Azacitidine prolongs overall survival and reduces infections and hospitalizations in patients with WHO-defined acute myeloid leukaemia compared with conventional care regimens: an update
P Fenaux1, GJ Mufti2, E Hellström-Lindberg3, V Santini4, N Gattermann5, G Sanz6, AF List7, SD Gore8, JF Seymour9, J Backstrom10, L Zimmerman10, D McKenzie10, CL Beach10 and LB Silverman11
1Hôpital Avicenne, Université Paris 13, Bobigny, France
2Department of Haematological Medicine, Kings College London,
London, UK
3Karolinska University Hospital, Stockholm, Sweden
4Hematology, Azienda Ospedaliera Careggi, Firenze, Italy
5Heinrich-Heine-University, Düsseldorf, Germany
6Department of Hematology, Hospital Universitario La Fe, Valencia,
Spain
7H Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
8The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins,
Baltimore, MD, USA
9Department of Haematology, Peter MacCallum Cancer Institute,
Victoria, Australia
10Celgene, Overland Park, KS, USA
11Pediatric Oncology, Dana-Farber Cancer Institute, Boston, MA, USA
Correspondence to P Fenaux. Email: pierre.fenaux@avc.aphp.fr
Abstract
Azacitidine (AZA), as demonstrated in the phase III trial (AZA-001), is the
first MDS treatment to significantly prolong overall survival (OS) in higher
risk MDS pts ((2007) Blood 110 817). Approximately, one-third of
the patients (pts) enrolled in AZA-001 were FAB RAEB-T (≥20–30% blasts)
and now meet the WHO criteria for acute myeloid leukaemia (AML) ((1999) Blood
17 3835). Considering the poor prognosis (median survival <1 year)
and the poor response to chemotherapy in these pts, this sub-group analysis
evaluated the effects of AZA versus conventional care regimens (CCR) on OS and
on response rates in pts with WHO AML.
Methods
The AZA-001 trial enrolled higher risk MDS pts (FAB: RAEB, RAEB-T, CMML and
IPSS: Int-2 or High). Prior to randomization, site investigators pre-selected
(based on age, performance status and co-morbidities) one of three CCR: best
supportive care only (BSC); low-dose ara-C (LDAC) or intensive chemotherapy
(IC). Pts were subsequently randomized 1:1 to AZA (75 mg/m2/d SC x 7d
q 28d) or CCR; pts randomized to CCR received their investigator pre-selected
treatment. Karyotypes were reclassified using AML standards: favourable (inv
16, t(8;21)), unfavourable (-7/7q- or complex) and intermediate (all others
including normal). OS was assessed by Kaplan-Meier (KM) methods and Cox
proportional hazards model, and IWG AML criteria (2003 J Clin Oncol
214642-9) were used to assess morphologic complete remissions (CR). Efficacy
analyses included all WHO AML pts randomized. All pts were followed until death
or study closure.
Results
Of 358 enrolled pts, 113 met the definition for WHO AML (median: 23% blasts) of whom 86% were considered unfit for IC and were pre-selected by investigators to receive a low-intensity regimen (BSC or LDAC). Fifty-five of the 113 pts were randomized to AZA and 58 pts to CCR. AZA and CCR groups had comparable baseline demographic and clinical characteristics. Of the 58 pts randomized to CCR, five withdrew without receiving treatment, and 53 were treated with their investigator pre-selected treatment as follows: IC (19%; 10/53), LDAC (34%; 18/53) and BSC (47%; 25/53). Of the 55 pts randomized to AZA, two withdrew without receiving treatment. Median age was 70 years; 24% had an unfavourable karyotype, 72% had an intermediate karyotype (including 46% normal); no pts had a favourable karyotype. Median follow-up for OS was 20.1 months. Median (min–max) number of treatment cycles was eight (1–39) for AZA, 2.5 (1–3) for IC; 5.5 (1–14) for LDAC; and six months (2–19) for BSC. KM median OS was 24.5 versus 16.0 months, respectively, in the AZA and CCR groups, hazard ratio (HR)=0.47, 95% CI, 0.28 to 0.79, p=0.004.
Figure: The overall survival rates in the azacitidine and conventional care regimen groups over a 40 month period.
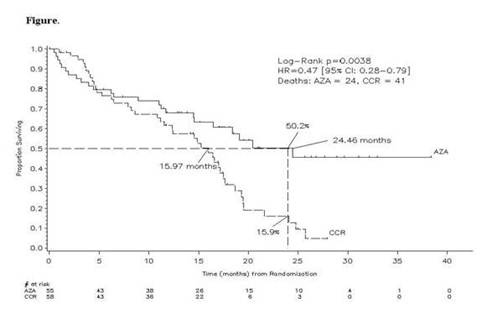
The OS rates at two years were 50% and 16% (Figure),
respectively, in the AZA and CCR groups, p=0.0007. There was no statistical
difference in the morphologic CR rate between the AZA (18%, 10/55) and CCR
groups (16%, 9/58; p=0.80). OS results in cytogenetic intermediate pts showed a
significant HR, favouring the AZA group (N=38) over CCR (N=43,
HR= 0.47 [95% CI: 0.24, 0.91], p=0.024) but not in pts with unfavourable
cytogenetics: AZA (N=14) versus CCR (N=13, HR=0.66 (95% CI: 0.26,
1.68), p=0.381); however, pt numbers were low. WHO AML pt outcome measures
showed significant benefits with AZA: fewer infections requiring IV antibiotics
per pt-year in the AZA group (0.58) versus CCR (1.14, HR=0.51 [95% CI 0.29,
0.78], p=0.003) and reduced rates of hospitalization in the AZA group (3.4 per
pt-year) versus CCR (4.3 per pt-year, HR=0.79 [95% CI 0.62, 1.00], p=0.028).
AZA was generally well tolerated.
Conclusion
Azacitidine significantly prolongs OS with significant improvements in important pt outcomes in elderly WHO AML pts with low-marrow blast counts, who currently have limited therapeutic options. Trials are ongoing to confirm the effect of AZA in elderly AML pts with more proliferative disease.
Conflicting interests
Fenaux: Celgene: Consultancy, Honoraria, Research Funding; Ortho Biotech: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Cephalon: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria, Research Funding. Mufti: Celgene: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Hellström-Lindberg: Celgene: Consultancy, Research Funding. Santini: Celgene: Honoraria; Novartis: Honoraria; J&J: Honoraria. Gattermann: Celgene: Research Funding, Speakers Bureau. Sanz: Celgene: Membership on an entity's Board of Directors or advisory committees. List: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Gore: Celgene: Consultancy, Equity Ownership, Research Funding. Seymour: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Backstrom: Celgene: Employment. Zimmerman: Celgene: Employment. McKenzie: Celgene: Employment. Beach: Celgene: Employment. Silverman: Celgene: Speakers Bureau.






