Effects of the COVID-19 pandemic on delays in diagnosis-to-treatment initiation for breast cancer in Brazil: a nationwide study
João Henrique Fonseca do Nascimento1,a, Cleonice Nascimento da Silva1,b, André Gusmão-Cunha1,2,c, Marinho Marques Silva Neto1,d and André Bouzas de Andrade1,3,e
1Life Sciences Department, Universidade do Estado da Bahia (UNEB), Salvador 41150-000, Brazil
2Anesthesiology and Surgery Department, Universidade Federal da Bahia (UFBA), Salvador 40026-010, Brazil
3Hospital Santa Izabel (HSI), Bahia Cancer Institute, Salvador 40050-410, Brazil
ahttps://orcid.org/0000-0001-8750-6116
bhttps://orcid.org/0000-0002-8356-6426
chttps://orcid.org/0000-0001-7762-168X
dhttps://orcid.org/0000-0002-9728-7268
ehttps://orcid.org/0000-0002-4010-0415
Abstract
Background: Short period from diagnosis to breast cancer (BC) treatment initiation remains challenging for the public health system in Brazil, which may have been further affected by the coronavirus disease-2019 (COVID-19) pandemic. This study assessed BC diagnosis-to-treatment intervals (DTi) in Brazil and the possible effects of the COVID-19 outbreak on delays.
Methods: The Painel de Monitoramento de Tratamento Oncológico database was queried to obtain the number of Brazilian patients with a BC confirmed diagnosis and initiating cancer treatment in the pre-COVID-19 (2013–2019) and during the COVID-19 (2020–2021) periods, adopting a 60-day limit as timely treatment. A p-value of <0.05 was considered significant.
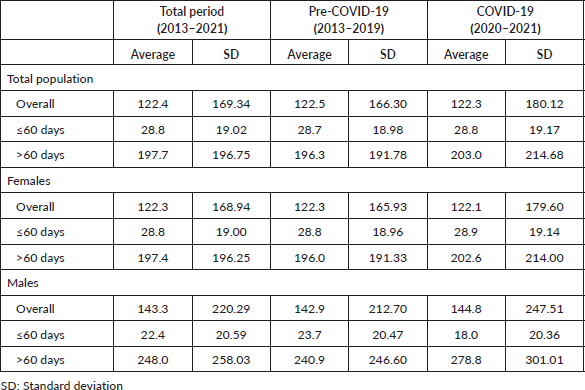
Results: A total of 315,951 cases were included (females: 99.3% and males: 0.7%), of which 251,667 and 64,284 records were computed before and during the COVID-19 years, respectively. Most patients failed to perform the first cancer treatment within 60 days (>60: 51.8%). We observed an upward trend in the number of BC treatments provided in the pre-COVID-19 years (r2 = 0.9575; p < 0.05), but the volume of treatments exhibited an average reduction of 24.6% yearly during the COVID-19 pandemic. The average DTi in days was 122.4, 122.5 and 122.3 in the total period studied, before and during the COVID-19 outbreak, respectively. The arrival of COVID-19 in Brazil increased the chances of treatment delay (OR = 1.043; p < 0.05) and inverted the proportion of early/advanced stages at BC diagnosis (55.8%/44.2%–48.4%/51.6%).
Conclusion: COVID-19 has imposed changes in BC care in Brazil, reducing the number of treatments provided by the Brazilian public health system, increasing the chances of delayed treatment initiation despite no differences in DTi averages being identified, and raising the proportion of advanced-stage diagnoses.
Keywords: breast cancer, COVID-19, time-to-treatment, treatment delay
Correspondence to: André Bouzas de Andrade
Email: abandrade@uneb.br
Published: 07/07/2023
Received: 16/04/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Breast cancer (BC) is the most frequent malignant neoplasm in Brazilian women, excluding non-melanoma skin cancer, and also figures as the first cause of female death by cancer in the country [1–3]. In Brazil, 66,280 new BC cases were estimated for 2022, accounting for nearly a third of all malignancies and corresponding to an adjusted incidence rate of 43.74 cases per 100,000 Brazilian women [1, 2, 4]. BC also affects men, yet rarely, representing about 1% of all cases [1, 5].
BC can be curable in up to 80% of patients when detected early and treatment started promptly [6–9]. The period from diagnosis confirmation to treatment initiation is the time interval that guidelines in oncology typically recommend minimising for better outcomes and overall survival, and, in this perspective, the Brazilian Government decreed Federal Law number 12,732 in November 2012, also known as the 60-Day Law, in an attempt to decrease treatment delays and its consequences subsequently [3, 9–11]. This legislation dictates the maximum interval of 60 days that a patient with any cancer has to wait to initiate his/her oncological treatment, counted from the diagnostic confirmation of the malignant neoplasm by histopathological analysis [3, 10, 11]. The law was reinforced and came into effect in 2013, when the Brazilian Ministry of Health instituted the National Policy for Cancer Prevention and Control through Ordinance No. 874/2013, within the scope of the Unified Health System (Sistema Único de Saúde – SUS) [10, 12].
Management based on intervals shorter than 60 days for cancer treatment onset after diagnostic confirmation remains challenging in Brazil, which could be affected by the current coronavirus disease-2019 (COVID-19) pandemic [3, 10, 13, 14]. Therefore, this population-based study aims to assess the waiting time between BC diagnosis and treatment initiation from the effective year of the 60-Day Law implementation and to evaluate the possible effects of the COVID-19 outbreak on delays in starting BC treatment in Brazil.
Methods
This retrospective, observational and nationwide study, in time series, evaluated the impacts of the COVID-19 pandemic on diagnosis-to-treatment initiation intervals, staging and therapeutic approaches for BC in Brazil. This investigation was conducted with secondary data from a government database. Data on the initiation of oncological treatment regarding Brazilian patients with BC were obtained at the Oncology Treatment Monitoring Panel (Painel de Monitoramento de Tratamento Oncológico – PAINEL-Oncologia), linked to the Unified Health System Department of Informatics (DATASUS) platform (available at <http://tabnet.datasus.gov.br/cgi/dhdat.exe?PAINEL_ONCO/PAINEL_ONCOLOGIABR.def>). This public domain source is a free-access and online database that gathers most information on the time interval for cancer treatment onset after a confirmed diagnosis. The PAINEL-Oncologia is a nationwide and population-based database that assembles patient data from the Ambulatory Information System (Sistema de Informações Ambulatoriais) – through the Individualised Outpatient Production Bulletin (Boletim de Produção Ambulatorial – Individualizado) and the High Complexity Procedure Authorisation (Autorização de Procedimento de Alta Complexidade) form, the Hospital Information System (Sistema de Informações Hospitalares) and the Cancer Information System (Sistema de Informações de Câncer). All these information and notification forms are reported by the State and Municipal Health Secretariats through the records of the patients’ national health card (CNS) in the SUS register, and managed by the Ministry of Health. Furthermore, the PAINEL-Oncologia platform serves as a public tool for monitoring compliance with Brazilian Federal Law No. 12,732/2012. Given this, a relevant delay in time to treatment initiation for this study was defined as more than 60 days.
