gy in 10 fractions to PTV 1 in phase II.
Hypoxic sub-volume delineation (Arm 3): The HSV delineation will be done for patients in arm 3 using baseline FMISO. The HSV will be contoured as per baseline FMISO and adjusted according to the second FMISO scan done between the fourth and the fifth week of radiation treatment. A planning CT will also be repeated at the time for adjusting the HSV to account for temporal changes. The HSV, thus, delineated will be isotropically enlarged by 3 mm to form a biological target volume (BTV). The BTV will be truncated from bone, air and organs at risk (OAR) by 3mm. The BTV will be prescribed 30 Gy in 10 fractions.
Adjusting HSV in phase 2: The HSV will not be adapted based on the response, but will be adjusted only to account for geometrical changes due to image registration in the new scan taking into account – OARs, air and bone. The boost volume will primarily remain consistent with the baseline HSV.
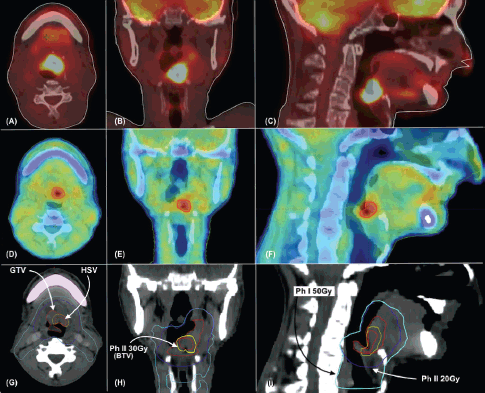
Figure 2. (a–c) shows 18F FDG PET CT images in axial, coronal and sagittal views, respectively. (d–f) shows 18F FMISO PET CT images in axial, coronal and sagittal views, respectively. (g–i), target delineation as labeled in axial, coronal and sagittal views, respectively. GTV – Gross Tumour Volume, HSV – Hypoxic Sub-Volume, Ph I 50 Gy – Volume receiving 50 Gy in 25 fractions in phase 1, Ph II 20Gy – Volume receiving 20 Gy in 10 fractions in phase 2. Ph II 30Gy – Volume receiving 30 Gy in 10 fractions in phase 2.
Organ at risk: Dose constraints to OAR and planning risk volume will be taken from quantitative analysis of normal tissue effects in the clinic (QUANTEC) and RTOG protocols (wherever QUANTEC did not provide constraints). The patients in ‘Arm 3’ will be subjected to isotoxic dose escalation, with constraints remaining the same as Arm 2.
Treatment planning: All patients will be treated on TrueBeam™ version 1.6 (Varian Medical Systems Inc., Palo Alto, CA, USA) equipped with HD-120 leaf MLC (28 pairs of 5 mm and 32 pairs of 2.5 mm). Plans were made on Varian Eclipse™ Version 15 (Varian Medical Systems Inc., Palo Alto, CA, USA) using 7–9 co-planar static fields with collimator angle 0. IMRT plans will be optimised using a dose-volume optimiser and calculated using the Anisotropic Analytical Algorithm.
Adherence to protocol and concomitant care
Patients will be monitored weekly to assess their tolerance to treatment. Any patient exhibiting grade 3 or higher toxicity will require urgent therapeutic intervention. The most anticipated side effects are mucositis and dysphagia. Patients will be evaluated weekly for the need for nutritional support and feeding tube placement, as well as on an as-needed basis. In rare instances of severe toxicities not responding to conservative measures, a treatment break may be necessary. The resumption of radiotherapy will be contingent upon the resolution of major toxicities, with the aim of restarting radiotherapy as soon as clinically feasible.
Outcome measures: Oncological outcomes will be collected by a designated team of head and neck specialists (radiation oncologist, surgeon and radiologist)
Response assessment: The patients would be assessed for the first time after 12 weeks of completion of definitive radiotherapy. The patients would be assessed by 18-FDG PET CT (± MRI) and direct laryngoscopy. The need for biopsy versus repeat imaging at a short interval will be taken by a multispeciality clinic taken into view the clinical examination, laryngoscopy findings (including narrow band imaging) and radiological findings.
Primary endpoint: locoregional control (LRC): LRC is defined as the absence of tumour recurrence or progression within the primary tumour site and the regional lymph nodes, as determined by clinical evaluation, imaging studies and/or biopsy confirmation. LRC will be assessed at predefined time points, with the primary time point being 2 years post-treatment. Locoregional recurrence within 2 years post-treatment is associated with significant morbidity and reduced quality of life. Therefore, 2-year LRC serves as a clinically meaningful endpoint to guide treatment decisions and optimise outcomes for patients receiving escalated radiation doses.
