Clinicopathological characteristics and treatment outcomes of oesophageal cancer patients in Uganda
Siraji Obayo1, Yusuf Mulumba1, Cheryl L Thompson2,3, Michael K Gibson4, Matthew M Cooney2,3 and Jackson Orem1
1Uganda Cancer Institute, Upper Mulago Hill Road PO Box 3935, Kampala, Uganda
2Case Western Reserve University, Case Comprehensive Cancer Centre, Cleveland, OH 44106, USA
3Case Western Reserve University, University Hospitals Seidman Cancer Center, Cleveland, OH 44106, USA
4Vanderbilt University Medical Centre, Vanderbilt-Ingram Cancer Center, Nashville, TN 37232, USA
Abstract
Background: Oesophageal cancer is the seventh most common cancer and the sixth leading cause of cancer death worldwide, and its incidence varies globally. In Uganda, the incidence and trend are on the increase. However, there is a paucity of published data regarding this population’s oesophageal cancer clinicopathologic characterisation and treatment outcomes.
Objectives: To study the patients’ clinicopathologic characteristics and treatment outcomes of oesophageal cancer over 10 years at the Uganda Cancer Institute.
Methods: Patients’ charts with histologically confirmed diagnoses of oesophageal cancer for 2009–2019 were identified. Case information, which included patient demographics, history of alcohol use or smoking, tumour location, histological type, tumour grade, clinical TNM (Tumour, Node, Metastasis) staging treatment exposure and treatment outcomes, was evaluated retrospectively. The median survival time was estimated with the Kaplan-Meier method and the median follow-up period was estimated using the reverse Kaplan-Meier.
Results: 1,965 oesophageal cancer patients were identified; 1,380(70.23%) were males and 585(29.77 %) females, their mean age was 60.20 years (±12.66). Most males had a history of both alcohol consumption and smoking 640(46.38%). The lower third of the oesophagus was the most common anatomical location 771(39.24%). The majority had squamous cell carcinoma histological type 1,783(90.74%) followed by adenocarcinomas 182(9.26%) in the distal oesophagus. Poorly differentiated tumour grade 743(37.81%) was predominant. The majority of the patients were in stage IVB, 733(37.30%), and most patients were planned for the best supportive care, 731(37.20%). Radiation alone was offered to 621(31.60%) and feeding gastrostomy to 249(12.70%). Treatment outcomes: at the time of the current analysis, 58.68% had died, 1.48% were alive and 39.84% were lost to follow-up. The median follow-up period was 65 months (IQR:35.83–83.30) with a median survival time of 4.47 months (95% CI: 4.17–4.80).
Conclusion: Treatment outcomes of Ugandan oesophageal cancer patients seeking care are poor as most patients present with advanced disease. There is a significant loss of follow-up after treatment initiation. Therefore, reduction in exposure to known modifiable risk factors, early detection and timely referral for treatment strategies are needed to improve outcomes of these patients in our population. Designing interventions to improve treatment adherence is necessary.
Keywords: oesophageal cancer, clinicopathological characteristics, treatment outcomes, Uganda
Correspondence to: Siraji Obayo
Email: obayos@yahoo.com
Published: 19/07/2023
Received: 14/04/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Oesophageal cancer is the seventh most common cancer and the sixth leading cause of cancer death worldwide [1]. More than 80% of cases and deaths from oesophageal cancer occur within developing countries [2–4]. The incidence of oesophageal cancer varies globally, with a higher incidence in areas such as Eastern Asia, South Central Asia, South Africa, Eastern Africa and Northern Europe [1–4]. In Uganda, one of the countries comprising the East African sub-region, the incidence of oesophageal cancer is increasing [5–9]. In Uganda, oesophageal cancer ranks sixth and is the third most common cause of cancer-related death, accounting for 8.7% [9]. Treatment of oesophageal cancer depends on the stage of the disease and the patient’s functional status at presentation. Curative treatment usually involves combined modality strategies with surgery, chemotherapy, radiation or endoscopic therapy for mucosal cancers [10–30]. Palliative treatment aims to improve dysphagia, nutrition and quality of life; this can be achieved with radiation, brachytherapy, chemoradiation or endoscopic therapy, which may include dilation and stenting, chemical or ablative debulking, and enteral feeding [10–12, 30–50]. Despite the availability of radiation, chemotherapy, surgical and endoscopic services at Uganda Cancer Institute, a national referral cancer treatment centre and rising oesophageal cancer incidence in Uganda, there is limited data about clinicopathologic features and treatment outcomes for this tumour type. This study aimed to characterise oesophageal cancer patients seeking care over 10 years regarding clinicopathological characteristics and treatment outcomes. Therefore, data obtained from this study will be the first important step to better understand the clinicopathological features and the associated treatment outcomes of oesophageal cancer in Uganda and enhance oesophageal cancer care in our population.
Methods
This was a retrospective chart review study of confirmed oesophageal cancer patients referred to the Uganda Cancer Institute, a national referral cancer centre, between 2009 and 2019 for treatment. Data collected on each patient’s chart included age, sex, history of alcohol use or smoking, tumour location, histological type, grade, stage, treatment exposures and outcomes. For patients who were no longer in contact with the staff through clinic visits, the patients or their next of kin were contacted through phone calls for patients’ survival status and the dates were recorded. Data were collected and stored using the RedCAP database. This study was approved by the Ugandan National Council for Science and Technology and the Uganda Cancer Institute.