The data were retrieved based on the 10th Revision of the International Disease Classification (ICD-10), using the C.50 code, recorded on DATASUS as ‘Malignant neoplasm of the breast’; therefore, cases of carcinoma in situ were not included. The following variables were analysed: the total and the annual number of BC cases, the diagnosis-to-treatment interval (DTi) in days, the number of patients with first cancer treatment performed in 60 days or less (≤60) and more than 60 days (>60), regions of Brazil (North, Northeast, Midwest, Southeast and South), age, gender, treatment modalities (surgery, chemotherapy, radiotherapy and other) and staging (I, II, III and IV). Clinical staging of BC was reported by health professionals and services following the Malignant Tumour Classification System used by the American Joint Committee on Cancer (AJCC). When information such as time to initiate treatment, modality of treatment, staging or age was marked as ‘unknown’ or ‘missing data’, this case was not counted during the statistical analysis. The study period ranged from January 2013 to December 2021, which encompassed the pre-COVID-19 years (2013–2019) and the first 2 years of the COVID-19 pandemic (2020–2021) in Brazil. Although the COVID-19 pandemic was only declared in March 2020, the PAINEL-Oncologia platform provides year-by-year data and therefore cases in January and February 2020 (pre-COVID-19 months) were included in that respective year since the data retrieved from 2020 comprised the number of cases for the full year.
The distribution of the variables was assessed by the Shapiro-Wilk test and the Q–Q plot. The Levene’s test for equality of variances assessed the homogeneity of variances. Descriptive statistics such as average, standard deviation (±SD), median, interquartile range (IQR) with 25th (Q1) and 75th (Q3) percentiles, odds ratio (OR) and confidence intervals (CI) were used to describe the numbers and proportions, applying a chi-square test and a Fisher’s exact test to perform the data analyses. Depending on the normality of the variable distribution, a nonparametric Mann-Whitney test and a Student’s
T-test were used to compare differences between groups. The level of significance was set at 5% (two-sided p-value < 0.05). Prais-Winsten linear regression [15] (Y = a + bX) was performed to evaluate the temporal trends and to provide the adjusted r2-values, adopting a confidence interval of 95%. To assess stability, growth or decrease of numbers over the years, the percentage change was calculated using the formula: ((next year's value − previous year's value)/previous year's value) × 100. The PAST software (Øyvind Hammer, UIO, v. 4.03) and the R software (RStudio, Inc., v. 4.0.3) were used to perform the statistical analyses.
The approval of the Ethics Committee in Research is waived since the secondary data were obtained from an online, public domain and governmental source, without identification of patients, following the current criteria of the guidelines in Resolution no. 510/2016 (07 April 2016) of the Brazilian National Council of Health, and as stated by the National Commission of Research Ethics in Brazil (available at <http://conselho.saude.gov.br/web_comissoes/conep/index.html>).
Results
In Brazil, 362,028 BC treatments were initiated from 2013 to 2021. After preliminary screening, 46,077 cases were excluded due to a lack of detailed treatment onset information, resulting in a set of 315,951 cases (Figure 1) (females = 313,825; 99.3% and males = 2,126; 0.7%). From the total, 251,667 treatments were initiated in the pre-COVID-19 years (average = 35,952/year), while 64,284 BC treatment records were computed during the COVID-19 outbreak (average = 32,142/year). The majority of patients failed to initiate BC treatment within the mandated 60-day limit after the confirmed diagnosis (≤60 = 48.2%; n = 152,330 versus >60 = 51.8%; n = 163,621) (Table 1). Excluding 2013, Figure 2 demonstrates that the annual frequency of patients with a waiting time lengthener than 60 days remained superior throughout the period.
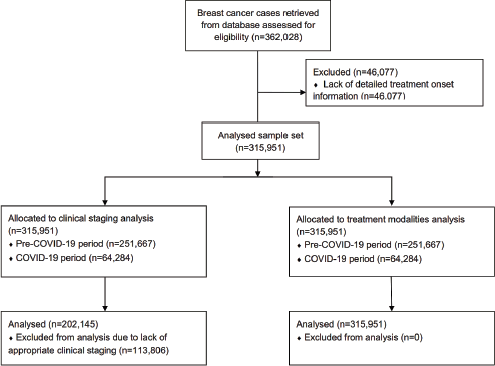
Figure 1. Patient flow diagram for analyses of clinical staging and treatment modalities.
Table 1. Number of patients initiating BC (C.50) treatment in Brazil, by gender and time intervals.
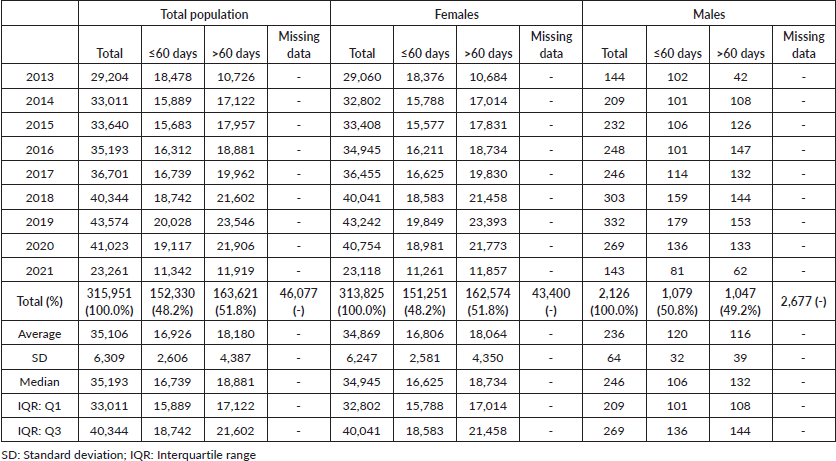
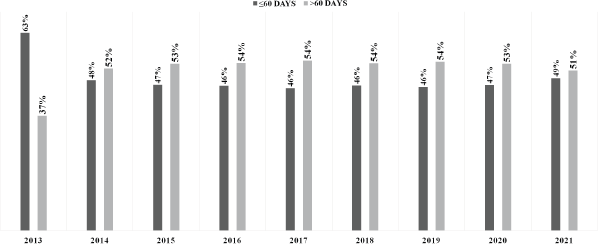
Figure 2. Frequency distributions of time intervals between BC (C.50) diagnosis and treatment initiation in Brazil.
Regions of Brazil
Figure 3 exhibits the Brazilian regional differences in the average proportion of patients waiting more than 60 days to initiate their BC treatment after diagnostic confirmation, also comparing the pre-COVID-19 (Figure 3b) and the COVID-19 (Figure 3c) periods, where the average increment was more prominent in the Midwest (MW) (48.3%–56.2%; p < 0.05), followed by the Northeast (NE) (48.6%–52.5%; p < 0.05), South (S) (44.7%–48.3%; p < 0.05), North (N) (55.3%–58.3%; p < 0.05) and Southeast (SE) (55.8%–55.7%; p < 0.05).
