Cancer of the anal canal, a reality in the Colombian coffee region. Clinical-epidemiological review 2000–2019
Carlos Raúl Villegas Mejía1a, Manuel Villegas Jaramillo2b and Pedro Villegas Jaramillo2c
1Clinical Oncology and Radiotherapy, Oncology Service, Oncologists of West SAS, Caldas 170004641, Colombia
2University of Manizales, Health Faculty, School of Medicine, Manizales, Caldas 170004641, Colombia
ahttps://orcid.org/0000-0002-0103-6844
bhttps://orcid.org/0000-0001-8672-3370
chttps://orcid.org/0000-0001-5445-4989
Abstract
Introduction: Anal cancer is a rare pathology which has increased over the last few decades, and, therefore, gained importance for the quality of life of affected individuals. Thus, a review has been conducted in the Colombian coffee region (Departments of Caldas, Quindío y Risaralda) describing its behaviour and clinical-epidemiological profile.
Materials and methods: Descriptive review of 437 patients of Western SAS Oncologists between January 2000 and December 2019 with a diagnosis of anal cancer.
Results: 62% of cases presented in women with a median age of 62 years, 30% in the sixth decade; centred at 65% in three main cities designated as capitals (Manizales, Pereira and Armenia); 62% as localised disease, with 40% stage II-A and 6% as initial metastasis; 29% presented positive ganglia, particularly N1a; squamous cell or epidermoid histology in 90%; 16% poorly differentiated; 5% related to Human Immunodeficiency Virus infection; localisation in the medial area of the anal canal in 63% of cases; 83% completed treatment, and 92% of them received chemotherapy/radiation therapy with 87% based on the Nigro protocol; finally, 11% presented with relapse in the liver in 10% of cases and 55% local.
Conclusion: Four hundred and thirty-seven patients evaluated over 20 years with follow up at median 34.13 months (standard deviation 41.75) with median survival at later ages decreasing to 62% in patients older than 80 years, and differences in survival in localised disease at 78% in comparison to 46% in advanced metastasis. Finally, the overall 5-year survival rate is 69% with a median survival of 191 months in the study.
Keywords: anal cancer, anal canal, profile, epidemiology, review
Correspondence to: Carlos Raúl Villegas Mejía
Email: caravim@hotmail.com
Published: 09/02/2021
Received: 23/08/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Anal cancer is a rare pathology and is considered infrequent, accounting for 1%–2.6% of digestive cancers and 2%–4% of anal-colorectal tumours [1], with increased incidence worldwide [2] and an increasing rate of 2.9%/year since 1992, according to The Surveillance, Epidemiology, and End Results Program of National Cancer Institute (SEER)-USA [3]. In Europe, nearly 2,000 men and 2,300 women are diagnosed each year [4].
According to the Global Cancer Observatory (GLOBOCAN), an agency belonging to the International Agency for Research on Cancer of the World Health Organisation, the global incidence rate is >1.2 per 100,000 women and 0.6–0.89 per 100,000 men. In the USA, the rate is 1 per 100,000 men and 1.8 women, with an overall rate of 1.4 per 100,000 inhabitants and mortality of 0.2 per 100,000 inhabitants. In comparison, in South America, the incidence is 0.57 per 100,000 for men and 1.20 per 100,000 in women with an overall rate of 0.92 per 100,000 inhabitants and a mortality rate of 0.19 per 100,000 inhabitants [5].
In reviewing South American cases, references are found from Brazil—a retrospective of 25 years with 49 cases [6]; from Venezuela—a report of results from 96 cases from 2000 to 2013 [7], and a previous report of 56 patients, and finally, in Chile, the mortality rate was reported at a minimum of 0.11 in 2003, increasing to 0.21 per 100,000 in 2010 [8].
In Colombia, according to GLOBOCAN, an incidence of 557 new cases was reported (0.55%), occupying 26th place, and a mortality rate of 84 cases (0.18%) with a prevalence of 1,395 cases (prop.2.82) in 2018: for Colombian statistics, a 15-year review effected at the Bogota National Cancer Institute shows 18% of cases [10], and a report of the results of the analysis of 82 patients of Oliveros R et al [11] published in 1990, treated by the gastroenterology service at the Bogota National Cancer Institute from 1935 to 1981.