Secondary endpoints
Overall Survival (OS): The duration of OS is defined as the time from the date of randomisation to death from any cause. If there is no death, the OS duration will be censored at the time of analysis.
Locoregional relapse-free survival (LRFS): LRFS is defined as the time from the date of randomisation to the first histopathologically confirmed relapse of locoregional disease. If there is no confirmed recurrence, the LRC duration will be censored at the time of analysis. Death from any cause will be considered as an event in LRFS.
Acute toxicity: Acute adverse events will be assessed and recorded according to the NCI CTCAE version 5.0 [40]. Evaluations will be conducted at baseline, mid-treatment (week 4), immediately post-treatment, 2 weeks post-radiotherapy and subsequently at 3 months post-treatment.
Late toxicity assessment: Long-term toxicities of the upper aerodigestive tract will be recorded at 1 and 2-year follow-ups. They will be assessed using the RTOG long-term toxicity assessment [41].
Patient-reported outcome: The data will be collected by the trial coordinator and, research nurse by collecting proformas filled by patients.
European Organisation for Research and Treatment of Cancer (EORTC) head and neck questionnaires: The Hindi versions of the EORTC QLQ-C30 [42] and QLQ-H and N35 questionnaires [43, 44] will be obtained from the QoL unit, EORTC Data Centre in Brussels, Belgium. The EORTC QLQ-C30 questionnaire consists of five functional scales that include physical, role, cognitive, emotional and social; three symptom scales that include fatigue, pain and nausea/vomiting; a global QoL scale; and six single items that include dyspnea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties. The QLQ-H and N 35 questionnaires consisted of seven multiple-item scales that assess the symptoms of pain, swallowing ability, senses (taste/smell), speech, social eating, social contact and sexuality and six single-item scales that assess the presence of symptomatic problems associated with teeth, mouth opening, dry mouth (xerostomia), sticky saliva, coughing and feeling ill. All the scales of the EORTC QLQ-C30, QLQ-H and N35 range from 0 to 100.
Dysphagia assessment: Dysphagia is commonly evaluated through functional assessments of swallowing, such as videofluoroscopy or fiberoptic endoscopic evaluation. Furthermore, tools that quantify the severity and impact of dysphagia are essential for determining treatment outcomes and rehabilitation requirements. Various instruments are available for assessing dysphagia, including the 10-item Eating Assessment Tool [45], the Sydney Swallow Questionnaire [46], the Swallowing Quality of Life Questionnaire (SWAL-QOL) [47] and the MD Anderson Dysphagia Inventory (MDADI) [48]. The MDADI includes global, emotional, functional and physical subscales, each designed to capture different aspects of how dysphagia impacts quality of life. The global assessment question provides a straightforward method for evaluating overall dysphagia severity. It was specifically chosen because of its relatively shorter 20-point questionnaire and easier implementation, especially when compared to SWAL-QOL or dysphagia handicap index [49].
Sample size
Estimated LC rates 59% and 84% in the control and treatment arm, respectively, in the phase II study by Welz et al [29]. For achieving an 80% power (i.e., 1−β = 0.8) at the 5% level of significance (i.e., α = 0.05) with the equal allocation (i.e., k = 1), the sample size for Arm 2 and 3 is 62 patients in each arm. This study is designed as a Phase II trial aimed at evaluating the preliminary efficacy and safety of the intervention. As such, the primary focus is on detecting a signal of LRC rather than establishing a long-term survival benefit. The sample size of 124 participants (62 per group) is consistent with Phase II study designs, which typically involve smaller cohorts to efficiently assess early efficacy. Due to the exploratory nature of this trial, the follow-up period was set at 2 years to capture early control rates, which are increasingly recognised as predictive of long-term outcomes in salivary ductal cancers. The 2-year endpoint aligns with the study’s goal of generating data to inform the feasibility of subsequent Phase III trials.