COVID-19 and trends
The overall period (2013–2021) exhibited no significant trend (p = 0.63), and no significant tendency was also observed among females (p = 0.64) and males (p = 0.42). However, the pre-COVID-19 years (2013–2019) showed an upward trend in the number of BC treatments provided by the SUS (Y = −4,418,731 + 2,201X; r2 = 0.9575; p < 0.05; Figure 4a), also observed among females (Y = −4,365,840 + 2,183X; r2 = 0.9574; p < 0.05; Figure 4b) and males (Y = −53,969 + 26X; r2 = 0.9269; p < 0.05; Figure 4c), with an average growth of +7.0% (±0.041), +6.9% (±0.041) and +15.8% (±0.163) per year, respectively. However, this trend was followed by an abrupt decrease during COVID-19 pandemic, with an average reduction of 24.6% (±0.26) yearly (2019–2020: −5.9%; 2020–2021: −43.3%). Similar findings were observed among females (average = −24.5%/year; SD = 0.265) and males (average = −32.9%/year; SD = 0.197) in the years 2020–2021. The regression coefficient showed that the arrival of COVID-19 in Brazil imposed an average reduction of 4,367 BC treatments provided yearly (Y = 39,811 − 4,367X), as seen in Figure 5.
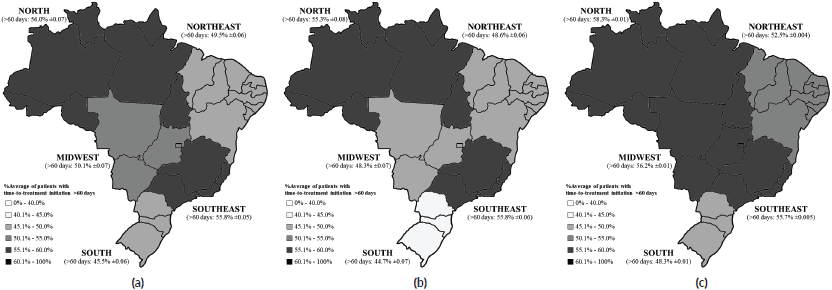
Figure 3. Brazilian regional differences in the average proportion of patients waiting longer than 60 days to initiate treatment (a) in the overall period (2013–2021), (b) in the pre-COVID-19 (2013–2019) and (c) during the COVID-19 (2020–2021) years.
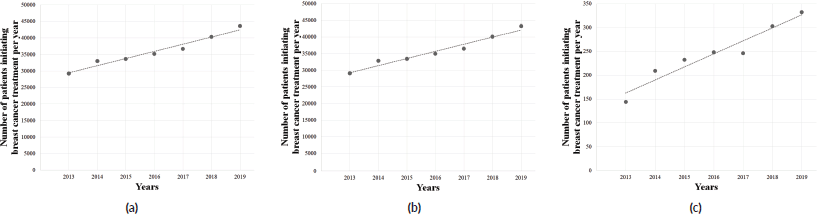
Figure 4. Linear regression analysis of the number of patients initiating BC (C.50) treatment in Brazil, in the pre-COVID-19 years, in the (a) total population, (b) females and (c) males. Data cover a 7-year range.
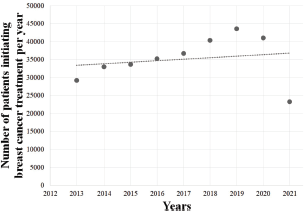
Figure 5. Linear regression analysis of the number of patients initiating BC (C.50) treatment in Brazil in 2013–2021. Data cover a 9-year range.
Patients were associated with a greater chance of initiating BC treatment later than 60 days during COVID-19 outbreak (OR = 1.0427; 95% CI = 1.024–1.060; p < 0.05), and a similar association was observed among females (OR = 1.0436; 95% CI = 1.025–1.061; p < 0.05), but not among males (OR = 0.9092; 95% CI = 0.733–1.127; p = 0.41). Further, the >60 days group showed an upward trend in the pre-pandemic years (overall: Y = −3,405,205 + 1,698X; r2 = 0.8726; p < 0.05; Figure 6a; females: Y = −3,375,158 + 1,683X; r2 = 0.8736; p < 0.05; Figure 6b; males: Y = −30,329 + 15X; r2 = 0.6870; p < 0.05; Figure 6c) – with an average growth of +15.4% (±0.217), +15.4% (±0.216) and +32.6% (±0.618) per year, respectively – however, the set of patients in the ≤60 days group did not display similar trend (Figure 7; p = 0.2).
Diagnosis-to-treatment intervals
Time intervals between diagnosis and first BC treatment varied greatly in the years 2013–2021, from patients who had a DTi of 0 days to patients who waited more than 2 years to start treatment after a confirmed BC diagnosis. Table 2 shows that the overall average DTi was 122.4 (±169.3) days yearly, and there was no difference comparing DTi before and during the COVID-19 pandemic (122.5 versus 122.3 days; p = 0.801). COVID-19 significantly lengthened the annual average DTi in the >60 days group (196.3 versus 203.0 days; p < 0.05), but no difference was observed in the group of 60 days or less (p = 0.422) (Table 2). Men with BC exhibited a longer average DTi than women (143.3 versus 122.3 days/year; p < 0.05) (Table 2). When assessing the two periods and gender, COVID-19 increased the chances of significant delays among women (OR = 1.0436; 95% CI = 1.025–1.061; p < 0.05), but not among men (p = 0.41).

Figure 6. Linear regression analysis of the number of patients waiting longer than 60 days to initiate BC (C.50) treatment in Brazil, in the pre-COVID-19 years, in the (a) total population, (b) females and (c) males. Data cover a 7-year range.
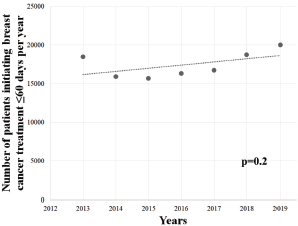
Figure 7. Linear regression analysis of the number of patients waiting 60 days or less to initiate BC (C.50) treatment in Brazil in the total population. Data cover a 7-year range.
Table 2. DTi averages in days for BC (C.50), by gender and time intervals.
Age and gender
The overall average age was 56.6 (±15.22) years old, with a peak age and peak age group of 50 and 50–54, respectively (Table 3). Male patients were significantly older than females (63.6 versus 56.6 years old; p < 0.05), including in the groups of ≤60 (male = 62.3 versus female = 55.6; p < 0.05) and >60 days (male = 63.9 versus female = 57.2; p < 0.05); and also, before (male = 63.9 versus female = 56.6; p < 0.05) and during (male = 62.7 versus female = 56.4; p < 0.05) COVID-19 outbreak (Table 3). By using the peak age of 50 years as a reference, Table 4 shows that the effect of aging increased the chances of delayed treatment initiation, and this increment was more substantial during the COVID-19 pandemic.
