Male breast cancer: a 30 year retrospective analysis from a tertiary cancer care centre
Abhishek Soni, Yashpal Verma, Ashok Chauhan, Paramjeet Kaur, Vivek Kaushal and Diptajit Paul
Department of Radiation Oncology, Pt. B. D. Sharma PGIMS, Rohtak 124001, Haryana, India
Abstract
Background: Male breast cancer (MBC) is one of the rare malignancies that account for less than 1% of all malignancies in males. However, the clinicopathological characteristics of MBC are not entirely similar to female breast cancer; but still, it is treated in line with the female breast cancer protocols.
Aims: To retrospectively analyse trends in MBC as to its distribution, presentation, treatment, and outcome.
Material and method: A total of 106 patients with MBC from 1991 to 2020 were analysed retrospectively. Frequency distribution analysis of the demographic and clinicopathological data and treatment variables was done.
Results: Median age of presentation was 57 years; ranging from 30 to 86 years. Either of the sides was almost equally affected with an R: L ratio of 1.2:1. The average duration of complaint was 26.2 months (range 1–240 months). History of gynaecomastia was noted in 18 patients, significant benign prostate hypertrophy in 13, and hypertension needing medical treatment in 14 patients. The majority of the patients were smokers (72/106) and alcoholics (43/106). Five patients reported positive family history. 21 patients had metastatic disease at presentation and received palliative treatment. Stage II was seen in 36.8%, stage III in 43.4%, and stage IV in 19.8% of patients. Node positives were 63.2%. Pathology was invariably (90.5%) infiltrative ductal carcinoma. Radiation was administered in 85.8% of the patients, chemotherapy in 72.6% of patients, and hormonal treatment was given in 47.2% of patients. The median overall survival (OS) was 78 months. OS at 5 and 10 years was 78% and 58% respectively.
Conclusion: Despite the possibility of MBC being apparent at an early stage, patients present with locally advanced disease. Radical surgery with adjuvant/neoadjuvant chemotherapy and adjuvant radiotherapy remains the gold standard. Cancer education campaigns must be run to catch the early disease and to radically treat the disease.
Keywords: breast cancer, chemotherapy, male, uncommon, radiotherapy
Correspondence to: Abhishek Soni
Email: abhisheksoni246@gmail.com
Published: 18/05/2023
Received: 04/02/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Male breast cancer (MBC) occurs all over the world, but the highest incidence is seen in sub-Saharan countries [1]. MBC is rare cancer representing 1% of all breast malignant neoplasms analysed every year in the United States. In 2021, 2,670 new cases of MBC were analysed in the United States, and around 500 men died from it [2]. The lifetime risk of breast cancer is around 1 out of every 1,000 for a man, while it is around 1 out of 8 for a female. Around the world, the female-to-male incidence ratio is 125:1 [3]. As of now, while the rate of female breast cancer has decreased by 42%, a 28% decline in MBC has been observed [4]. The incidence of breast cancer increases consistently with age in males as well as in females. The typical age of new breast cancer incidence is 5 years more for males (67 years) than for females (62 years) [5]. Other risk factors for MBC incorporate a family background of breast cancer, dark ethnicity, breast or chest exposure to radiation, conveying an inclining germline hereditary change (e.g., BRCA2, BRCA1, CHEK2, PALB2), utilisation of exogenous oestrogen, and diseases related with hyperestrogenism (e.g., Klinefelter syndrome) [3]. Men with Klinefelter condition (XXY) have a 14–50 times risk of developing MBC and account for 3% of all MBC cases [6]. Men with persistent liver issues like cirrhosis, constant liquor abuse, and schistosomiasis; history of mumps, orchitis, undescended testis, or testicular injury; and feminisation, whether hereditary or ecological, are at more risk of developing breast cancer. Gynaecomastia itself does not give an impression of being a single risk factor [6].
There are some lacunae with regard to the ideal management of MBC and its treatment has been extrapolated to a great extent from research led on female breast cancer. Studies that focus on MBC should subsequently recommend the standard of care, but till now they have not been able to address all important questions regarding MBC. There are no randomised studies because of the low number of patients continued in any institution and retrospective studies are additionally not very many. Initial trials proposed that MBC had a poor prognosis in comparison to breast cancer in females [7–9]. The MBC treatment is a combination of surgery, radiation, chemotherapy, and hormonal therapy. The lack of breast tissue in men makes it challenging to achieve safe margins in MBC, especially in small tumors. In India, MBC patients present at a locally advanced stage and it is very difficult to achieve a negative margin. Thus, it mandates adjuvant treatment in MBC patients.