Data collection and analysis
All data will be collected in the central registry of the institute and anonymised. All statistical analyses were performed using the Statistical Package for Social Science System (SPSS v26, SPSS Inc, Chicago, IL) or equivalent software. Continuous variables will be written as mean (along with standard deviation) and median (along with interquartile range). Categorical variables will be represented as frequencies and percentages. Inter-cohort testing will be performed using chi-square for categorical variables and independent-t/nonparametric tests for continuous variables. Time-to-event endpoints will be measured from the date of randomisation to the date of failure or last follow-up. The LRC rate at 2 years will be estimated using the Kaplan–Meier method, with the time to locoregional failure defined as the duration from the start of radiation therapy to the first documented recurrence or progression within the primary tumour site or regional lymph nodes. The LRFS and OS, as mentioned earlier, death from any cause will be considered a failure. The LRFS and the OS rates will be estimated using the Kaplan–Meier product-limit method for each arm. Their distributions will be compared between treatment arms (Arms 2 and 3) with a two-sided log-rank test ≤ 0.05. The clinicopathological characteristics, survival outcomes and treatment modalities will be analysed using the univariate and multivariate Cox proportional hazards model. All eligible patients will be included in the analysis in the arm to which they were randomly assigned (intent-to-treat).
Interim analyses
Interim analyses are not planned for this study to avoid the potential introduction of Type I and Type II errors. Conducting interim analyses could increase the likelihood of false-positive results, leading to incorrect conclusions about the treatment’s efficacy. Additionally, it could result in false negative findings, potentially overlooking beneficial effects that emerge later in the treatment course.
Oversight and data monitoring committee
Organisational structure, protocol revision, publication and managing general administrative function will be carried out by principal investigator and co-principal investigator. A separate trial committee (see title page for members) will be tasked to ensure quality checks on the radiation plan, data entry and 6-monthly report and audit. Any unexpected or grade 5 toxicity will be reported to the Serious Adverse Events (SAEs) Committee, which will monitor and evaluate the potential causes of these adverse effects.
Recruitment strategy: The trial will be conducted at a tertiary care centre specialising exclusively in oncology. Based on the annual case volume managed by the head and neck multispecialty clinic, the projected sample collection is expected to be completed within 2 years.
Retention strategy: Patients will be advised on the importance of maintaining diligent follow-up and completing the health-related quality of life questionnaire. In the event of a missed follow-up, efforts will be made to contact patients via telephone, including offering video consultations, to ensure the completion of the required forms. Long-term care will be provided to those whose side effects are considered to be beyond standard of care treatment, as assessed by the SAE committee.
Trial status
The trial is currently in the recruitment phase, actively enrolling patients since March 2024, with an expected completion date of March 2026. The protocol in use is version 1.2, dated August 28, 2023.
Discussion
The trial is currently ongoing and in the recruitment phase, with completion of recruitment anticipated by March 2026. A significant challenge for the trial is the procurement of the FMISO radiotracer, which may potentially delay the project timeline. To date, most patients are tolerating the treatment well, with no serious SAEs reported.
Ethics approval and consent to participate
The study is being conducted following approval from the IRB and the Scientific Review Board. Before enrollment, informed consent forms, signed by the patients, will be collected by the principal investigator, research coordinator or an assigned researcher. All data will be anonymised, with no patient identifiers retained in the research database. Additionally, a separate clinical data record will be maintained in the institute’s registry. Any amendments to the protocol will be promptly reported to the IRB and the funding agency.
Acknowledgments
The authors would like to thank all the support given by the institute and their respective departments. They would also like to thank all the trial patients.
Conflicts of interest
The authors have no competing interests.
Funding
The study has been funded by Varian Medical Systems, Inc.
Consent for publication
Yes, including for ancillary studies if planned.
Availability of data and material
Individual patient data and statistical analysis required to support the protocol can be provided upon request and the final decision to share will be subject to steering committee approval. The investigator and the participating site will ensure data protection and prevent any disclosure outside the scope of the research.
Author contributions
ST and MG (Munish Gairola) have contributed equally to all aspects of the protocol, study design, writing, conceptualisation and are the co-PI and PI of the study, respectively. ST, MV, PA and AN have contributed to constructing the statistical design for the study. MG (Munish Gairola), MM, PSC and MG (Manoj Gupta) have been involved in analysing PET metrics and segmentation. SR, AB (Akash Bellige), AS, VY, VA,
DKS, IA and RS have helped in reviewing the literature and designing proper design parameters. KSC, JSS, AP, MA, AA, RB and AKD have maintained the quality check on trial study parameters. SS, DV, AB (Abhishek Bhadri) and HV have maintained radiation quality check and control. All authors have read and contributed to various aspects of patient care, protocol implementation and manuscript writing.