Table 3. Average age, peak age and peak age group in years old of patients initiating treatment.
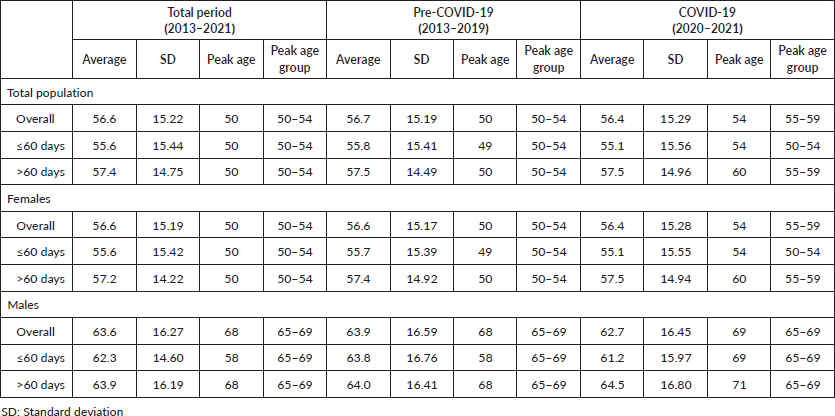
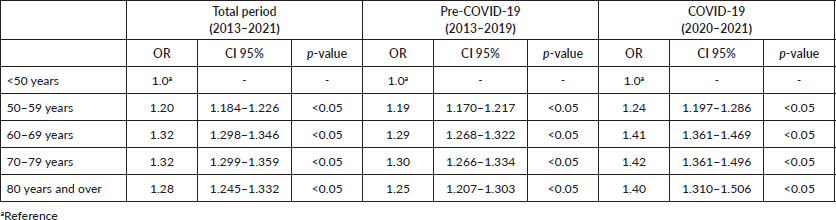
Table 4. Age comparison for the chances of waiting longer than 60 days for treatment initiation.
BC stages
As for properly staged cases (n = 202,145; Figure 1; Table 5), 54.04% of the general population were early cases of BC (stages I and II) and 45.96% were advanced cases (stages III and IV), and a similar proportion was observed in the pre-pandemic years (n = 154,380; early = 55.78% and advanced = 44.22%). However, advanced stages outnumbered the proportion of early-stage disease during the COVID-19 outbreak (n = 47,765; early = 48.41%; advanced = 51.59%). Moreover, with the arrival of COVID-19 in Brazil, the early-stage disease showed an average reduction in the annual diagnostic frequency (I: −11.8% and II: −2.0%), but advanced-stage disease at diagnosis increased by +30.2% for stage III and +17.5% for stage IV (Table 6). This finding is supported by the observation that COVID-19 imposed a greater overall chance of diagnosing advanced BC cases more frequently than in early stages compared to the pre-COVID-19 period (OR = 1.3441; 95% CI = 1.316–1.372; p < 0.05), and this association remained significant among females (OR = 1.3460; 95% CI = 1.318–1.374; p < 0.05) but not among males (p = 0.29). Beyond, COVID-19 increased the chances of detecting metastases at diagnosis in females (OR = 1.1003; 95% CI = 1.068–1.133; p < 0.05) but not in males (p = 0.2). Early stages had a greater chance of delayed treatment onset (OR = 2.2813; 95% CI = 2.240–2.323; p < 0.05) than advanced cases, including before (OR = 2.2895; 95% CI = 2.242–2.337; p < 0.05) and during the COVID-19 pandemic (OR = 2.2212; 95% CI = 2.140–2.305; p < 0.05).
Modalities of treatment
Chemotherapy was the most frequently performed first BC treatment from 2013 to 2021 (60.0%), with an average of 21,062.9 (±4,051.6) treatments yearly, followed by surgery (33.1%; average = 11,613.4/year; SD = 3,238.6), and radiotherapy (6.7%; average = 2,362.0/year; SD = 450.6). Twenty thousand nine hundred thirteen (n = 20,913) patients were diagnosed with BC only after a non-oncological surgery, and this procedure was later counted as their first cancer treatment, which we understood as revised diagnoses, and they were excluded from the statistical analysis. As seen in Table 7, the highest percentage of patients awaited between 121 and 300 days for the first BC treatment to be performed (chemotherapy = 22.2% and radiotherapy = 35.9%), except for surgery, in which 19.6% of patients displayed a 0-day DTi. A minimal proportion of patients exhibited a 0-day interval in other treatment groups (chemotherapy = 2.9% and radiotherapy = 2.3%). The majority of patients who initiated BC treatment with surgery had the procedure performed within 60 days (≤60/>60: 57.7%/42.3%), but similar proportion was not found in other treatment modalities (chemotherapy = 41.3%/58.7% and radiotherapy = 22.4%/77.6%).
Table 5. Clinical staging of patients initiating BC (C.50) treatment, by gender and time intervals.
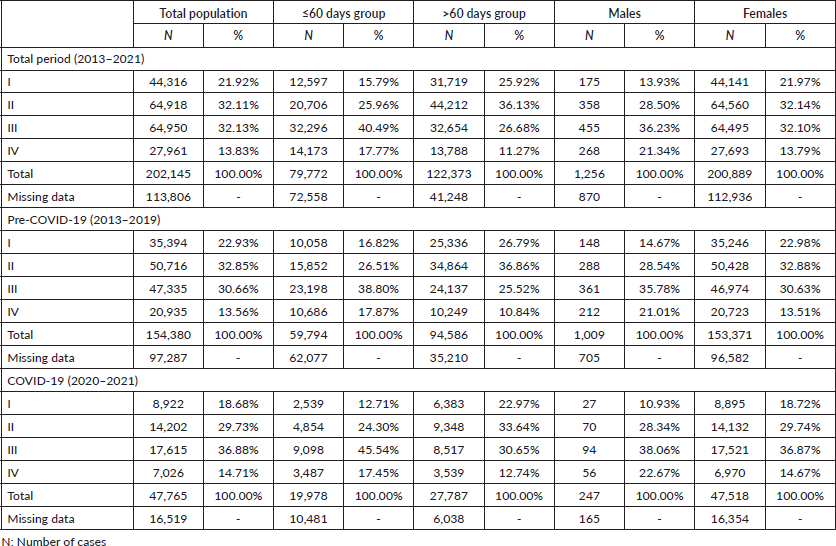
Table 6. Annual average numbers of patients initiating BC (C.50) treatment in Brazil, by staging and gender.