Indications for postmastectomy radiotherapy (PMRT) in MBC are in accordance with the guidelines for female breast cancer. PMRT seems to decrease locoregional recurrence (LRR) in MBC; in any case, the effect on overall survival (OS) is unknown [10–13]. Few studies have depicted the advantage of PMRT in MBC patients [14–17]. Many retrospective studies assessed the role of adjuvant hormonal treatment, and these studies have demonstrated that most MBC patients can benefit from adjuvant tamoxifen in terms of recurrence and survival [18–22]. Although chemotherapy has been indicated to treat high-risk patients with MBC, there is a lack of data to help it. A few studies recommended that adjuvant and systemic treatment in MBC ought to be equivalent to female breast cancer [18–20, 23]. In this review, we retrospectively analysed the clinicopathological characteristics and treatment outcomes of MBC patients at a single institution. The hallmark of the present article is that it demonstrates the MBC data of 30 years’ time period, which, to the best of our knowledge, is the first study on MBC in India spanning over three decades.
Materials and methods
A total of 8,154 breast cancer cases were registered from 1991 to 2020, out of which 106 MBC cases were identified constituting 1.3% of total breast cancer cases. These were retrospectively analysed for patient‑related characteristics such as age, comorbidity, family history, tumour size, nodal status, stage, histology, oestrogen/progesterone, and human epidermal growth factor receptor 2 (HER‑2) status (Tables 1 and 2). All parameters were entered into a computerised database. As ours is a government institution, immunohistochemistry could not be performed in all patients due to logistic issues and also it was not available in the early phase of the study.
PMRT was delivered to patients with a T3 tumour, close/positive margins, and positive nodes. Standard two tangential opposed fields were used to irradiate the chest wall (CW). Radiotherapy was delivered with 50 Gy in 25 fractions over 5 weeks to CW. The dose was prescribed at the midpoint of the central axis. For supraclavicular fossa, the dose delivered was 50 Gy in 25 fractions over 5 weeks with a single‑incident field. Radiation therapy was delivered using a Cobalt-60 machine. Tamoxifen was prescribed to estrogen receptor (ER)/progesterone receptor (PR)‑positive patients for 5 years as per female breast cancer guidelines and letrozole which was prescribed for a further 3–5 years in females, was not prescribed in MBC cases [5]. Patients were followed up at regular intervals: every 3 months till 1 year, every 4 months till 3 years, every 6 months till 5 years, and yearly thereafter. Further tests were done only if they had symptoms or evidence of recurrent or metastatic disease. Treatment outcomes analysed were LRR, disease‑free survival (DFS), and OS. LRR was defined as any recurrence in the skin or soft tissue over CW or a recurrence in the regional lymphatic sites (axilla, internal mammary nodes, infraclavicular, and supraclavicular). DFS was defined as the time duration from surgery to the first recurrence. OS was defined as the time duration from pathologic diagnosis to death or last follow‑up with any death defined as an event. DFS and OS were estimated using Kaplan–Meier method and compared between patients receiving and not receiving adjuvant treatment using the log‑rank test. All statistical tests were two-tailed, and the differences were considered statistically significant if p < 0.05. Statistical analysis was performed using Statistical Package for Social Sciences software’s recent version.
Results
Table 1 shows the patients characteristics. The median age was 57 years. Most (67%) of the patients belonged to a rural region. In all patients, the lump was the presenting complaint and the other complaints were pain and discharge. More than half of the patients presented with right side MBC. The mean duration of symptoms was 26.2 months. Comorbidities and family history were present in 42.5% and 4.7% patients, respectively. Out of 106 patients, 67.9% were chronic smokers and 40.6% were alcoholics. Table 2 depicts the tumour characteristics of the patients. The mean tumour size was 5.8 cm. The nipple was involved in 57.5% patients. The majority (90.5%) patients had invasive ductal carcinoma histology, others were undifferentiated and papillary. Out of 106 patients, the most common stage was T3 (51.9%), and the least common was T4A (7.5%). A total of 63.2% of patients were node positive. Out of 67 node-positive patients, 62.7% were N2A, 25.3% were N3B and 12% were N3C. 21 patients (19.8%) presented with metastasis. Out of 106 patients, 30.2% presented in stage IIIA, 24.5% in stage IIA, 19.8% in stage IV, 12.3% in stage IIB, 11.3% in stage IIIC, and 1.9% in stage IIIB. Early, locally advanced, and metastatic diseases were seen in 36.8%, 43.6%, and 19.8% patients, respectively. ER, PR, and HER-2neu positive patients were seen in 41.5%, 25.5%, and 8.5% patients, respectively. ER, PR, and HER-2neu cannot be performed for all patients due to logistic issues.