Statistical analysis
Mean values and SDs were calculated for continuous variables and counts of categorical variables described the distributions of clinicopathologic variables, treatment exposure and outcomes. The relationship between outcomes by stage and treatment was determined by cross-tabulation. The care period at the Uganda Cancer Institute was calculated from the date patients were enrolled to when they were no longer in contact with the clinic or the date of death. The median survival time was estimated with the Kaplan-Meier method and the median follow-up period was estimated using the reverse Kaplan-Meier.
Results
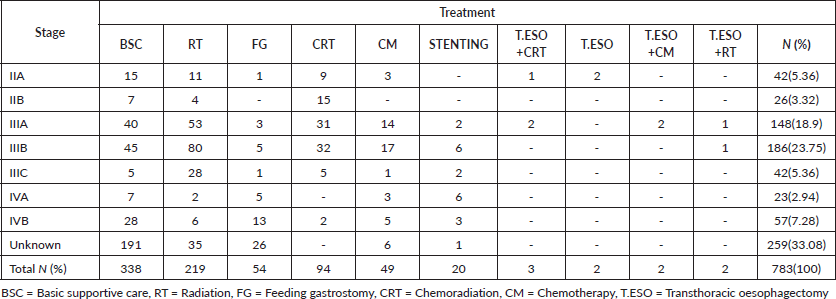
Over the study period, 1,965 oesophageal patient cases were identified, 1,380(70.23%) were males and 585(29.77%) were females with a mean age of 60.20 years (±12.66). Most males had a history of both alcohol consumption and smoking 640(46.38%), among females majority neither used alcohol nor smoked 363(62.05%). The most common tumour location was the lower third 771(39.24%), followed by the middle third 727(36.99%), then the upper third of the oesophagus 467(23.77%) and the majority had squamous cell histological type 1,783 (90.74%), followed by adenocarcinoma 182(9.26%) in the distal oesophagus. Tumour grades were as follows, poorly differentiated 743(37.81%), moderately differentiated 613(31.20%) and well differentiated 609(30.99%) (Table 1). Staging of the patients, the majority were in stage IVB, 733(37.30%), followed by stage IVA, 421(21.42%), unknown stage 266(13.54%), stage IIIB, 239 (12.16%), then stage IIIA patients 163(8.30%) (Table 1).
Table 1. Clinicopathologic characteristics of oesophageal cancer patients enrolled into care.
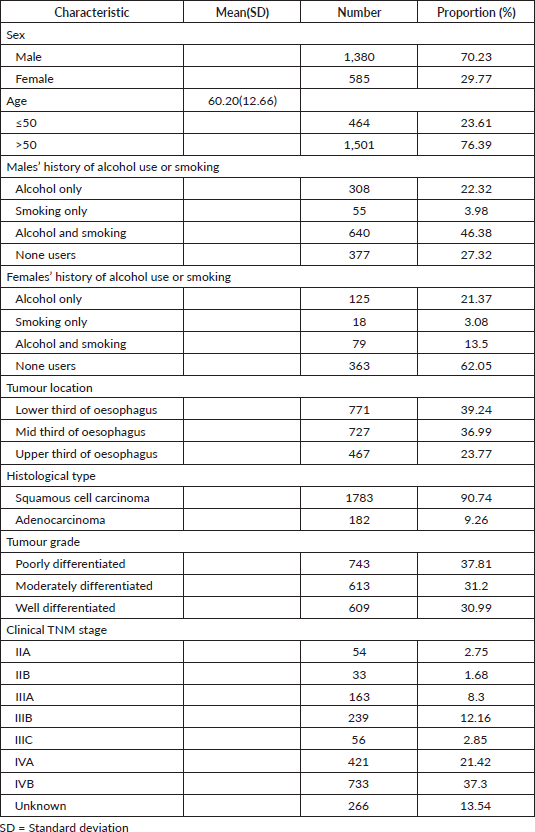
Treatment, most got basic supportive care, 731(37.20%), followed by radiation alone 621(31.60%), Feeding gastrostomy alone 249(12.70%) for nutritional purposes then chemoradiation was at 162(8.24%). Survival status of the patients was as follows, dead 1,153(58.68%), followed by those who were lost to follow-up, 783(39.84%), and 29(1.47%) were alive at the closure of this study (Table 2). The median follow-up period was 65 months with an IQR of 35.83–83.30 months and the median survival time after diagnosis with oesophageal cancer was 4.47 months (95% of CI 4.17–4.80).
For most living oesophageal cancer patients, 10(34.48%) were in stage IIA and 6(20.69%) were in stage IIB. The majority of stage IIA and IIB patients had chemoradiation 9(31.03%) followed by transthoracic oesophagectomy plus chemoradiation 4(13.79%), as their treatment. Of those in stage IVA 5(17.24%) who were still alive on chemotherapy alone, 2(6.90%), basic supportive care, 2(6.90%) or had oesophageal stenting, 1(3.44%), had been in care for about 1.7 months by the end of our study. One patent in stage IIIB had gotten oesophageal stenting with no proper rationale from an outside primary healthcare hospital before enrollment (Table 3).