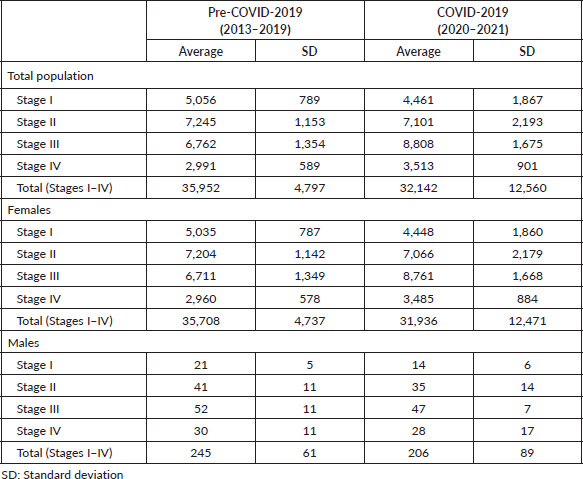
Table 7. Frequency distribution of treatment modalities by time intervals for BC (C.50) treatment initiation.
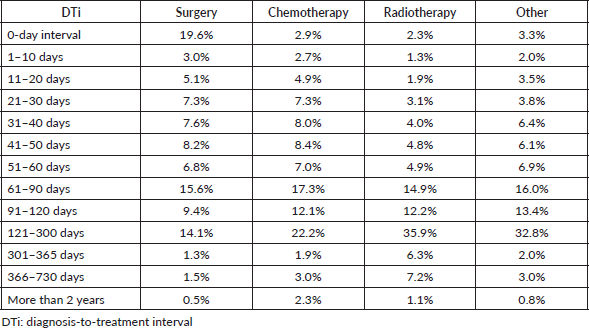
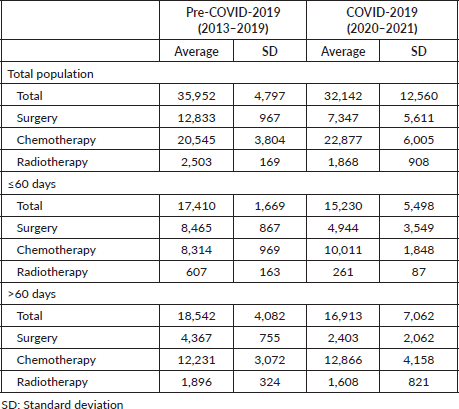
Despite exceeding the 60-day limit, surgery exhibited a significantly shorter average DTi (82.0 days) compared to chemotherapy (133.8 days; p < 0.05), and radiotherapy (179.4 days; p < 0.05) (Table 8). As seen in Table 8, significantly shorter average DTi were found when comparing surgery to other modalities in both pre-COVID-19 and during COVID-19 years. Besides, while the COVID-19 pandemic significantly shortened the average DTi for surgery (84.1–70.8 days; p < 0.05) and chemotherapy (134.9–130.6 days; p < 0.05), radiotherapy significantly lengthened the average DTi during the COVID-19 outbreak in Brazil (173.8–205.5 days; p < 0.05) (Table 8). Compared to surgery, all other modalities were associated with greater chances of delayed treatment onset in the years 2013–2021 (Table 9). Table 9 also shows that surgery and chemotherapy were associated with lower chances of significant delays during the COVID-19 years compared with the pre-COVID-19 period. Table 10 shows that most treatment modalities displayed a reduction in the annual volume of treatments performed during COVID-19, except for chemotherapy. The regression coefficient showed an average reduction of 5,142 BC surgeries per year with the arrival of COVID-19 in Brazil (Y = 18,072 − 5,142X; p < 0.05), but this finding was not observed among other therapeutic modalities (chemotherapy: p = 0.5 and radiotherapy: p = 0.07).
Table 8. DTi averages in days for BC (C.50) treatment initiation, by treatment modalities.
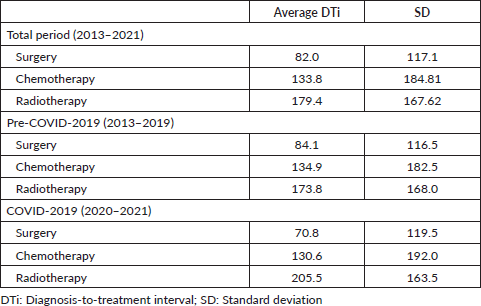
Table 9. Treatment modalities comparison for the chances of waiting longer than 60 days for treatment initiation.
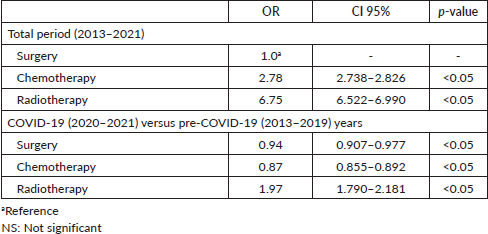
Table 10. Annual average number of treatment modalities on patients initiating BC (C.50) treatment, by time intervals.
Discussion
The rapid rise in the number of severe cases of COVID-19 required public policies to redistribute available hospital beds, healthcare workforce and medical equipment, which underprioritised several non-emergency medical conditions, and cancer was one of them [14, 16]. Our results showed an important reduction in the volume of BC treatments provided during the first 2 years of the pandemic in Brazil. Moreover, COVID-19 imposed a greater chance of delayed treatment onset, intensified the already significant odds of age-related delays, increased the percentage of delayed BC treatment onset across the regions of Brazil, and also worsened the delay in the group that was already starting therapy with a DTi lengthier than 60 days. Linear regression identified an upward trend in the number of patients with delayed treatment onset in the pre-COVID-19 years, highlighting that the number of patients initiating BC treatment beyond the maximum term determined by Brazilian law was increasing even before the pandemic. This observation seems to be a recurrent problem in BC care in Brazil.
A 473-patient retrospective study in Northeast Brazil between 2009 and 2011, which implies a time before both COVID-19 and the 60-Day Law, showed a median interval of 71.5 days between diagnosis and treatment onset for BC [17]. Shafaee et al. [18], by evaluating 963 BC patients in Southeast Brazil (2009–2011) showed that the average time from diagnosis to the first overall treatment and the first systemic treatment was 86.8 and 151.9 days, respectively.
The relevance of cancer treatment prompt initiation for better outcomes and lower chances of recurrence is well-established, and Brazil recognises this importance, to the point of creating a federal law regulating a medical practice of short intervals between diagnosis and cancer treatment initiation [10, 11, 13, 14]. Previous reports have shown that most BC patients still have not been receiving timely treatment in Brazil even after the announcement of the law [2, 19–21].
A 470-patient prospective study (2014–2015) conducted at the Cancer Hospital III in Rio de Janeiro/Brazil demonstrated that delayed treatment was identified in 89.1% of BC cases and the median DTi was 127 days [2]. dos Santos Andrade et al. [22], in a 304-patient study in Northeastern Brazil (2016–2019), showed that the average DTi was 62.4 days for 77.3% of cases, of which 42.0% had a delay lengthener than 90 days. In 2022, the 60-Day Law completed 10 years in force, yet our investigation evidenced that most Brazilian cases of BC still start treatment with delays of 60 days or more.