Adjuvant radiotherapy, chemotherapy, and tamoxifen were received by 85.8%, 72.6%, and 47.2% men, respectively. Chemotherapy regimens used were CMF (Cyclophosphamide 600 mg/m2, Methotrexate 40 mg/m2, and 5‑FU 600 mg/m2) in 24.7% patients in the early part of the study period till 1996, FAC (5‑FU 600 mg/m2, Adriamycin 50 mg/m2, and Cyclophosphamide 600 mg/m2) in 49.4% patients till 2008, and anthracyclines and taxanes in 25.9% patients from 2008 afterwards (Figure 1). The median number of courses of chemotherapy was 4.5. Among 21 patients with metastatic disease, 16 men received chemotherapy, 4 became operable, 8 also received radiotherapy and tamoxifen and 5 patients with poor general condition were given only supportive care. In radically treated 85 men, neoadjuvant chemotherapy with FAC and FAC + taxanes regimen was given to 49.4% and 25.9% of patients, respectively. Complete response and PR were seen in 65.9% and 34.1% of patients, respectively. Mastectomy was done in 85.9% and wide local excision in 14.1% of patients.
The median follow‑up was 60 months (range of 4–278 months). For the entire cohort, LRR occurred in 9.4% and distant metastasis in 19.8% of patients. PMRT reduced LRR, and improved OS and DFS in low‑ as well as high‑risk patients. For the entire cohort, the median DFS was 43 months, and the 5 and 10‑year survival rates were 78% and 58%, respectively. The median OS was 78 months. DFS at 10 years was 54% and 24% with and without PMRT (p = 0.007), respectively (Figure 2). OS at 10 years was significantly better in men with PMRT (64%) as compared to 42% without PMRT (p = 0.022) (Figure 2). The OS was better in the early stages and decreased in the advanced stages (Figure 3). Statistically significant improvement in OS with PMRT was seen across all stages and 10-year OS for stage II was 91% versus 58%, for stage III – 78% versus 41% and for stage IV – 32% versus 19%, with and without PMRT, respectively, p < 0.05 (significant). 10-year DFS for stage II was 72% versus 41%, for stage III – 55% versus 26% and for stage IV – 19% versus 11%, with and without PMRT, respectively, p < 0.05 (significant). Hormonal therapy also significantly improved DFS and OS at 10 years (57% versus 14.5%, p = 0.004 and 62% versus 39%, p = 0.045, respectively) (Figure 4). Only 1 (1%) patient developed a second malignancy in the contralateral breast after 8 years of follow‑up.
Table 1. Patient characteristics of the MBC patients.
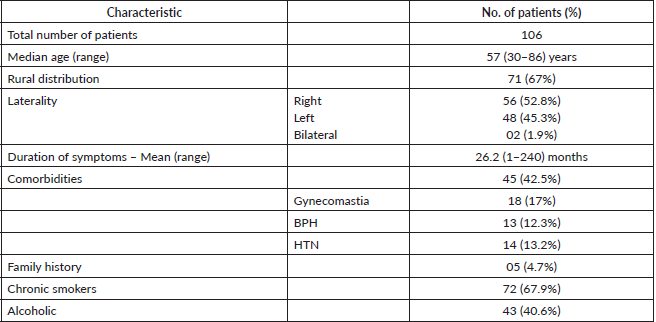
Table 2. Tumour characteristics of the MBC patients.
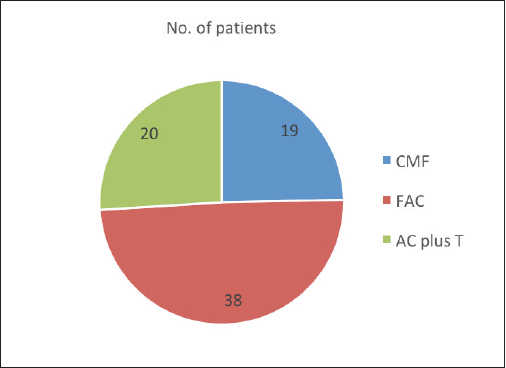
Figure 1. Chemotherapy regimens administered to the MBC patients.
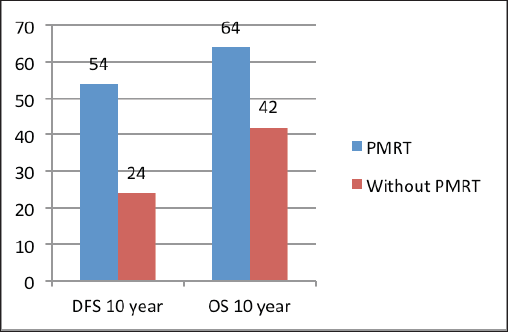
Figure 2. DFS and OS at 10 years with and without PMRT.