With respect to local statistics, according to the Manizales Quinquennial Population Cancer Registry 2003–2007, the incidence is 0.8% (12 cases) in men and 0.6% (11 cases) in women, with 0.7% overall. During the 5-year period from 2008 to 2012, it was 0.5%, with an incidence rate of 0.9 per 100,000 inhabitants [12] and more specifically, in the area of the Caldas Department, a frequency of 0.7% is found (26th place) [13].
Anal cancer is an uncommon neoplasm; however, its incidence has recently increased due to Human Immunodeficiency Virus (HIV), making it the fourth most common cancer in this population with a general annual rate of 1 per 100,000 inhabitants to 35 per 100,000 inhabitants in HIV( ) men. Other factors include anal sex, promiscuity, immunosuppression, effects of Human Papillomavirus (HPV) attributed to HPV-16 serotype, the cause in both genders of 73% of cases, with the HPV-18 serotype attributed to 3.4%–7% [14]. According to the Centers for Disease Control and Prevention, these main serotypes [15] are present in 79% of anal cancers, attributed to the use and abuse of cigarettes, which causes an increase in cases in both sexes with worldwide predominance in males in this population [16]. The Colombian Coffee Sector (Caldas, Quindío and Risaralda Departments) does not escape this reality due to the scarcity of epidemiological data. In view of this, an analysis of the effects of anal cancer in the Western SAS Oncologists’ area of influence was performed to provide evidence of the magnitude of the problem, its clinical profile, epidemiology, treatment and finally, the results attained for this pathology.
Materials and methods
A retrospective analysis was performed from the database of the Western SAS Oncologists, a private organisation dedicated to cancer awareness, treatment and monitoring in this geographical area, from 01/01/2000 to 31/12/2019, using International Disease Classification codes 10th edition (CIE-10) with C21.0, C21.1, C21.2 and C21.8 related to anal cancer [17]. The CIE-10 was employed as a base classification as the institutional standard for the classification of cancer patients who were examined, and with compromised histology as an exclusion criterion, thus raising doubts about anatomical location in the final selection of a population base for the analysis of 437 cases.
To begin, general clinical and demographic aspects were analysed (age, occupation, procedure, department, municipality, clinical stage, oncological classification by tumour, nodules and metastasis (approved by the 2017 investigatory team at tumour-node-metastasis (TNM) to avoid confusion and time variation), HIV, Karnofsky and symptom duration); anatomical variables (histology, histological grade, prognostic factors, anatomical location within the canal); therapeutic variables (surgery, type of non-surgical treatment, gap in radiation therapy, initial and rescue chemotherapy plans, radiation therapy dose and sessions, site of recurrence, time at recurrence, number of rescue chemotherapy treatments and a description of the therapy as completed or not) and finally, based on the results of intervention and survival (overall monitoring and survival according to different variables analysed). These factors may be analysed and defined as risk factors according to the results obtained. The date of diagnosis was analysed in 5-year increments to evaluate the patterns, age by 10- and 20-year period and the clinical stage as defined by the most recent classification of the American Joint Committee on Cancer (AJCC) from 2017 [18].
The histological grade was classified as well differentiated, moderately differentiated and poorly differentiated, defined by an institutional or external pathology team, and was based on the pathological findings following established guidelines for the speciality, which is outside the scope of this analysis. The treatment was based on surgery, radiation treatment, chemotherapy, radiation and chemotherapy together or none. Recurrence, or clinical evidence of tumour activity, was evaluated by the oncologist or, when in doubt clinically, based on a positive biopsy report confirming tumour activity following treatment, or without the necessity of a pathological study if a lesion, defined by paraclinical methods such as endoscopy or radiology, was present, with all of the foregoing occurring following a disease-free period of 12 months or more. The persistence of the tumour was defined as aforementioned, without a disease-free period, after completing the principle treatment. The gap variable was described as the time, in days, of radiation therapy interruption, with a goal of permitting functional recovery of the patient from generally morbidity from chemotherapy/radiation therapy [6]. Symptom duration is given in months.
Monitoring was defined as the period from the date of diagnosis to the final completed treatment, and survival was defined as the period from the end of treatment to remission or death. The final stage was determined using categories of alive or dead, sub-classified as active, lost and deceased, with or without tumour activity.