References
1. Pignon JP, le Maitre A, and Maillard E, et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients Radiother Oncol 92(1) 4–14 https://doi.org/10.1016/j.radonc.2009.04.014 PMID: 19446902
2. Posner MR, Hershock DM, and Blajman CR, et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer N Engl J Med 357 1705–1715 https://doi.org/10.1056/NEJMoa070956 PMID: 17960013
3. Brockstein B, Haraf DJ, and Rademaker AW, et al (2004) Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience Ann Oncol 15 1179–1186 https://doi.org/10.1093/annonc/mdh308 PMID: 15277256
4. Bourhis J, Le Maître A, and Baujat B, et al (2007) Meta-Analysis of Chemotherapy in Head, Neck Cancer Collaborative Group. Meta-Analysis of Radiotherapy in Carcinoma of Head, Neck Collaborative Group. Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma Collaborative Group Individual patients’ data meta-analyses in head and neck cancer Curr Opin Oncol 19 188–194 https://doi.org/10.1097/CCO.0b013e3280f01010 PMID: 17414635
5. Leeman JE, Li JG, and Pei X, et al (2017) Patterns of treatment failure and postrecurrence outcomes among patients with locally advanced head and neck squamous cell carcinoma after chemoradiotherapy using modern radiation techniques JAMA Oncol 3 1487–1494 https://doi.org/10.1001/jamaoncol.2017.0973 PMID: 28542679 PMCID: 5710194
6. Tandon S, Gairola M, and Ahlawat P, et al (2019) Failure patterns of head and neck squamous cell carcinoma treated with radical radiotherapy by intensity-modulated radiotherapy technique using focal volume and dosimetric method Head Neck 41(6) 1632–1637 https://doi.org/10.1002/hed.25586
7. Gray LH, Conger AD, and Ebert M, et al (1953) The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy Br J Radiol 26 638–648 https://doi.org/10.1259/0007-1285-26-312-638 PMID: 13106296
8. Nordsmark M, Overgaard M, and Overgaard J (1996) Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck Radiother Oncol 41 31–39 https://doi.org/10.1016/S0167-8140(96)91811-3 PMID: 8961365
9. Rischin D, Fisher R, and Peters L, et al (2007) Hypoxia in head and neck cancer: studies with hypoxic positron emission tomography imaging and hypoxic cytotoxins Int J Radiat Oncol Biol Phys 69(2) S61–S63 https://doi.org/10.1016/j.ijrobp.2007.05.043 PMID: 17848298
10. Rischin D, Hicks RJ, and Fisher R, et al (2006) Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group study 98.02 J Clin Oncol 24(13) 2098–2104 https://doi.org/10.1200/JCO.2005.05.2878 PMID: 16648512
11. Kikuchi M, Yamane T, and Shinohara S, et al (2011) 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma Ann Nucl Med 25(9) 625–633 https://doi.org/10.1007/s12149-011-0508-9 PMID: 21720778
12. Löck S, Kamierska R, and Seidlitz A, et al (2017) Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging Radiother Oncol 124 533–540 https://doi.org/10.1016/j.radonc.2017.08.010 PMID: 28843726
13. Mortensen LS, Johansen J, and Kallehauge J, et al (2012) FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial Radiother Oncol 105 14–20 https://doi.org/10.1016/j.radonc.2012.09.015 PMID: 23083497
14. Zegers CML, Hoebers FJP, and van Elmpt W, et al (2016) Evaluation of tumour hypoxia during radiotherapy using [(18)F]HX4 PET imaging and blood biomarkers in patients with head and neck cancer Eur J Nucl Med Mol Imaging 43 2139–2146 https://doi.org/10.1007/s00259-016-3429-y PMID: 27251643 PMCID: 5047929
15. Bittner MI, Wiedenmann N, and Bucher S, et al (2016) Analysis of relation between hypoxia PET imaging and tissue-based biomarkers during head and neck radi- ochemotherapy Acta Oncol 55 1299–1304 https://doi.org/10.1080/0284186X.2016.1219046 PMID: 27593107
16. Norikane T, Yamamoto Y, and Maeda Y, et al (2014) Correlation of (18)F-fluoromisonidazole PET findings with HIF-1α and p53 expressions in head and neck cancer: comparison with (18)F-FDG PET Nucl Med Commun 35 30–35 https://doi.org/10.1097/MNM.0000000000000010