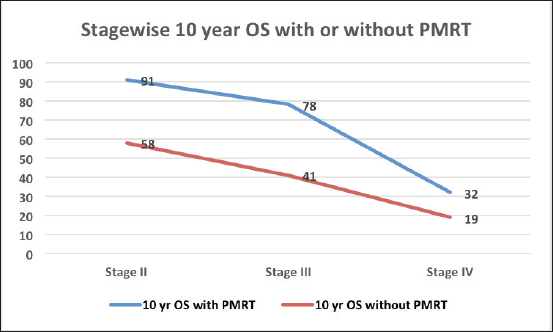
Figure 3. OS at 10 years with and without PMRT in various stages of MBC.
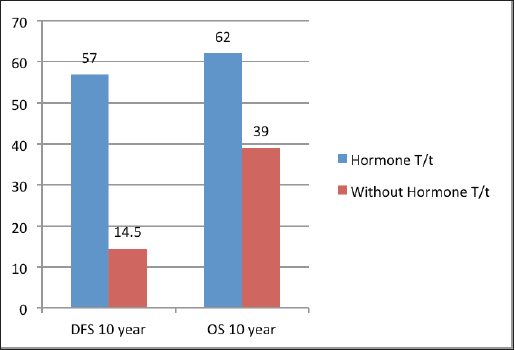
Figure 4. DFS and OS at 10 years with and without hormonal treatment.
Discussion
The incidence of MBC patients in the present study was 1.3%, which was slightly higher than the worldwide average of 1%, but it is in close relation to the Indian study from Patna, Bihar by Pawar et al [24]. However, higher incidence is reported by Shah et al [25] (4.1% from Kashmir) and Khandelwal et al [26] (1.9% from Punjab). This might be due to geographical variations. In the present study, the patients were 10 years younger than those demonstrated in the Western studies. The median age in this study was 57 years [7] versus 67 years from West literature [25]. In the present study, patients presented late with mean duration of symptoms 26.2 months and in the advanced stage with mean tumour size of 5.8 cm contrasted with Western patients [25]. The patients in this study were also included from the 1990s and at that time, the Indian male patients were too shy to report to the physician regarding any changes in the breast; and so, the mean duration of symptoms was so high. Most patients presented at a locally advanced stage because of ignorance, obliviousness, elective medicines use (desi and ayurvedic treatment), and distant cancer centres. A family history was seen in 4.7% of patients in this study. Nigerian study by Ahmed et al [28] reported 2.5% familial risk. The familial risk from Indian studies was 15.4% from Bengaluru [28], 10% from Delhi by Gogia et al [30], and no familial risk was reported by Sundriyal et al [34] from Delhi. This might be because of topographical contrasts. There is not a lot of information from India particularly on familial cancer risk in MBC as Indian patients might have less familial risk. Another explanation might be underreporting, yet its possibilities are less because there is a standard format for recording the patient history and it incorporates family history as one of its parts. Although recommended for such cases, still genetic testing for BRCA mutation was not possible in the present study due to logistic issues.
In this study, approximately three fourth of the total MBC patients had central quadrant disease, and 57.5% had nipple involvement. These findings are similar to the available literature [31]. More than 90% of patients were having infiltrative ductal carcinoma (IDC) histology, which is consistent with female breast cancer and study by Khandelwal et al [26] at Tata Medical Centre. The literature reports that 90% of MBCs express ER and 81% express PR, contrasted with 75% and 66% in females with BC [23, 25]. Some Indian studies also reported ER/PR positivity in the range of 77%–89% [28–30, 32, 33]. However, the present study reported a relatively less rate of ER/PR expression. The reason behind this was no hormone receptor testing facilities in our institution in the early 1990s. Incidence of triple-negative MBC patients was 20.8% in the present study which was consistent with 22% incidence in female breast cancer [29].