Most dead patients, 676(58.63%), were in stage IVB. Not surprisingly majority had basic supportive care 274(23.76%), palliative radiation 211(18.30%) with 20 Gy in 4 Gy fractions daily, 5 consecutive workdays, feeding gastrostomy 119(10.32%) for nutrition purposes then palliative chemotherapy 45(3.90%), oesophageal stenting 21(1.82%) and chemoradiation 6(0.52%) for their disease (Table S1). One patient in stage IIA had radiation alone due to his advanced age of 88 years to relieve symptoms of dysphagia and chest pain and passed on after 11.93 months. The other in stage IIA was 56 years had chemoradiation and died after 75 months of follow-up, the cause of death was uncertain.
Table 2. Treatment and outcomes of oesophageal cancer patients enrolled into care.
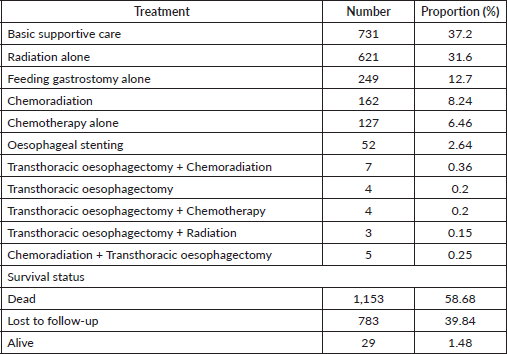
Table 3. Oesophageal cancer patients in care who were still alive by stage and treatment.
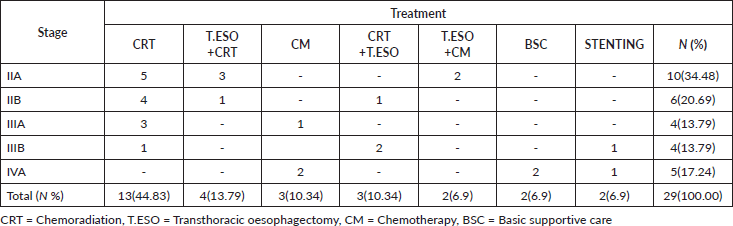
Lost to follow-up patients mostly were in the unknown stage, 259(33.08%), followed by those in stage IIIB 186(23.75%). For those in stage IIIB, the majority had radiation alone, 80(10.22%) then lost to follow-up, while those in the unknown stage most had basic supportive care, 191(24.39%) then lost to follow-up (Table S2).
Discussion
This study reviewed oesophageal cancer patients’ clinicopathological presentation and treatment outcomes at Uganda’s National Referral Cancer Centre. Out of 1,965 cases reviewed, males were predominant at 1,380(70.23%). The male predominance demonstrated in this study is like other studies performed in Africa, particularly in the Sub-Saharan African region [51, 52]. The male predominance could be explained by the fact that most of the known risk factors for oesophageal cancer are related to behaviour-smoking and excessive alcohol consumption, of which men are known to be worse consumers than women as demonstrated in our study and earlier studies in Africa and China [53–58].
The lower third of the oesophagus was the most frequent anatomical site for oesophageal cancer (39.24%). With (9.26%) being adenocarcinoma and (29.98%) squamous cell carcinoma histological type. This result is consistent with previous studies [59–67], which found a lower third of the oesophagus as the most anatomical site. Our finding differs from other studies [68–73], which reported the middle third of the oesophagus as the most common site for oesophageal cancer. However, we could not establish the reasons for the variation in this anatomical distribution pattern.
Our data demonstrate squamous cell carcinoma is the most common histology representing over >90% of all cases, which is in keeping with other studies from the East African countries of Tanzania, Kenya and Uganda. Southern African countries of Malawi, Mozambique and South Africa. Iran in South Central Asia, and China in East Asia [68, 74–84]. Most tumour grade in our study was poorly differentiated (high grade), keeping with reports from China and Netherlands [72, 85]. This could probably be among the reasons for the high stages in our patients at presentations, as this tumour grade tends to metastasise fast.
In our study, more than 50% of the patients were in stage IV; this finding is consistent with studies from Ethiopia, Zambia, India and the United States of America that reported most of the patients being in stage IV at presentation for care [62, 70, 86–88]. The late-stage presentation and poor performance status are why most of our patients had basic supportive care, among other palliative treatment modalities. 13.54% of the patients in our study had no imaging studies to stage their disease; hence their, stages were unknown, this could have been probably due to the burden of cost in care for the illness, among other reasons in our setting.
Treatment exposure, this study shows most patients had the best supportive care, palliative radiotherapy (20 Gy in 4 Gy fractions) and feeding gastrostomy insertion as their commonest treatments. This is not surprising as the majority of the patients presented for care in the late stage. Our results are in accordance with reports from Mozambique, the Netherlands, the United States of America and Ethiopia, where most patients had the best supportive care, palliative radiotherapy and feeding gastrostomy insertion [81, 85, 89–91].
Treatment outcomes, in our study, death was at 58.68% mostly in stages IVB and IVA, followed by a loss to follow-up patients (39.85%) mostly in the unknown stage and stage IIIB then alive (1.48%) most in stage IIA at the end of this study. This is similar to a study in Brazil that showed death in more than 50% of treated oesophageal cancer patients in advanced stages of the disease [92], as well as other studies from Uganda and the United States of America that showed poor adherence to treatment [93, 94].
A recent Ugandan study reported that depleted financial resources within the first few treatment visits, high transport costs and long distances to the Uganda Cancer Institute hindered patients’ promptness from seeking care and adhering to the treatment schedules. Participants reported that patients would postpone health-seeking visits and miss appointments for their treatments because of a lack of money for transport and upkeep [93].