The COVID-19 outbreak appears to be associated with controversial findings regarding delays in time to BC treatment initiation worldwide, as reported by Hawrot et al. [13] in a retrospective study with 366 patients in Philadelphia/US, which, despite an 18.8% reduction in the number of new BC diagnoses, no difference was found in the average DTi comparing 2018 (44.7 days) to 2020 (44.4 days). Caswell‑Jin et al. [23] assessed 19,329,646 women and men newly diagnosed with BC in the US and showed that the proportion of patients with DTi longer than 60 days in the pre-COVID time (January/2017–March/2020: 18.9%) firstly decreased between April and May 2020 (15.7%), but then increased from June 2020 to February 2021 (19.1%). Li et al. [24] identified a lengthened DTi in 8,397 BC patients during quarantine restrictions in Hubei (3.5–7.7 days) and other provinces in China (5.7–7.7 days). On the other hand, a comparative analysis placed at the Instituto do Câncer do Estado de São Paulo/Brazil, with 268 BC patients, demonstrated that the median interval for the first cancer centre visit after breast tumour biopsy was lower during the pandemic (September/2020–January/2021: 5.4 months) than previous to it (September/2019–January/2020: 6.7 months) [25]. Similar to Hawrot et al. [13], our results showed a non-significant difference in the waiting time between the pre-COVID-19 (122.5 days) and during the COVID-19 (122.3 days) years, yet the averages were still more than double the 60-day limit recommended by Brazilian law.
One in every six Brazilian women who died of cancer in 2019 was due to BC, which emphasises the magnitude of the BC mortality burden in Brazil [5, 26]. The mantra ‘early detection saves lives’ may correlate with the tenet that late BC treatment initiation kills patients, and this might be explained by a constellation of factors, such as tumour growth, lymph node invasion and progression to local or distant metastases [6, 9]. A recent issue of the US Department of Health and Human Services estimated that malignant breast tumour doubles in size with medians between 45 and 260 days [9]. Unfortunately, our results showed that the average DTis for Brazilian BC patients in the overall period, before and during the COVID-19 pandemic were 122.4, 122.5 and 122.3 days, respectively. Although no differences were observed in the average waiting times for BC treatment onset between periods in our analyses, COVID-19 was associated with a greater chance of relevant delays (OR = 1.043; p < 0.05), and this controversial finding may be the result of differences in the number of patients in the sample sets before and during the pandemic years. We believe that the small number of cases in the patient collective assembled in 2020–2021 (n = 64,284) compared to the sample set gathered in the pre-COVID-19 years (n = 251,667) may have interfered with the DTi interval averages and standard deviations and could explain the lack of DTi intervals differences between these periods.
The regression analyses showed an upward trend in the number of BC treatments offered in the pre-pandemic years, which highlights that Brazilian public health was increasingly supplying treatments for newly diagnosed BC patients (growth = 7.0%/year); yet, our results evinced that COVID-19 imposed a drastic reduction in the volume of treatments that were being provided by the SUS. Furthermore, we found a significantly greater chance of Brazilian patients starting BC treatment with an interval longer than 60 days during the COVID-19 pandemic, and this might be related to also the upward trend found in the group of patients with delayed treatment onset in the pre-COVID-19 years.
Brazil is a considerably large country with a continental dimension, and this wide territorial area may be the root of inequalities in the healthcare structure between regions [3, 27]. South Brazil presents a remarkably high incidence of BC [1, 5], and this region exhibited the lowest percentages of patients with delayed treatment initiation in our analyses, even when facing COVID-19. The Southeast showed stability in the percentage of patients with delayed treatment onset between the two periods.
The South of Brazil displays the highest Basic Education Development Index (IDEB = 6.17), the highest per capita income (US$ 320.73), the highest percentage of working people with formal employment (70.6%) and the highest Human Development Index (HDI = 0.756), and similarities are found in the Southeast (IDEB = 6.03; per capita income = US$ 299.40; formal employment = 65.1%; and HDI = 0.754) [27]. These demographic features indicate a proper socioeconomic development of these regions, which might reflect a better public health structure, and it is relevant for our analysis since less access to health care is associated with socioeconomic vulnerabilities in Brazil [3, 8, 27].
The North and the Northeast exhibit the worst sociodemographic indicators in Brazil (N: IDEB = 5.29; per capita income = US$ 176.71; formal employment = 45.4%; HDI = 0.684; NE: IDEB = 5.12; per capita income = US$ 163.48; formal employment = 40.7%; and HDI = 0.660) [27], which may explain why these regions held the highest proportions of patients with delayed treatment. The Midwest displays demographic indicators that suggest adequate regional development (IDEB = 5.73; per capita income = US$ 261.17; formal employment = 59.1%; and HDI = 0.730) [27], yet, this region experienced the worst impact on delays for BC treatment onset during the COVID-19 outbreak, with the greatest percentage increase between periods (+7.86%).
Male cases computed only 0.7% of our sample, however, men were older and exhibited longer waiting times. Moreover, women began to exhibit a higher frequency of advanced BC diagnoses with the arrival of COVID-19 in Brazil, yet significant chances of men displaying advanced-stage disease at diagnosis more often during the pandemic were not observed since these men had already been bearing a higher proportion of advanced disease even before COVID-19.
Some reports have implied that male patients are more frequently diagnosed with advanced BC and have a worse prognosis due to poor awareness that BC can affect men, lack of attention by men (and even by health professionals) at breast symptoms onset, the experience of embarrassment at breast symptom onset, and all these factors combined may result in delays in male BC care [19, 28, 29]. A retrospective study in Hong Kong (1998–2018) showed that the average interval between symptom onset and the first consultation for 56 men with BC was 12.4 months, which could reach up to 120 months [29]. Researchers have demonstrated little interest in publishing about the occurrence of BC in men, yet male BC incidence appears to be slowly rising in developing countries in the last decades [28], and BC incidence in Brazilian men tripled from 1998 to 2008 [19]. Our results showed that the number of newly-diagnosed male BC treatments provided annually increased by 3.64% per year between 2013 and 2021, which, in turn, also reflects an increase in the BC incidence in Brazilian men from our sample.
As aforementioned, the proportion of advanced-stage disease in female BC diagnosis increased with the arrival of COVID-19 in Brazil and imposed a 34.4% higher chance of diagnosing advanced disease, yet early-stage patients exhibited a greater chance of delayed treatment onset during and even before the pandemic, which places women with potentially curable diseases at risk for upstaging and worse outcomes. Lengthy time to treatment initiation is often associated with the risk of BC upstaging in developing countries, even to a status of incurable disease [3, 9].
A report on the impact of the COVID-19 pandemic on BC in São Paulo/Brazil also observed an increment in the proportion of advanced-stage disease during the pandemic (2019–2020: early/advanced = 72.9%/36.1%; 2020–2021: early/advanced = 47.0%/53.0%) [25]. These observations might be explained by the ‘stay-at-home’ strategies initially adopted by Brazilian state governments in an attempt to restrain the transmission of the coronavirus, for instance, the lockdown policy, the lower circulation of non-COVID-19 patients in healthcare services and the postponement of screening tests, which, ultimately, may have delayed patient evaluation and led to more advanced-stage presentations at diagnosis [23–25, 30].