In our institution, PMRT is prescribed to patients when indicated like in T3/T4 tumour, close/positive margins, and positive nodes [32]. In this study, PMRT was administered to most MBC patients because of the locally advanced stage at initial presentation. PMRT was seen to improve DFS and OS. LRR rates without PMRT was 14%–25% in low-risk patients and 25%–50% in high-risk patients. The LRR rate with PMRT was 3.5%–13%. Yu et al. [13] from Canada reported improved LRR with PMRT in patients with high-risk MBC patients (positive node, high stage, ≤2 mm or positive margin). But it did not demonstrate OS benefit with PMRT. LRR rate without PMRT in the present study was higher (32.7% without PMRT and 9.4% with PMRT) in comparison to some other studies [9, 17, 34, 35]. This might be due to the bigger tumour size and advanced stage of presentation in our patients. The LRR reported to be 3.5% by Chakravarthy and Kim [9], 4.7% with PMRT and 11.5% without PMRT by Cutuli et al [17], 6.9%–10.3% in low-risk patients without PMRT & 17%–20% in high-risk patients by Yu et al [13], 11.3% with PMRT by Selcukbiricik et al [37] from Turkey, 18% with PMRT by Perkins et al [16]. Cutuli et al [36] reported LRR rates of 7.3% with PMRT and 13% without PMRT. Conversely, Hultborn et al [38] reported an LRR rate of 30% in patients with greater than equal to 5 cm tumour or metastases with perinodal development. Iyer et al [39] demonstrated that neoadjuvant CTRT in MBC in limited patients reduced LRR and improved OS and DFS. The 5-year local recurrence-free survival (LRFS) in this study was 63%, which is similar to those demonstrated in the literature (55%–69%) [10, 12–17, 21, 34, 39–43]. The LRFS rate was better in patients treated with PMRT in comparison to surgery alone (p = 0.038), but there was no survival difference (p = 0.72) [14]. Our outcomes are in line with different studies of MBC patients and in favour of PMRT versus surgery alone [13, 17, 34, 41, 42].
Neoadjuvant chemotherapy showed no advantage in DFS or OS. DFS at 10 years with and without neoadjuvant chemotherapy was 62% and 57%, respectively (p = 0.66). Additionally, OS at 10 years with and without neoadjuvant chemotherapy was 64% and 59%, respectively (p = 0.77). A total of 77 (72.6%) patients went through chemotherapy in this study. Recurrence rates were lower in patients who got both chemotherapy and hormonal treatment contrasted to chemotherapy alone 35% versus 78%. Giordano et al [23] reported advantage of chemotherapy with 10-year OS of 43% in patients with positive nodes.
Standard hormonal therapy in ER/PR-positive patients is tamoxifen. A few studies have shown a decreased risk of local recurrence and mortality in MBC patients who got tamoxifen [35, 44, 45]. Ribeiro and Swindell [45] reported that 5-year DFS and OS were 61% and 41% versus 56% and 28% with 1–2 years tamoxifen post mastectomy versus mastectomy alone, respectively. Similarly, Goss et al [46] reported advantage in both DFS and OS. In the present study, tamoxifen was prescribed for 5 years and hormonal therapy improved 10-year DFS (57% versus 14.5%) and 10-year OS (62% versus 39%); which is in similar lines to the literature [23, 25, 44, 45]. This might be due to the fact that hormone receptor-positive MBC patients have better prognosis as compared to other subtypes. There is no data on the extended utilisation of tamoxifen in MBC. If tamoxifen is contraindicated, aromatase inhibitors are not administered as sole agent, however, aromatase inhibitors may be co-administered with gonadotropin-releasing hormone analogues [5].
The limitation of the present study was retrospective nature with a small number of patients from a single institution. In one-third of our patients, ER/PR status was not known, yet the larger part (70%) were getting hormonal treatment. Of four different studies with a limited number of patients and follow-ups from India, three announced that ER/PR was communicated in 77%–89% of MBC patients [28–30, 46]. Subsequently, the administration of tamoxifen to 70% of patients in this study might be legitimate. In any case, three different studies did not report the result of PMRT or other adjuvant treatments [28, 30, 46]. Due to retrospective nature and small sample size, it is difficult to analyse the effects of different adjuvant treatment strategies on LRR and OS rates. Furthermore, the present study was conducted over three decades and thus, change in demography and culture affected patient’s characteristics. Also, the evolving diagnostic approaches with ER/PR/HER-2-neu status and imaging modalities, staging system, and treatment strategies affected results of the present study.
The strength of our study is that it is the largest data set from India on MBC with a good median follow‑up of 60 months in all patients treated on a cobalt machine with conventionally fractionated radiotherapy. The recurrence rate in MBC patients in this study is equivalent to female BC patients [47, 48]. The available literature also supports that patient gender does not seem to be a prognostic factor in local disease recurrence and PMRT further improved DFS and OS in female BC; therefore, it is reasonable to consider PMRT in MBC patients with high‑risk features [32, 33, 48–56].
Conclusion
MBC patients present at a locally advanced stage with a long median duration of symptoms despite the possibility of being diagnosed at an early stage. Radical surgery with chemotherapy and post-mastectomy radiation treatment remains the standard practice as in the females. Patients in this study were younger as compared to the Western series. PMRT reduced the LRR rate in MBC. PMRT and hormonal therapy significantly improved DFS and OS in MBC patients. However, ER/PR-positive patients might have been responded better to hormonal treatment. Chemotherapy had no impact on DFS and OS. However, future prospective trials with a large sample size may enlighten the path to successful treatment with minimal recurrence. This study necessitates health education and cancer awareness in the rural parts of India as 70% of the population of India belongs to a rural background. So, effective cancer education campaigns and/or programs must be run for early presentation and better cure of MBC.