Qualitative variables were analysed as proportions, and quantitative variables are calculated using standard deviations. Survival calculations were performed using the Kaplan–Meier estimator and the log-Rank test. Statistical analysis was based on Epi Info 6.04™, Version 3.5.4, and IBM SPSS, Version 22®.
The project was approved by the Medical Director and the Research Department of Western SAS Oncologists, with the approval and supervision of the appropriate ethics committee, who permitted the inclusion of their business name in the publication. Given that there is no alternative therapeutic intervention or external information particular to the accepted management of these patients, it is considered a study without risk following Article 11 of the 1993 Resolution 8430 of the Colombian Ministry of Health, and current regulations pertaining to health research adhering to the Guide of the International Conference of Tripartite Harmonisation, Harmonisation of Good Clinical Practices and the Helsinki Declaration (Version 64a of the General Assembly, Fortaleza, Brazil, October 2013).
Results
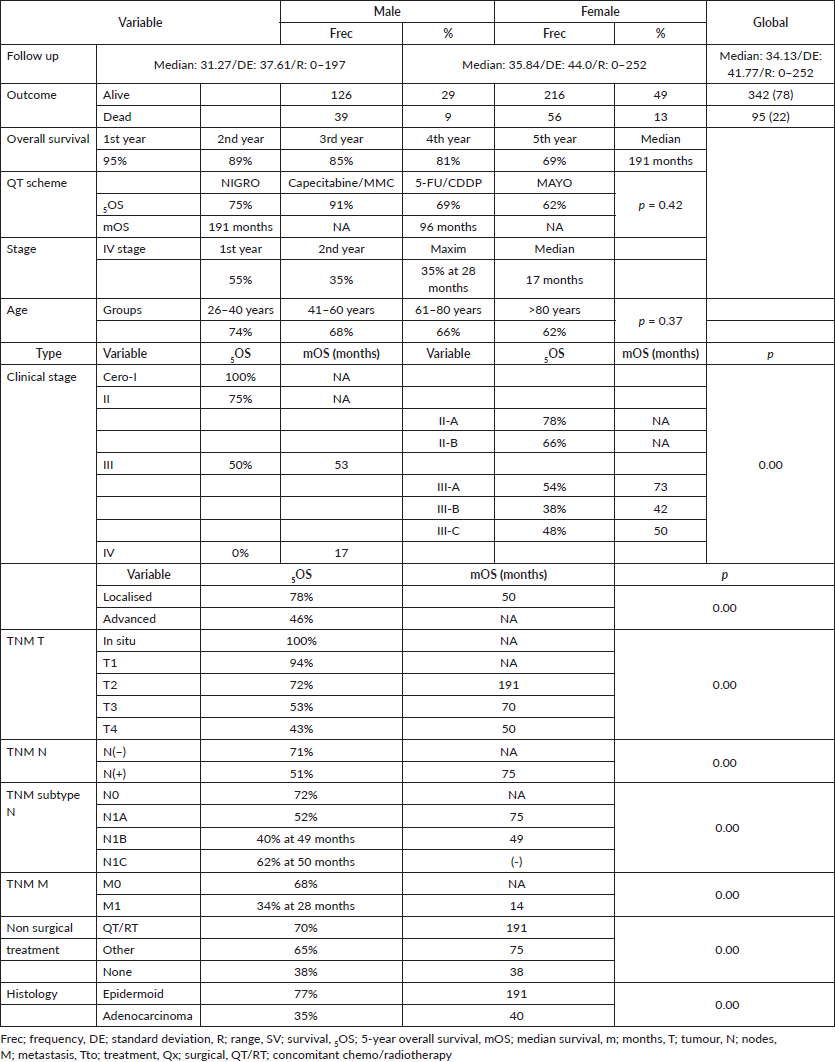
Anal cancer constitutes 2% of colorectal anal tumours, presenting twice as often in females (62%) and tripling in frequency beginning in the fifth decade (Table 1), with survival diminishing as age increases, from 75% in the 26–40-year-old age group to 62% for ≥80 years, displaying an inverse relationship between age and survival (p = 0.37) (Figure 1).