However, their study was an interview-based qualitative one in which only 16 patients and 17 healthcare professionals were involved and could not establish the magnitudes of associations and characteristics of the participants likely to be associated with the concepts that were being explored.
To overcome transport costs and long-distance travel, the Ugandan government, through the Uganda Cancer Institute, is opening functional regional cancer centers in the East Central (Mbale), Acholi (Gulu), West Nile (Arua) and Southwestern (Mbarara) sub-regions. This will reduce non-adherence to treatment regimens due to long-distance travel costs and consequently improve treatment outcomes.
Limitations
This study has some limitations. Only a tertiary national referral hospital was involved, thus cases only seen at the regional and district hospitals or self-referred patients seeking treatment outside the country that never reported to the Uganda Cancer Institute for care may have been missed, and thus case ascertainment may be incomplete. We cannot exclude diagnostic bias based on interest, expertise, and access to diagnostic facilities. Lost to follow-up patients whose phones were no longer on the network, and we could not confirm their actual vital status that may have been missed, thus underestimating their treatment outcomes.
Conclusion
This study confirmed the clinicopathological characteristics and treatment outcomes data of oesophageal cancer in Uganda, which validates the predominance of high-grade oesophageal squamous cell carcinoma with the late presentation, poor treatment outcomes and significant loss of follow-up in this context. This study demonstrates the need to prioritise oesophageal cancer on the national health agenda, including promoting preventative strategies, cost-effective early assessment, detection and timely treatment and designing interventions to improve treatment adherence such as using appointment reminders via mobile phones and other technologies, enhancing referral pathways and patient navigation is necessary.
Acknowledgment
Our patients, staff of the GI Clinic, Endoscopy Unit and Medical Records Unit, Uganda Cancer Institute, mainly Nabirye Oliver, for her input in accessing the charts and calling up patients or their next of kin for patients’ vital status.
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
Research support from the Conquer Cancer Foundation of the American Society of Clinical Oncology as part of the Long-term International Fellowship (LIFe) Award.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide Int J Cancer 136(5) 359–386 https://doi.org/10.1002/ijc.29210
3. Wong MCS, Hamilton W, and Whiteman DC, et al (2018) Global incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries Sci Rep 8(1) 4522 https://doi.org/10.1038/s41598-018-19819-8 PMID: 29540708 PMCID: 5852053
4. Cheng ML, Zhang L, and Borok M, et al (2015) The incidence of oesophageal cancer in Eastern Africa: identification of a new geographic hot spot? Cancer Epidemiol 39(2) 143–149 https://doi.org/10.1016/j.canep.2015.01.001 PMID: 25662402 PMCID: 4470609
5. Obayo S, Luswa L, and Orem J, et al (2017) Gastrointestinal malignancies at five regional referral hospitals in Uganda Afri Health Sci 17(4) 1051–1058 https://doi.org/10.4314/ahs.v17i4.13
6. Wabinga H, Nambooze S, and Amulen PM, et al (2014) Trends in the incidence of cancer in Kampala, Uganda1991-2010 Int J Cancer 135(2) 432–439 https://doi.org/10.1002/ijc.28661 PMID: 24615279
7. GBD 2017 Oesophageal Cancer Collaborators (2020) The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 Lancet Gastroenterol Hepatol 5(6) 582–597 https://doi.org/10.1016/S2468-1253(20)30007-8
8. Huang J, Koulaouzidis A, and Marlicz W, et al (2021) Global burden, risk factors, and trends of oesophageal cancer: an analysis of cancer registries from 48 countries Cancer 13(1) 141 https://doi.org/10.3390/cancers13010141
9. Ferlay J, Ervik M, and Lam F, et al (2020) Global Cancer Observatory: Cancer Today (Lyon: International Agency for Research on Cancer)
10. Evans JA, Early DS, and Chandraskhara V, et al (2013) The role of endoscopy in the assessment and treatment of oesophageal cancer Gastrointest Endosc 77(3) 328–334 https://doi.org/10.1016/j.gie.2012.10.001 PMID: 23410694
11. Asombang AW, Chishinga N, and Nkhoma A, et al (2019) Systematic review and meta-analysis of oesophageal cancer in Africa: epidemiology, risk factors, management and outcomes World J Gastroenterol 25(31) 4512–4533 https://doi.org/10.3748/wjg.v25.i31.4512 PMID: 31496629 PMCID: 6710188