At the time of writing, no previous nationwide study statistically evaluated the effects of COVID-19 on the waiting time for BC treatment onset in Brazil and its regions and assessed the impact of the pandemic on BC staging and procedures. The time interval to the first cancer treatment after a confirmed diagnosis is used as a quality-of-service parameter in many healthcare centres worldwide [9, 16, 31, 32]. In our investigation, none of the treatment options fully complied with the 60-Day Law, and, despite the surgical modality having computed better waiting times, our diagnosis-to-surgery intervals might be sufficient to impact the survival of Brazilian patients with BC. An 8,860-patient retrospective study with BC cases in the US (1997–2006) showed an 80% 5-year survival in women with a diagnosis-to-surgery interval longer than 42 days, which was considerably lower than the 90% 5-year survival in those who underwent surgery in less than 14 days after diagnostic confirmation [32].
Our patients waited an overall average of 82 days to undergo surgery, however, the averages were 84 and 70 days before and during the COVID-19 pandemic, respectively. In early 2020, the American College of Surgeons (ACS) [33] initially recommended the immediate suspension of elective procedures or, at least, surgeons should curtail the performance of elective surgeries in the face of the COVID-19 spread worldwide. The Brazilian College of Surgeons and the Brazilian Society of Oncological Surgery soon adopted the ACS recommendation and instructed a reduction in the volume of elective surgeries nationwide, including oncological surgeries, although reinforced the need for a case-by-case evaluation [33, 34]. Later 2020, the Brazilian Ministry of Health announced the resumption of oncological surgeries in the SUS routine with the premise that ‘cancer patients cannot wait’ [34]. In light of these events, Bonadio et al. [25] hypothesised that non-oncological patients were not referred to surgical centres during the periods of restrictions, facilitating the access of cancer patients to surgery and shortening the diagnosis-to-surgery interval. Nevertheless, our analyses have shown a significant reduction in the number of BC surgeries performed during COVID-19.
Our data also implied that one in almost every five patients who had surgery as the first treatment modality of choice exhibited a 0-day DTi. This finding suggests that this patient underwent surgery on the same day that received the diagnosis confirmation of BC, which does not represent the most common approach to invasive malignant tumours [9]. Conversely, the 0-day DTi may also reflect a misapplication of codes on public health system reporting forms that feed the database, either from the ICD-10 or the SUS procedures list, which, ultimately, have biased our data analyses regarding surgery. Furthermore, radiotherapy as a first treatment for BC is unusual and accounted for only 6.7% of first treatments in our sample. We believe that these are patients for whom surgery was not immediately available, and radiotherapy enabled better control of critical symptoms, such as local haemostasis.
Limitations exist within the scope of this investigation and including the retrospective and observational design of the study and the database, whose structure did not allow us to evaluate patients regarding ethnicity, level of education, per capita income, access to health insurance, history of BC or other malignancy, the first and the number of symptoms noticed, the circumstance of BC detection and referral source. Besides, the database did not provide the number of cases with ‘missing data’ by year and by gender, so it was not possible to measure whether there were temporal or gender differences in the completion of data in our investigation. The database did not examine information about the initial staging at diagnosis and the reassessment for upstaging after a long waiting time for treatment initiation. Clinical staging of BC in the Brazilian public health practice follows the classification system established by the AJCC; however, the platform did not disclose if the reports complied with the AJCC staging system changes for BC stated in 2018. Further, it was not possible to differentiate the intention of chemotherapy as palliative or neoadjuvant, nor to evaluate tumour size, angiolymphatic or perineural invasion, histologic grade, molecular subtypes and laterality. Therefore, the present study could not associate these features with delays in BC treatment onset during COVID-19, which, in turn, constitutes grounds for performing more robust, multicentre and primary studies.
Conclusion
In conclusion, our results show that despite Federal Law No. 12,732/2012 completing 10 years in operation in 2022, most BC patients still initiate treatment with delays longer than 60 days after the diagnostic confirmation, especially if the presentation is with early-stage disease, advanced age or the patient is a man. Our time series analysis indicated that the number of patients with delayed treatment onset has been predominant since 2014, with increasing trends throughout the years, and the panorama worsened with the arrival of COVID-19 in Brazil when the odds of detecting metastases and the frequency of advanced disease outnumbered early-stage disease at diagnosis. The ideal prospect to improve the BC mortality burden in post-COVID-19 Brazil requires significant investments in cancer care nationwide, effective resumption of BC screening programs, and the development of public policies for educating men and women about BC diagnosis and the importance of timely presentation and treatment. Undoubtedly, the 60-Day Law is a milestone in the history of Brazilian public health as an effort to reduce health system delays to cancer treatment initiation, but surveillance still needs to be improved to ensure that this law is properly implemented.
Acknowledgments
The authors are thankful to the Academic League of Internal and Surgical Medicine – Trauma League (LAMIC-LT) and its esteemed members for fruitful debates and for stimulating the medical research.
Conflicts of interest
There is no conflict of interest
Funding
None
Author contributions
Conception and design: All authors. Collection and assembly of data: JHFN and CNS. Data analysis and interpretation: All authors. Manuscript writing: JHFN. Revised the language/article: All authors. Final approval of manuscript: All authors.