Regarding clinical staging, localised stages (E.C. II and TNM:T2) constituted 54%, with the sub-type II-A in the majority (40%); 26% with TNM N1a; and only 6% as initial metastasis, with 50% at the locoregional level and 13% of these at the bone level. Epidermoid histology was 90%. Differences in 5-year overall survival (5OS) between well- and poorly-differentiated were 10% (68% versus 58%, p = 0.05). 28% of the population presented with an adverse prognostic factor, i.e. the presence of ganglia demonstrated by our results and favouring negative ganglia versus positive (5OS: 72% versus 51%, p = 0.00), similar to comparisons of TNM N0 versus N1a, at 20% (p = 0.00), with differences of 12% between sub-types II-A and II-B (5OS: 78% versus 66%, p = 0.00), and defining sub-type II-B and TNM T4 (Figures 2 and 3) as the same as ulceration as the principle adverse prognostic factors with differences in comparison to those not presenting factors (p = 0.03). Medial location in the canal was 2/3 higher than lateral (5OS: 70% versus 55%, p = 0.56). Karnofsky ≥ 80% doubled in the 50%–70% survival group (70% versus 35%, p = 0.00) (Table 2).
87% of the group who completed treatment received the Nigro protocol, in comparison to 6% receiving the Mayo protocol, 4% receiving Capecitabine/Mitomicina C (MMC) as a variant of the Nigro protocol and 3% receiving 5-Fluoropirimidina (5-FU)/Cisplatin (CDDP), with no statistically significant differences between Nigro, 5-FU/CDDP and Mayo (5OS: 75% versus 68% versus 62%, p = 0.34), though favouring the alternate protocol of Capecitabine/MMC in comparison to Nigro (91% versus 75%, p = 0.58). Comparing the protocols designated as second line, with overall rates of response nearing 70% and mOS of 12 months for 5-FU/Cisplatin, slightly less than Nigro (Figure 4). As with other adverse factors, status as described by the Karnofsky scale becomes, in the majority of neoplasms, a further prognostic factor.
The total dose for radiation therapy in the group who completed treatment was 49.08 Gys, with differences within the group of greater or less than 45.00 Gys, favouring the larger applied dose (5OS: 77% versus 68%, p = 0.01) (Table 3).
Table 1. Clinical and demographic characteristics.
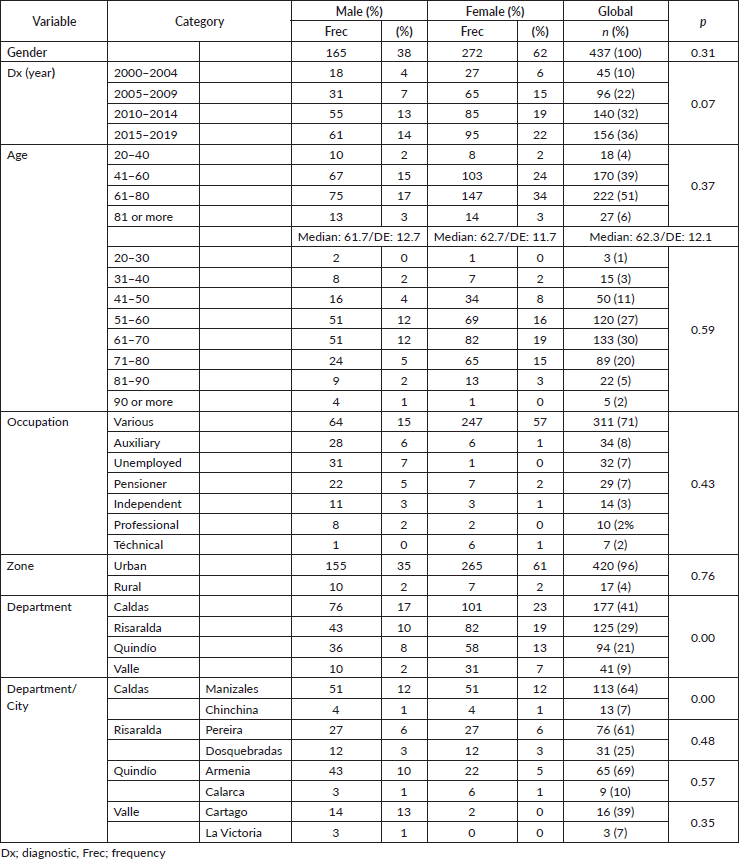
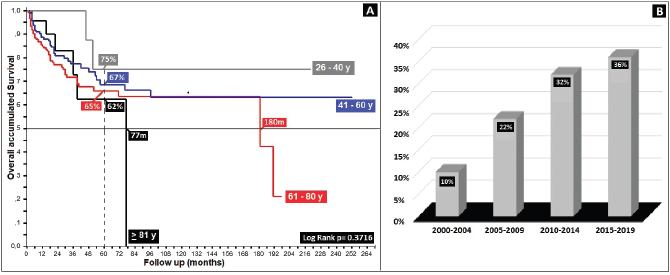
Figure 1. Age distribution in patients with anal cancer by age. (a): Distribution of survival by age groups in twenties. (b): Behaviour of anal cancer according to five-yearly.