Conflicts of interest
Nil.
Funding
Nil.
References
1. Ly D, Forman D, and Ferlay J, et al (2013) An international comparison of male and female breast cancer incidence rates Int J Cancer 132(8) 1918–1926 https://doi.org/10.1002/ijc.27841 PMCID: 3553266
2. Siegel RL, Miller KD, and Fuchs HE, et al (2022) Cancer statistics CA Cancer J Clin 72 7–33 https://doi.org/10.3322/caac.21708 PMID: 35020204
3. Giordano SH (2018) Breast cancer in men N Eng J Med 378 2311–2320 https://doi.org/10.1056/NEJMra1707939
4. Anderson WF, Jatoi I, and Tse J, et al (2010) Male breast cancer: a population-based comparison with female breast cancer J Clin Oncol 28(2) 232–239 https://doi.org/10.1200/JCO.2009.23.8162 PMCID: 2815713
5. Giordano SH, Cohen DS, and Buzdar AU, et al (2004) Breast carcinoma in men: a population-based study Cancer 101 51–57 https://doi.org/10.1002/cncr.20312 PMID: 15221988
6. Fentiman IS, Fourquet A, and Hortobagyi GN (2006) Male breast cancer Lancet 367(9510) 595–604 https://doi.org/10.1016/S0140-6736(06)68226-3 PMID: 16488803
7. Ying MW, Agrawal A, and Cheung KL (2005) The ‘‘other half’’ of breast cancer: a review of male breast cancer J Mens Health Gend 2 406–413 https://doi.org/10.1016/j.jmhg.2005.10.012
8. Crichlow RW, Kaplan EL, and Kearney WH (1972) Male mammary cancer: an analysis of 32 cases Ann Surg 175 489–494 https://doi.org/10.1097/00000658-197204000-00005 PMID: 4336485 PMCID: 1355204
9. Chakravarthy A and Kim CR (2002) Post‑mastectomy radiation in male breast cancer Radiother Oncol 65 99–103 https://doi.org/10.1016/S0167-8140(02)00210-4 PMID: 12443805
10. Schuchardt U, Seegenschmiedt MH, and Kirschner MJ, et al (1996) Adjuvant radiotherapy for breast carcinoma in men: a 20‑year clinical experience Am J Clin Oncol 19 330–336 https://doi.org/10.1097/00000421-199608000-00002 PMID: 8677899
11. Stranzl H, Mayer R, and Quehenberger F, et al (1999) Adjuvant radiotherapy in male breast cancer Radiother Oncol 53 29–35 https://doi.org/10.1016/S0167-8140(99)00122-X
12. Robison R and Montague ED (1982) Treatment results in males with breast cancer Cancer 49 403–406 https://doi.org/10.1002/1097-0142(19820115)49:2<403::AID-CNCR2820490232>3.0.CO;2-T PMID: 7053837
13. Yu E, Suzuki H, and Younus J, et al (2012) The impact of post‑mastectomy radiation therapy on male breast cancer patients – a case series Int J Radiat Oncol Biol Phys 82 696–700 https://doi.org/10.1016/j.ijrobp.2011.01.010
14. Erlichman C, Murphy KC, and Elhakim T (1984) Male breast cancer: a 13‑year review of 89 patients J Clin Oncol 2 903–909 https://doi.org/10.1200/JCO.1984.2.8.903 PMID: 6086848
15. Atahan L, Yildiz F, and Selek U, et al (2006) Postoperative radiotherapy in the treatment of male breast carcinoma: a single institute experience J Natl Med Assoc 98 559–563 PMID: 16623069 PMCID: 2569252
16. Perkins GH, Middleton LP, and Garcia SM, et al (2002) Male breast cancer, outcomes and predictors of local regional failure in patients treated without radiation therapy Breast Cancer Res Treat 76 S121
17. Cutuli B, Lacroze M, and Dilhuydy JM, et al (1995) Male breast cancer: results of the treatments and prognostic factors in 397 cases Eur J Cancer 31A 1960–1964 https://doi.org/10.1016/0959-8049(95)00366-5 PMID: 8562148
18. Joshi MG, Lee AK, and Loda M, et al (1996) Male breast carcinoma: an evaluation of prognostic factors contributing to a poorer outcome Cancer 77 490–498 https://doi.org/10.1002/(SICI)1097-0142(19960201)77:3<490::AID-CNCR10>3.0.CO;2-# PMID: 8630956
19. Borgen PI, Senie RT, and McKinnon WM, et al (1997) Carcinoma of the male breast: analysis of prognosis compared with matched female patients Ann Surg Oncol 4 385–388 https://doi.org/10.1007/BF02305550 PMID: 9259964