References
1. Instituto Nacional de Câncer José Alencar Gomes da Silva (2020) Estimativa 2020 – Incidência de Câncer no Brasil | INCA – Instituto Nacional de Câncer (Rio de Janeiro: Ministério da Saúde)
2. Medeiros GC, Thuler LCS, and Bergmann A (2021) Determinants of delay from cancer diagnosis to treatment initiation in a cohort of Brazilian women with breast cancer Health Soc Care Community 29 1769–1778 https://doi.org/10.1111/hsc.13284 PMID: 33438787
3. Bukowski A, Gioia S, and Chavarri-Guerra Y, et al (2017) Patient navigation to improve access to breast cancer care in Brazil J Glob Oncol 3 433–437 https://doi.org/10.1200/JGO.2016.006726 PMID: 29094079 PMCID: 5646893
4. Silva JDDE, de Oliveira RR, and da Silva MT, et al (2021) Breast cancer mortality in young women in Brazil Front Oncol 10 3149 https://doi.org/10.3389/fonc.2020.569933
5. Instituto Nacional de Câncer José Alencar Gomes da Silva (2022) Incidência | INCA – Instituto Nacional de Câncer (Rio de Janeiro: Ministério da Saúde) [https://www.inca.gov.br/controle-do-cancer-de-mama/dados-e-numeros/incidencia]
6. Harbeck N, Penault-Llorca F, and Cortes J, et al (2019) Breast cancer Nat Rev Dis Primers 5 1–31 https://doi.org/10.1038/s41572-019-0111-2
7. Li Y, Zhou Y, and Mao F, et al (2019) The influence on survival of delay in the treatment initiation of screening detected non-symptomatic breast cancer Sci Rep 9 1–7
8. McLaughlin JM, Anderson RT, and Ferketich AK, et al (2012) Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer J Clin Oncol 30 4493–4500 https://doi.org/10.1200/JCO.2012.39.7695 PMID: 23169521 PMCID: 3518728
9. Bleicher RJ (2018) Timing and delays in breast cancer evaluation and treatment Ann Surg Oncol 25 2829–2838 https://doi.org/10.1245/s10434-018-6615-2 PMID: 29968031 PMCID: 6123282
10. Paulino E, de Melo AC, and Nogueira-Rodrigues A, et al (2018) Gynecologic cancer in Brazil and the law of sixty days J Gynecol Oncol 29 e44 https://doi.org/10.3802/jgo.2018.29.e44 PMID: 29533026 PMCID: 5920227
11. Brasil (2012) Lei No 12.732, de 22 de Novembro de 2012 Presidência da República – Casa Civil
12. Brasil (2013) Portaria N° 874, de16 de Maio de 2013 Ministério da Saúde – Governo Federal
13. Hawrot K, Shulman LN, and Bleiweiss IJ, et al (2021) Time to treatment initiation for breast cancer during the 2020 COVID-19 pandemic JCO Oncol Pract 17 534–540 https://doi.org/10.1200/OP.20.00807 PMID: 33710914 PMCID: 8457793
14. Araujo SEA, Leal A, and Centrone AFY, et al (2020) Impact of COVID-19 pandemic on care of oncological patients: experience of a cancer center in a Latin American pandemic epicenter Einstein (Sao Paulo) 19 eAO6282 https://doi.org/10.31744/einstein_journal/2021AO6282 PMID: 33338192 PMCID: 7793126
15. Antunes JLF and Cardoso MRA (2015) Using time series analysis in epidemiological studies Epidemiol Serv Saúde 24 565–576 https://doi.org/10.5123/S1679-49742015000300024
16. Duarte MBO, Argenton JLP, and Carvalheira JBC (2022) Impact of COVID-19 in cervical and breast cancer screening and systemic treatment in São Paulo, Brazil: an interrupted time series analysis JCO Glob Oncol 8 e2100371 https://doi.org/10.1200/GO.21.00371
17. Alves Soares Ferreira N, Melo Figueiredo de Carvalho S, and Engrácia Valenti V, et al (2017) Treatment delays among women with breast cancer in a low socio-economic status region in Brazil BMC Womens Health 17 13 https://doi.org/10.1186/s12905-016-0359-6 PMID: 28222726 PMCID: 5320774
18. Shafaee MN, Silva LR, and Ramalho S, et al (2022) Breast cancer treatment delay in safetynet health systems, Houston versus Southeast Brazil Oncologist 27 344–351 https://doi.org/10.1093/oncolo/oyac050 PMID: 35348756 PMCID: 9074991
19. Coutinho de Medeiros G, Gomes Chagas Teodózio C, and Alves Nogueira Fabro E, et al (2020) Fatores Associados ao Atraso entre o Diagnóstico e o Início do Tratamento de Câncer de Mama: um Estudo de Coorte com 204.130 Casos no Brasil Rev Bras Cancerol 66 e-09979 https://doi.org/10.32635/2176-9745.RBC.2020v66n3.979
20. Lopes TCR, Gravena AAF, and Demitto M de O, et al (2017) Delay in diagnosis and treatment of breast cancer among women attending a reference service in Brazil Asian Pac J Cancer Prev 18 3017–3023
21. Chaves IM dos A, Oliveira VAA de, and Araujo DN, et al (2020) Oncological treatment in Brazil: a gender and region are associated to starting the therapeutics Braz J Oncol 17 e-20200045
22. dos Santos Andrade LS, de Melo Santos TT, and Case de Oliveira ME, et al (2021) Shorter delay to treatment by integrated diagnostic services and NGO-provided support among breast cancer patients in two Brazilian referral centres J Public Health Res 10 jphr.2021.1880 https://doi.org/10.4081/jphr.2021.1880 PMID: 33709643 PMCID: 8314677
23. Caswell-Jin JL, Shafaee MN, and Xiao L, et al (2022) Breast cancer diagnosis and treatment during the COVID-19 pandemic in a nationwide, insured population Breast Cancer Res Treat 194 475–482 https://doi.org/10.1007/s10549-022-06634-z PMID: 35624175 PMCID: 9140322
24. Li J, Wang H, and Geng C, et al (2020) Suboptimal declines and delays in early breast cancer treatment after COVID-19 quarantine restrictions in China: a national survey of 8397 patients in the first quarter of 2020 EClinicalMedicine 26 100503 https://doi.org/10.1016/j.eclinm.2020.100503 PMID: 32989430 PMCID: 7511845
25. Bonadio RC, Messias AP, and Moreira OA, et al (2021) Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil Ecancermedicalscience 15 1299 https://doi.org/10.3332/ecancer.2021.1299 PMID: 34824622 PMCID: 8580713
26. Nogueira LR and Kluthcovsky ACGC (2022) Análise da mortalidade por câncer de mama no Brasil e regiões, 2005 a 2019 Res Soc Dev 11 e23211931628 https://doi.org/10.33448/rsd-v11i9.31628
27. Instituto Brasileiro de Geografia e Estatística (2022) IBGE | Brasil | Panorama [https://cidades.ibge.gov.br/brasil/panorama]
28. Ruddy KJ and Winer EP (2013) Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship Ann Oncol 24 1434–1443 https://doi.org/10.1093/annonc/mdt025 PMID: 23425944
29. Co M, Lee A, and Kwong A (2020) Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer Cancer Med 9 3305–3309 https://doi.org/10.1002/cam4.2953 PMID: 32167660 PMCID: 7221437
30. Vanni G, Tazzioli G, and Pellicciaro M, et al (2020) Delay in breast cancer treatments during the first COVID-19 lockdown. a multicentric analysis of 432 patients Anticancer Res 40 7119–7125 https://doi.org/10.21873/anticanres.14741 PMID: 33288611
31. Buzaid AC, Achatz MI, and Amorim GL da S, et al (2020) Challenges in the journey of breast cancer patients in Brazil Braz J Oncol 16 1–10
32. Smith EC, Ziogas A, and Anton-Culver H (2013) Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity JAMA Surg 148 516–523 https://doi.org/10.1001/jamasurg.2013.1680 PMID: 23615681
33. American College of Surgeons (2020) COVID-19: Elective Case Triage Guidelines for Surgical Care | ACS (ACS) [Online] [https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-case/]
34. Instituto Nacional de Câncer (2020) Importância e segurança de cirurgias oncológicas durante pandemia do novo coronavírus são temas de live do MS (Rio de Janeiro: Ministério da Saúde) [https://www.inca.gov.br/noticias/importancia-e-seguranca-de-cirurgias-oncologicas-durante-pandemia-do-novo-coronavirus-sao]