12. Ancona E, Ruol A, and Santi S, et al (2001) Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable oesophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone Cancer 91(11) 2165–2174 https://doi.org/10.1002/1097-0142(20010601)91:11<2165::AID-CNCR1245>3.0.CO;2-H PMID: 11391598
13. Medical Research Council Oesophageal Cancer Working Group (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial Lancet 359(9319) 1727–1733 https://doi.org/10.1016/S0140-6736(02)08651-8 PMID: 12049861
14. Ando N, Iizuka T, and Ide H, et al (2003) Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic oesophagus: a Japan clinical oncology group study J Clin Oncol 21(24) 4592–4596 https://doi.org/10.1200/JCO.2003.12.095 PMID: 14673047
15. Urschel JD and Vasan H (2003) A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable oesophageal cancer Am J Surg 185(6) 538–543 https://doi.org/10.1016/S0002-9610(03)00066-7 PMID: 12781882
16. Malthaner RA, Wong RK, and Rumble RB, et al (2004) Neoadjuvant or adjuvant therapy for resectable oesophageal cancer: a systematic review and meta-analysis BMC Med 2 35 https://doi.org/10.1186/1741-7015-2-35 PMID: 15447788 PMCID: 529457
17. Gebski V, Burmeister B, and Smithers BM, et al (2007) Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis Lancet Oncol 8(3) 226–234 https://doi.org/10.1016/S1470-2045(07)70039-6 PMID: 17329193
18. Pennathur A, Luketich JD, and Landreneau RJ, et al (2008) Long-term results of a phase II trial of neoadjuvant chemotherapy followed by oesophagectomy for locally advanced oesophageal neoplasm Ann Thorac Surg 85(6) 1930–1936 https://doi.org/10.1016/j.athoracsur.2008.01.097 PMID: 18498797
19. Pennathur A and Luketich JD (2008) Resection for oesophageal cancer: strategies for optimal management Ann Thorac Surg 85(2) 751–756 https://doi.org/10.1016/j.athoracsur.2007.11.078
20. Tepper J, Krasna MJ, and Niedzwiecki D, et al (2008) Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for oesophageal cancer J Clin Oncol 26(7) 1086–1092 https://doi.org/10.1200/JCO.2007.12.9593 PMID: 18309943 PMCID: 5126644
21. Pennathur A, Farkas A, and Krasinskas AM, et al (2009) Oesophagectomy for T1 oesophageal cancer: outcomes in 100 patients and implications for endoscopic therapy Ann Thorac Surg 87(4) 1048–1054 https://doi.org/10.1016/j.athoracsur.2008.12.060 PMID: 19324126 PMCID: 2912110
22. Jin HL, Zhu H, and Ling TS, et al (2009) Neoadjuvant chemoradiotherapy for resectable oesophageal carcinoma: a meta-analysis World J Gastroenterol 15(47) 5983–5991 https://doi.org/10.3748/wjg.15.5983 PMID: 20014464 PMCID: 2795187
23. Lv J, Cao XF, and Zhu B, et al (2010) Long-term efficacy of perioperative chemoradiotherapy on oesophageal squamous cell carcinoma World J Gastroenterol 16(13) 1649–1654 https://doi.org/10.3748/wjg.v16.i13.1649 PMID: 20355244 PMCID: 2848374
24. Sjoquist KM, Burmeister BH, and Smithers BM, et al (2011) Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis Lancet Oncol 12(7) 681–692 https://doi.org/10.1016/S1470-2045(11)70142-5 PMID: 21684205
25. Ando N, Kato H, and Igaki H, et al (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic oesophagus Ann Surg Oncol 19(1) 68–74 https://doi.org/10.1245/s10434-011-2049-9
26. Van Hagen P, Hulshof MC, and Van Lanschot JJ, et al (2012) Preoperative chemoradiotherapy for oesophageal or junctional cancer N Engl J Med 366(22) 2074–2084 https://doi.org/10.1056/NEJMoa1112088 PMID: 22646630
27. Almhanna K and Strosberg JR (2012) Multimodality approach for locally advanced oesophageal cancer World J Gastroenterol 18(40) 5679–5687 https://doi.org/10.3748/wjg.v18.i40.5679 PMID: 23155307 PMCID: 3484335
28. Vijayakumar M, Burrah R, and Hari K, et al (2013) Oesophagectomy for cancer of the oesophagus. A regional cancer centre experience Indian J Surg Oncol 4(4) 332–335 https://doi.org/10.1007/s13193-013-0260-9
29. Kidane B, Coughlin S, and Vogt K, et al (2015) Preoperative chemotherapy for resectable thoracic oesophageal cancer Cochrane Database Syst Rev 2015(5) CD001556 PMID: 25988291 PMCID: 7058152
30. Weidenbaum C and Gibson MK (2022) Approach to localized squamous cell cancer of the oesophagus Curr Treat Options Oncol 23(10) 1370–1387 https://doi.org/10.1007/s11864-022-01003-w PMID: 36042147 PMCID: 9526684
31. Luketich JD, Christie NA, and Buenaventura PO, et al (2000) Endoscopic photodynamic therapy for obstructing oesophageal cancer: 77 cases over a 2-year period Surg Endosc 14(7) 653–657 https://doi.org/10.1007/s004640000144 PMID: 10948303