17. Löck S, Linge A, and Seidlitz A, et al (2019) Repeat FMISO-PET imaging weakly correlates with hypoxia-associated gene expressions for locally advanced HNSCC treated by primary radiochemotherapy Radio- ther Oncol 135 43–50 https://doi.org/10.1016/j.radonc.2019.02.020
18. Zschaeck S, Löck S, and Hofheinz F, et al (2020) Individual patient data meta-analysis of FMISO and FAZA hypoxia PET scans from head and neck cancer patients undergoing definitive radio- chemotherapy Radiother Oncol 149 189–96 https://doi.org/10.1016/j.radonc.2020.05.022 PMID: 32417350
19. Boeke S, Thorwarth D, and Mönnich D, et al (2017) Geometric analysis of loco-regional recurrences in relation to pre-treatment hypoxia in patients with head and neck cancer Acta Oncol 56 1571–1576 https://doi.org/10.1080/0284186X.2017.1372626 PMID: 28891398
20. Zschaeck S, Haase R, and Abolmaali N, et al (2015) Spatial distribution of FMISO in head and neck squamous cell carcinomas during radio-chemotherapy and its cor- relation to pattern of failure Acta Oncol 54 1355–1363 https://doi.org/10.3109/0284186X.2015.1074720
21. Dirix P, Vandecaveye V, and De Keyzer F, et al (2009) Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with (18)F-FDG PET, (18)F-fluoromisonidazole PET, diffusion- weighted MRI, and dynamic contrast-enhanced MRI J Nucl Med 50 1020–1027 https://doi.org/10.2967/jnumed.109.062638 PMID: 19525447
22. Nishikawa Y, Yasuda K, and Okamoto S, et al (2017) Local relapse of nasopharyngeal cancer and Voxel- based analysis of FMISO uptake using PET with semiconduc- tor detectors Radiat Oncol 12 148 https://doi.org/10.1186/s13014-017-0886-9
23. Reuzé S, Schernberg A, and Orlhac F, et al (2018) Radiomics in nuclear medicine applied to radiation therapy: methods, pitfalls, and challenges Int J Radiat Oncol 102 1117–1142 [doi: 10.1016/j.ijrobp.2018.05.022] https://doi.org/10.1016/j.ijrobp.2018.05.022
24. Zwanenburg A (2019) Radiomics in nuclear medicine: robustness, reproducibility, standardization, and how to avoid data analysis traps and replication crisis Eur J Nucl Med Mol Imaging 46 2638–2655 https://doi.org/10.1007/s00259-019-04391-8 PMID: 31240330
25. Scrivener M, de Jong EE, and van Timmeren JE, et al (2016) Radiomics applied to lung cancer: a review Transl Cancer Res 5 398–409 https://doi.org/10.21037/tcr.2016.06.18
26. Rajendran JG, Hendrickson KR, and Spence AM, et al (2006) Hypoxia imaging-directed radiation treatment planning Eur J Nucl Med Mol Imaging 33(Suppl. 1) 44–53 https://doi.org/10.1007/s00259-006-0135-1 PMID: 16763816
27. Thorwarth D, Eschmann SM, and Paulsen F, et al (2007) Hypoxia dose painting by numbers: a planning study Int J Radiat Oncol Biol Phys 68 291–300 https://doi.org/10.1016/j.ijrobp.2006.11.061 PMID: 17448882
28. Lee NY, Mechalakos JG, and Nehmeh S, et al (2008) Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study Int J Radiat Oncol Biol Phys 70 2–13 https://doi.org/10.1016/j.ijrobp.2007.06.039
29. Welz S, Paulsen F, and Pfannenberg C, et al (2022) Dose escalation to hypoxic subvolumes in head and neck cancer: a randomized phase II study using dynamic [18F]FMISO PET/CT Radiother Oncol 171 30–6 https://doi.org/10.1016/j.radonc.2022.03.021 PMID: 35395276
30. Pigorsch SU, Wilkens JJ, and Kampfer S, et al (2017) Do selective radiation dose escalation, and tumour hypoxia status impact the loco-regional tumour control after radio-chemotherapy of head & neck tumours? The ESCALOX protocol Radiat Oncol 12(1) 45 https://doi.org/10.1186/s13014-017-0776-1 PMID: 28249612 PMCID: 5333380
31. Ferris RL, Flamand Y, and Weinstein GS, et al (2022) Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ locally advanced oropharynx cancer: an ECOG-ACRIN cancer research group trial (E3311) J Clin Oncol 40(2) 138–49 https://doi.org/10.1200/JCO.21.01752 PMCID: 8718241
32. Swisher-McClure S, Lukens JN, and Aggarwal C, et al A phase 2 trial of alternative volumes of oropharyngeal irradiation for de-intensification (AVOID): omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus–related squamous cell carcinoma of the oropharynx Int J Radiat Oncol Biol Phys 106(4) 725–732 PMID: 31785337