20. Donegan WL, Redlich PN, and Lang PJ, et al (1998) Carcinoma of the breast in males: a multi-institutional survey Cancer 83 498–509 https://doi.org/10.1002/(SICI)1097-0142(19980801)83:3<498::AID-CNCR19>3.0.CO;2-R PMID: 9690543
21. Izquierdo MA, Alonso C, and De Andres L, et al (1994) Male breast cancer. Report of a series of 50 cases Acta Oncol 33 767–771 https://doi.org/10.3109/02841869409083946 PMID: 7993644
22. Jaiyesimi IA, Buzdar AU, and Sahin AA, et al (1992) Carcinoma of the male breast Ann Int Med 117 771–777 https://doi.org/10.7326/0003-4819-117-9-771 PMID: 1416579
23. Giordano SH, Perkins GH, and Broglio K, et al (2005) Adjuvant systemic therapy for male breast carcinoma Cancer 104 2359–2364 https://doi.org/10.1002/cncr.21526 PMID: 16270318
24. Pawar SS, Kumar M, and Kishor K, et al (2021) Male breast cancer: a 5-year experience from a State Cancer Institute Int Surg J 8(9) 2595–2599 https://doi.org/10.18203/2349-2902.isj20213209
25. Shah P, Robbani I, and Shah O (2009) Clinicopathological study of male breast carcinoma: 24 years of experience Ann Saudi Med 29(4) 288–293 https://doi.org/10.4103/0256-4947.55314 PMID: 19584580 PMCID: 2841456
26. Khandelwal S, Goel P, and Sharma R, et al (2021) Presentation and spectrum of male breast cancer in a rural cancer center in a subunit of Tata Memorial Center, India Indian J Surg Oncol 12(2) 330–334 https://doi.org/10.1007/s13193-021-01306-8 PMID: 34295077 PMCID: 8272793
27. Giordano SH, Buzdar AU, and Hortobagyi GN (2002) Breast cancer in men Ann Intern Med 137 678–687 https://doi.org/10.7326/0003-4819-137-8-200210150-00013 PMID: 12379069
28. Ahmed A, Ukwenya Y, and Abdullahi A, et al (2012) Management and outcomes of male breast cancer in Zaria, Nigeria Int J Breast Cancer 2012 1–6 https://doi.org/10.1155/2012/845143
29. Chikaraddi SB, Krishnappa R, and Deshmane V (2012) Male breast cancer in Indian patients: is it the same? Indian J Cancer 49 272–276 https://doi.org/10.4103/0019-509X.104484 PMID: 23238143
30. Gogia A, Raina V, and Deo SV, et al (2015) Male breast cancer: a single institute experience Indian J Cancer 52 526–529 https://doi.org/10.4103/0019-509X.178399
31. Reis LO, Dias FG, and Castro MA, et al (2011) Male breast cancer Aging Male 14(2) 99–109 https://doi.org/10.3109/13685538.2010.535048 PMID: 21204612
32. Ram D, Rajappa SK, and Selvakumar VP, et al (2017) Male breast cancer: a retrospective review of clinical profile from a tertiary cancer care center of India South Asian J Cancer 6 141–143 https://doi.org/10.4103/sajc.sajc_2_17
33. Mitra D, Manna A, and Sikdar SK, et al (2007) Clinicopathological study and its prognostic implication in male breast carcinoma J Indian Med Assoc 105(12) 681–686
34. Sundriyal D, Kotwal S, and Dawar R, et al (2015) Male breast cancer in India: series from a cancer research center Indian J Surg Oncol 6 384–386 https://doi.org/10.1007/s13193-015-0473-1
35. Vinod SK and Pendlebury SC (1999) Carcinoma of the male breast: a review of adjuvant therapy Australas Radiol 43 69–72 https://doi.org/10.1046/j.1440-1673.1999.00601.x
36. Cutuli B, Le‑Nir CC, and Serin D, et al (2010) Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases Crit Rev Oncol Hematol 73 246–254 https://doi.org/10.1016/j.critrevonc.2009.04.002
37. Selcukbiricik F, Tural D, and Aydoğan F, et al (2013) Male breast cancer: 37‑year data study at a single experience center in Turkey J Breast Cancer 16 60–65 https://doi.org/10.4048/jbc.2013.16.1.60 PMID: 23593083 PMCID: 3625771
38. Hultborn R, Friberg S, and Hultborn KA, et al (1987) Male breast carcinoma. II. A study of the total material reported to the Swedish cancer registry 1958‑1967 with respect to treatment, prognostic factors, and survival Acta Oncol 26 327–341 https://doi.org/10.3109/02841868709104357