32. Litle VR, Luketich JD, and Christie NA, et al (2003) Photodynamic therapy as palliation for oesophageal cancer: experience in 215 patients Ann Thorac Surg 76(5) 1687–1692 https://doi.org/10.1016/S0003-4975(03)01299-2 PMID: 14602313
33. Petersen BT, Chuttani R, and Croffie J, et al (2006) Photodynamic therapy for gastrointestinal disease Gastrointest Endosc 63(7) 927–932 https://doi.org/10.1016/j.gie.2006.02.044 PMID: 16733105
34. Ndonga AK, Rucha MR, and Oigara R (2008) Oesophageal cancer and experience with endoscopic stent intubation at St. Mary’s Hospital, Nairobi Ann Afr Surg
35. Shenfine J, McNamee P, and Steen N, et al (2009) A randomized controlled clinical trial of palliative therapies for patients with inoperable oesophageal cancer Am J Gastroenterol 104(7) 1674–1685 https://doi.org/10.1038/ajg.2009.155 PMID: 19436289
36. Diamantis G, Scarpa M, and Bocus P, et al (2011) Quality of life in patients with oesophageal stenting for the palliation of malignant dysphagia World J Gastroenterol 17(2) 144–150 https://doi.org/10.3748/wjg.v17.i2.144 PMID: 21245986 PMCID: 3020367
37. Thumbs A, Borgstein E, and Vigna L, et al (2012) Self-expanding metal stents (SEMS) for patients with advanced oesophageal cancer in Malawi: an effective palliative treatment J Surg Oncol 105(4) 410–414 https://doi.org/10.1002/jso.23003
38. Jiang XJ, Song MQ, and Xin YN, et al (2012) Endoscopic stenting and concurrent chemoradiotherapy for advanced oesophageal cancer: a case-control study World J Gastroenterol 18(12) 1404–1409 https://doi.org/10.3748/wjg.v18.i12.1404 PMID: 22493556 PMCID: 3319969
39. Rueth NM, Shaw D, and D'Cunha J, et al (2012) Oesophageal stenting and radiotherapy: a multimodal approach for the palliation of symptomatic malignant dysphagia Ann Surg Oncol 19(13) 4223–4228 https://doi.org/10.1245/s10434-012-2459-3 PMID: 22752374
40. Yang C, Li J, and Fu W, et al (2014) Interventions for dysphagia in oesophageal cancer Cochrane Database Syst Rev 2014(10) CD005048 PMID: 25354795 PMCID: 8106614
41. Parthipun A, Diamantopoulos A, and Shaw A, et al (2014) Self-expanding metal stents in palliative malignant oesophageal dysplasia Ann Palliat Med 3(2) 92–103
42. Altuntaş B, Yekeler E, and Subaşı M, et al (2015) Palliation of malignant dysphagia with self expandable oesophageal stents Turk Gogus Kalp Dama 23 82–87 https://doi.org/10.5606/tgkdc.dergisi.2015.9596
43. Ramakrishnaiah VPN, Malage S, and Sreenath GS, et al (2016) Palliation of dysphagia in carcinoma oesophagus Clin Med Insights Gastroenterol 9 11–23 https://doi.org/10.4137/CGast.S30303 PMID: 27279758 PMCID: 4896534
44. Bhatnagar AR, Bhandari R, and Kumbhaj P, et al (2017) Role of palliative radiotherapy, chemotherapy and stents for dysphagia and quality of life improvement in advanced oesophageal cancer. The optimal management? Ann Oncol 28 III29–III30 https://doi.org/10.1093/annonc/mdx261.060
45. Kim KY, Tsauo J, and Song HY, et al (2017) Self-expandable metallic stent placement for the palliation of oesophageal cancer J Korean Med Sci 32(7) 1062–1071 https://doi.org/10.3346/jkms.2017.32.7.1062 PMID: 28581260 PMCID: 5461307
46. Włodarczyk JR and Kuzdzał J (2018) Stenting in palliation of unresectable oesophageal cancer World J Surg 42(12) 3988–3996 https://doi.org/10.1007/s00268-018-4722-7
47. Buckle GC, Mahapatra R, and Mwachiro M, et al (2021) Optimal management of oesophageal cancer in Africa: a systemic review of treatment strategies Int J Cancer 148(5) 1115–1131 https://doi.org/10.1002/ijc.33299
48. Birla R, Achim F, and Marica C, et al (2020) Palliation of dysphagia in patients with unresectable oesophageal tumours-current methods and indications-a review Dig Med Res 3 26 https://doi.org/10.21037/dmr-20-67
49. Koggel LM, Lantinga MA, and Siersema PD (2021) Palliation of malignant dysphagia: stent or radiotherapy Ann Oesophagus 4 41 https://doi.org/10.21037/aoe-2020-08
50. Mwachiro M, Parker R, and Lando J, et al (2022) Predictors of adverse events and early mortality after oesophageal stent placement in a low resource setting: a series of 3823 patients in Kenya Endosc Int Open 10(4) 479–487 https://doi.org/10.1055/a-1783-9829
51. Middleton DRS, Bouaoun L, and Hanisch R, et al (2018) Oesophageal cancer male to female incidence ratios in Africa: a systematic review and meta-analysis of geographic, time and age trends Cancer Epidemiol 53 119–128 https://doi.org/10.1016/j.canep.2018.01.020 PMID: 29414631 PMCID: 5871654
52. Kachala R (2010) Systematic review: epidemiology of oesophageal cancer in Sub Saharan Africa Malawi Med J 22(3) 65–70 https://doi.org/10.4314/mmj.v22i3.62190