33. Lee NY, Sherman EJ, and Schöder H, et al (2024) Intra-treatment hypoxia directed major radiation de-escalation as definitive treatment for human papillomavirus-related oropharyngeal cancer JCO 42(16_suppl) 6007 https://doi.org/10.1200/JCO.2024.42.16_suppl.6007
34. Chan A-W, Tetzlaff JM, and Altman DG, et al (2013) SPIRIT 2013 statement: defining standard protocol items for clinical trials Ann Intern Med 158 200–207 https://doi.org/10.7326/0003-4819-158-3-201302050-00583 PMID: 23295957 PMCID: 5114123
35. Chan A-W, Tetzlaff JM, and Gøtzsche PC, et al (2013) SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials BMJ 346 e7586 https://doi.org/10.1136/bmj.e7586 PMID: 23303884 PMCID: 3541470
36. Lee N, Schoder H, and Beattie B, et al (2016) Strategy of using intratreatment hypoxia imaging to selectively and safely guide radiation dose de-escalation concurrent with chemotherapy for locoregionally advanced human pap-illomavirus-related oropharyngeal carcinoma Int J Radiat Oncol Biol Phys 96 9–17 https://doi.org/10.1016/j.ijrobp.2016.04.027 PMID: 27511842 PMCID: 5035649
37. Carles M, Fechter T, and Grosu AL, et al (2021) (18)F-FMISO-PET hypoxia monitoring for head- and-neck cancer patients: radiomics analyses predict the outcome of chemo-radiotherapy Cancers (Basel) 13 3449 https://doi.org/10.3390/cancers13143449
38. Riaz N, Sherman E, and Pei X, et al (2021) Precision radiotherapy: reduction in radiation for oropharyngeal cancer in the 30 ROC trial J Natl Cancer Inst 113 742–751 https://doi.org/10.1093/jnci/djaa184 PMID: 33429428 PMCID: 8168141
39. Kazmierska J, Cholewinski W, and Piotrowski T, et al (2020) Assessment of tumour hypoxia, proliferation and glucose metabolism in head and neck cancer before and during treatment Br J Radiol 93 20180781 https://doi.org/10.1259/bjr.20180781 PMCID: 7055437
40. Common Terminology Criteria for Adverse Events (CTCAE) Version 5 US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. [https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf]
41. Cox JD, Stetz J, and Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys 31(5) 1341–1346 https://doi.org/10.1016/0360-3016(95)00060-C PMID: 7713792
42. Aaronson NK, Ahmedzai S, and Bergman B, et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology J Natl Cancer Inst 85(5) 365–376 https://doi.org/10.1093/jnci/85.5.365 PMID: 8433390
43. Sherman AC, Simonton S, and Adams DC, et al (2000) Assessing quality of life in patients with head and neck cancer: cross-validation of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Head and Neck module (QLQ-H&N35) Arch otolaryngol Head Neck Surg 126(4) 459–67 https://doi.org/10.1001/archotol.126.4.459
44. Singer S, Arraras JI, and Chie WC, et al (2013) Performance of the EORTC questionnaire for the assessment of quality of life in head and neck cancer patients EORTC QLQ-H&N35: a methodological review Qual Life Res 22(8) 1927–1941 https://doi.org/10.1007/s11136-012-0325-1
45. Belafsky PC, Mouadeb DA, and Rees CJ, et al (2008) Validity and reliability of the eating assessment tool (EAT-10) Ann Otol Rhinol Laryngol 117(12) 919–924 https://doi.org/10.1177/000348940811701210
46. Dwivedi RC, Rose SS, and Roe JWG, et al (2010) Validation of the Sydney swallow questionnaire (SSQ) in a cohort of head and neck cancer patients Oral Oncol 46 e10–e14 https://doi.org/10.1016/j.oraloncology.2010.02.004 PMID: 20219415
47. McHorney CA, Robbins JA, and Lomax K, et al (2002) The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity Dysphagia 17(2) 97–114 https://doi.org/10.1007/s00455-001-0109-1 PMID: 11956835
48. Chen AY, Frankowski R, and Bishop-Leone J, et al (2001) The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory Arch Otolaryngol Head Neck Surg 127(7) 870–876 PMID: 11448365
49. Silbergleit AK, Schultz L, and Jacobson BH, et al (2012) The dysphagia handicap index: development and validation Dysphagia 27(1) 46–52 https://doi.org/10.1007/s00455-011-9336-2