39. Iyer P, Balasubramanian A, and Selvaluxmy G, et al (2019) Neoadjuvant concurrent chemoradiation in male breast cancer: experience from a tertiary cancer center Indian J Cancer 56 37–40 https://doi.org/10.4103/ijc.IJC_44_18 PMID: 30950442
40. Stierer M, Rosen H, and Weitensfelder W, et al (1995) Male breast cancer: Austrian experience World J Surg 19 687–692 https://doi.org/10.1007/BF00295904 PMID: 7571664
41. Zabel‑du Bois A, Milker‑Zabel S, and Wannenmacher M, et al (2007) Postoperative radiotherapy of the chest wall in patients with male breast cancer Zentralbl Chir 132 391–395
42. Chung HC, Koh EH, and Roh JK, et al (1990) Male breast cancer – a 20‑year review of 16 cases at Yonsei University Yonsei Med J 31 242–250 https://doi.org/10.3349/ymj.1990.31.3.242 PMID: 2177938
43. Willsher PC, Leach IH, and Ellis IO, et al (1997) A comparison outcome of male breast cancer with female breast cancer Am J Surg 173 185–188 https://doi.org/10.1016/S0002-9610(97)89592-X PMID: 9124623
44. Gennari R, Curigliano G, and Jereczek‑Fossa BA, et al (2004) Male breast cancer: a special therapeutic problem. Anything new? (Review) Int J Oncol 24 663–670 PMID: 14767551
45. Ribeiro G and Swindell R (1992) Adjuvant tamoxifen for male breast cancer (MBC) Br J Cancer 65 252–254 https://doi.org/10.1038/bjc.1992.50 PMID: 1739625 PMCID: 1977740
46. Goss PE, Reid C, and Pintilie M, et al (1999) Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret hospital during 40 years: 1955‑1996 Cancer 85 629–639 https://doi.org/10.1002/(SICI)1097-0142(19990201)85:3<629::AID-CNCR13>3.0.CO;2-V PMID: 10091736
47. Shukla NK, Seenu V, and Goel AK, et al (1996) Male breast cancer: a retrospective study from a regional cancer center in Northern India J Surg Oncol 61 143–148 https://doi.org/10.1002/(SICI)1096-9098(199602)61:2<143::AID-JSO10>3.0.CO;2-A PMID: 8606547
48. Yadav BS, Sharma SC, and Singh R, et al (2007) Postmastectomy radiation and survival in patients with breast cancer J Cancer Res Ther 3 218–224 https://doi.org/10.4103/0973-1482.38997
49. Yadav BS, Sharma SC, and Patel FD, et al (2010) Therapeutic benefit of radiotherapy after surgery in patients with T1‑T2 breast tumor JRP 9 33–40 https://doi.org/10.1017/S1460396909990124
50. Macdonald G, Paltiel C, and Olivotto IA, et al (2005) A comparative analysis of radiotherapy use and patient outcome in males and females with breast cancer Ann Oncol 16 1442–1448 https://doi.org/10.1093/annonc/mdi274 PMID: 15972730
51. Rai B, Ghoshal S, and Sharma SC (2005) Breast cancer in males: a PGIMER experience J Cancer Res Ther 1(1) 31–33 https://doi.org/10.4103/0973-1482.16087
52. Shah S, Bhattacharyya S, and Gupta A, et al (2012) <a href=https://pubmed.ncbi.nlm.nih.gov/23997516/>Male breast cancer: a clinicopathologic study of 42 patients in Eastern India</a> Indian J Surg Oncol 3(3) 245–249 https://doi.org/10.1007/s13193-012-0163-1 PMCID: 3444579
53. Shah T, Shah N, and Vijay DG, et al (2020) Male breast cancer: current trends – a tertiary care centre experience Ind J Surg Oncol 11(1) 7–11 https://doi.org/10.1007/s13193-019-01021-5
54. Kaur RP, Kumar V, and Shafi G, et al (2019) A study of mechanistic mapping of novel SNPs to male breast cancer Med Oncol 36(70) 1–7 https://doi.org/10.1007/s12032-019-1290-0
55. Chhabra MK, Chintamani, and Kadyaprath G, et al (2019) Male breast cancer—an Indian multicenter series of 106 cases Indian J Surg 83 333–340 https://doi.org/10.1007/s12262-019-01953-w
56. Panda N, Das PK, and Mahapatra SS, et al (2017) Clinico pathological profile of male breast cancer treated in a regional cancer centre of Eastern India J Med Sci Clin Res 5(5) 21264–21267 https://doi.org/10.18535/jmscr/v5i5.10