53. Pampel F (2008) Tobacco use in Sub-Sahara Africa estimates from the demographic health surveys Soc Sci Med 66 1772–1783 https://doi.org/10.1016/j.socscimed.2007.12.003 PMID: 18249479 PMCID: 2679748
54. Middleton DRS, Mmbaga BT, and Menya D, et al (2022) Alcohol consumption and oesophageal squamous cell cancer risk in East Africa: finding from the large multicenter ESCCAPE case-control study in Kenya, Tanzania, and Malawi Lancet Glob Health 10(2) 236–245 https://doi.org/10.1016/S2214-109X(21)00506-4
55. Li Q, Hsia J, and Yang G (2011) Prevalence of smoking in China in 2010 N Engl J Med 364 2469–2470 https://doi.org/10.1056/NEJMc1102459 PMID: 21696322
56. Millwood IY, Li L, and Smith M, et al (2013) Alcohol consumption in 0.5 million people from 10 diverse regions of China: prevalence, patterns and socio-demographic and health-related correlates Int J Epidemiol 42 816–827 https://doi.org/10.1093/ije/dyt078 PMID: 23918852 PMCID: 3733702
57. Liu S, Zhang M, and Yang L, et al (2017) Prevalence and patterns of tobacco smoking among Chinese adult men and women findings of the 2010 national smoking survey J Epidemiol Community Health 71 154–161 https://doi.org/10.1136/jech-2016-207805
58. Yang X, Chen X, and Zhuang M, et al (2017) Smoking and alcohol drinking in relation to the risk of oesophageal squamous cell carcinoma: a population-based case-control study in China Sci Rep 7(1) 7249 https://doi.org/10.1038/s41598-017-17617-2
59. Wakhisi J, Patel K, and Buziba N, et al (2005) Oesophageal cancer in north rift valley of western Kenya Afr Health Sci 5(2) 157–163 PMID: 16006224 PMCID: 1831916
60. Deybasso HA, Roba KT, Nega B, et al (2021) Clinico-pathological findings and spatial distributions of oesophageal cancer in Arsi Zone, Central Ethiopia Cancer Manag Res 13 2755–2762 https://doi.org/10.2147/CMAR.S301978 PMCID: 8001180
61. Chetwood JD, Finch PJ, and Kankwatira A, et al (2018) Five-year single-centre experience of carcinoma of the oesophagus from Blantyre, Malawi BMJ Open Gastro 5(1) e000232 https://doi.org/10.1136/bmjgast-2018-000232
62. Hassen HY, Teka MA, and Addisse A (2021) Survival status of oesophageal cancer patients and its determinants in Ethiopia: a facility based retrospective cohort study Front Oncol 10 594342 https://doi.org/10.3389/fonc.2020.594342 PMID: 33659206 PMCID: 7917207
63. Tettey M, Edwin F, and Aniteye E, et al (2004) The changing epidemiology of oesophageal cancer in Sub-Saharan Africa-the case of Ghana Pan Afr Med J 13 6
64. Alidina A, Gaffar A, and Hussain F, et al (2004) Survival data and prognostic factors seen in Pakistani patients with oesophageal cancer Ann Oncol 15(1) 118–122 https://doi.org/10.1093/annonc/mdh014
65. Pedram A, Mahmodlou R, and Enshayi A, et al (2011) Oesophageal cancer in northwestern Iran Indian J Cancer 48(2) 165–169 https://doi.org/10.4103/0019-509X.82875 PMID: 21768660
66. Cherian JV, Sivavaman R, and Muthusamy AK, et al (2007) Carcinoma of the oesophagus in Tamil Nadu (South India):16 year trends from a tertiary center J Gastrointestin Liver Dis 16(3) 245–249 PMID: 17925916
67. Pun CB, Aryal G, and Basyal R, et al (2012) Histological pattern of oesophageal cancer at BP Koirala memorial cancer hospital in Nepal: a three year retrospective study J Pathol Nepal 2 277–281 https://doi.org/10.3126/jpn.v2i4.6877
68. Aledavood A, Anvari K, and Sabouri G (2011) Oesophageal cancer in northeast of Iran Iran J Cancer Prev 4(3) 125–129 PMID: 26328051 PMCID: 4551295
69. Mabula DM, Rambau PF, and Chalya PL, et al (2013) Endoscopic and clinicopathological patterns of oesophageal cancer in Tanzania: experiences from two tertiary health institutions World J Surg Onc 11 257 https://doi.org/10.1186/1477-7819-11-257
70. Asombang AW, Kasongo N, and Muyutu J, et al (2021) Descriptive analysis of oesophageal cancer in Zambia using the cancer disease hospital database: young age, late stage at presentation Pan Afr Med J 39 12 PMID: 34394803 PMCID: 8348283
71. Jiang YX, Zhang DW, and Chen Y, et al (2015) The characteristics of oesophageal squamous cell carcinoma: an analysis of 1317 cases in southeastern China Contemp Oncol (Pozn) 19(2) 137–141 PMID: 26034392 PMCID: 4444447
72. Song T, Wan Q, and Yu W, et al (2017) Pretreatment nutritional risk scores and performance status are prognostic factors in oesophageal cancer patients treated with definitive chemoradiotherapy Oncotarget 8(58) 98974–98984 https://doi.org/10.18632/oncotarget.21940 PMID: 29228742 PMCID: 5716782
73. Nam SY, Jeon SW, and Lee SJ, et al (2020) Clinical factors to predict the response to concurrent chemoradiotherapy and survival in oesophageal cancer patients Gut and Liver 14(4) 450–458 https://doi.org/10.5009/gnl19165 PMID: 32000467 PMCID: 7366147
74. Mmbaga EJ, Deardorff KV, and Mushi B, et al (2018) Characteristics of oesophageal cancer cases in Tanzania J Glob Oncol 4 1–10
75. Patel K, Wakhisi J, and Mining S, et al (2013) Oesophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors ISRN Oncol 2013 9
76. Dawsey SP, Tonui S, and Parker RK, et al (2010) Oesophageal cancer in young people: a case series of 109 cases and review of the literature PLoS One 5(11) 14080 https://doi.org/10.1371/journal.pone.0014080
77. Odera JO, Odera E, and Githang J, et al (2017) Oesophageal cancer in Kenya Am J Dig Dis 4(3) 23–33
78. Ocama P, Kagimu MM, and Odida M, et al (2008) Factors associated with carcinoma of the oesophagus at Mulago Hospital, Uganda Afr Health Sci 8(2) 80–84 PMCID: 2584326
79. Alema ON and Iva B (2014) Cancer of the oesophagus: histopathological sub-types in northern Uganda Afr Health Sci 14(1) 17–21 https://doi.org/10.4314/ahs.v14i1.4 PMID: 26060453 PMCID: 4449046
80. Mlombe YB, Rosenberg NE, and Wolf LL, et al (2015) Environmental risk factors for oesophageal cancer in Malawi: a case-control study Malawi Med J 27(3) 88–92
81. Come J, Castro C, and Morais A, et al (2018) Clinical and pathologic profiles of oesophageal cancer in Mozambique: a study of consecutive patients admitted to Maputo Central Hospital J Glob Oncol 4 1–9 PMID: 30398947 PMCID: 7010456
82. Ferndale L and Aldous C (2022) Oesophageal cancer in South Africa: a scoping review S Afr J Oncol 6(0) 217 https://doi.org/10.4102/sajo.v6i0.217
83. Liang H, Fan JH, and Qiao YL (2017) Epidemiology, etiology, and prevention of oesophageal squamous cell carcinoma in China Cancer Biol Med 14(1) 33–41 10.20892/j.issn.2095-3941.2016.0093>https://doi.org/10.20892/j.issn.2095-3941.2016.0093 PMID: 28443201 PMCID: 5365188
84. National Health Commission of the People's Republic of China (2019) Chinese guidelines for diagnosis and treatment of oesophageal carcinoma Chin J Cancer Res 31(2) 223–258 PMID: 31156297 PMCID: 6513746
85. Opstelten JL, de Wijkerslooth LR, and Leenders M, et al (2017) Variation in palliative care of oesophageal cancer in clinical practice: factors associated with treatment decisions Dis Oesophagus 30(2) 1–7
86. Ansari F and Rai A (2021) A retrospective study of the epidemiology of oesophageal cancer and its management in a Central Indian Institute Int Surg J 8(12) 3530–3534 https://doi.org/10.18203/2349-2902.isj20214484
87. Then EO, Lopeza M, and Saleem S, et al (2020) Oesophageal cancer: an updated surveillance epidemiology and end results database analysis World J Oncol 11(2) 55–64 https://doi.org/10.14740/wjon1254 PMID: 32284773 PMCID: 7141161
88. Nassri A, Zhu H, and Muftah M, et al (2018) Epidemiology and survival of oesophageal cancer patients in an American cohort Cureus 10(4) 2507
89. Kim S, DiPeri TP, and Guan M, et al (2020) Impact of palliative therapies in metastatic oesophageal cancer patients not receiving chemotherapy World J Gastrointest Surg 12(9) 377–389 https://doi.org/10.4240/wjgs.v12.i9.377 PMID: 33024512 PMCID: 7520571
90. Seraphin M, Silberstein PT, and Cichon G (2022) Trends in palliative care interventions for stage IV oesophageal cancer: an analysis of the NCDB J Clin Oncol 40(16) 24104 https://doi.org/10.1200/JCO.2022.40.16_suppl.e24104
91. Wondimagegnehu A, Hirpa S, and Abaya SW, et al (2020) Oesophageal cancer magnitude and presentation in Ethiopia 2012-17 PLoS One 15(12) 0242807 https://doi.org/10.1371/journal.pone.0242807
92. Cruz AIBM, Pinto LFR, and Thuler LCS, et al (2018) Profile of patients with oesophageal cancer diagnosed between 2001 and 2010 in Brazil Rev Bras Cancerol 64(4) 471–477 https://doi.org/10.32635/2176-9745.RBC.2018v64n4.195
93. Nakimuli E, Ssentongo J, and Mwaka AD (2021) Understanding health-seeking and adherence to treatment by patients with oesophageal cancer at the Uganda cancer Institute: a qualitative study BMC Health Serv Res 21 159 https://doi.org/10.1186/s12913-021-06163-3
94. Ambroggi M, Biasini C, and Del Giovane C, et al (2015) Distance as a barrier to cancer diagnosis and treatment: review of the literature Oncologist 20(12) 1378–1385 https://doi.org/10.1634/theoncologist.2015-0110 PMID: 26512045 PMCID: 4679078
Supplementary material
Table S1. Oesophageal cancer patients enrolled in care who died.
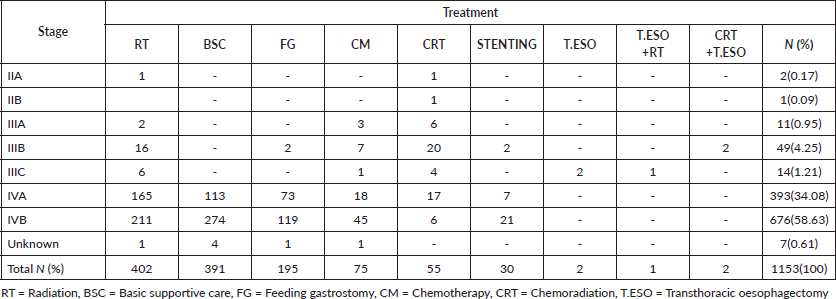
Table S2. Oesophageal cancer patients who were lost to follow-up by stage and treatment.