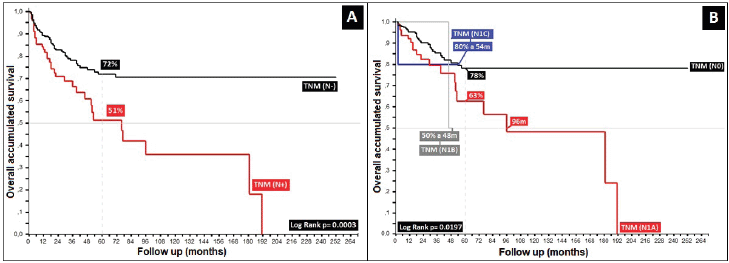
Figure 2. Distribution according to TNM (N) survival in anal cancer patients. (a): Distribution according to the presence of positive or negative nodes. (b): Anal cancer behaviour according to TNM (N).
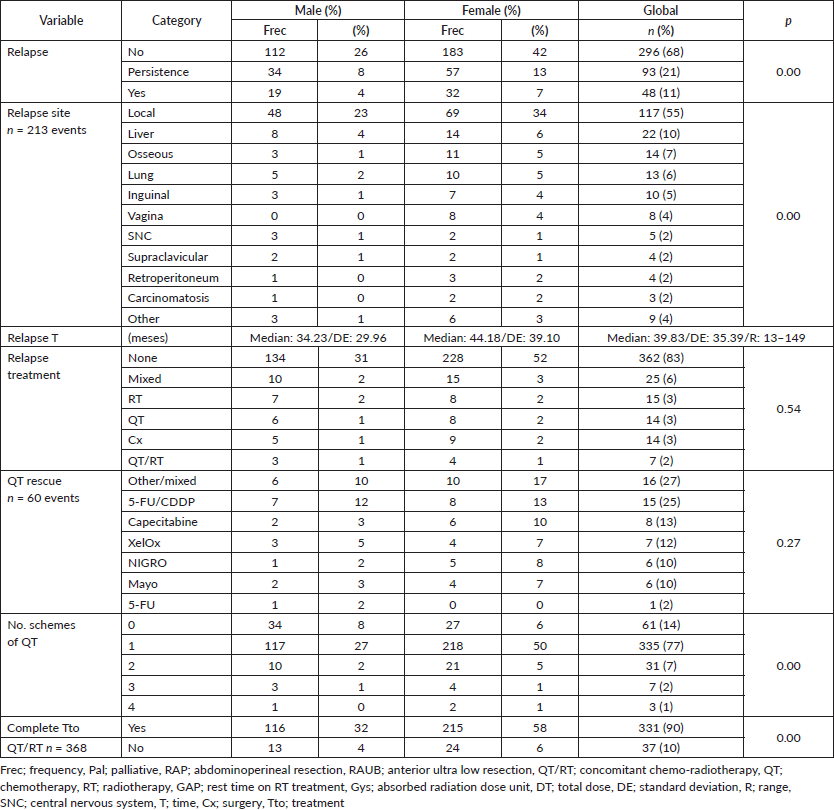
32% presented with recurrence, with a statistically significant difference in survival between persistence (17%) and recurrence (47%) (p = 0.00), with this difference being larger upon comparison between patients who did or did not present with recurrence (36% versus 90%, p = 0.00), along with differences between patients who completed treatment in comparison to those who did not: 52% in 5OS and a mean survival (mOS) of 177 months, favouring the group who completed treatment, to ultimately generate an overall survival of 95%, 89%, 85%, 81% and 69% from the first to the fifth year, and an mOS of 191 months (Table 4).
Figure 3. Distribution according to TNM (T) in patients with anal cancer. (a): Anal cancer behaviour according to TNM (T). (b): Distribution according to localised or advanced disease.
Table 2. Clinical and demographic characteristics.
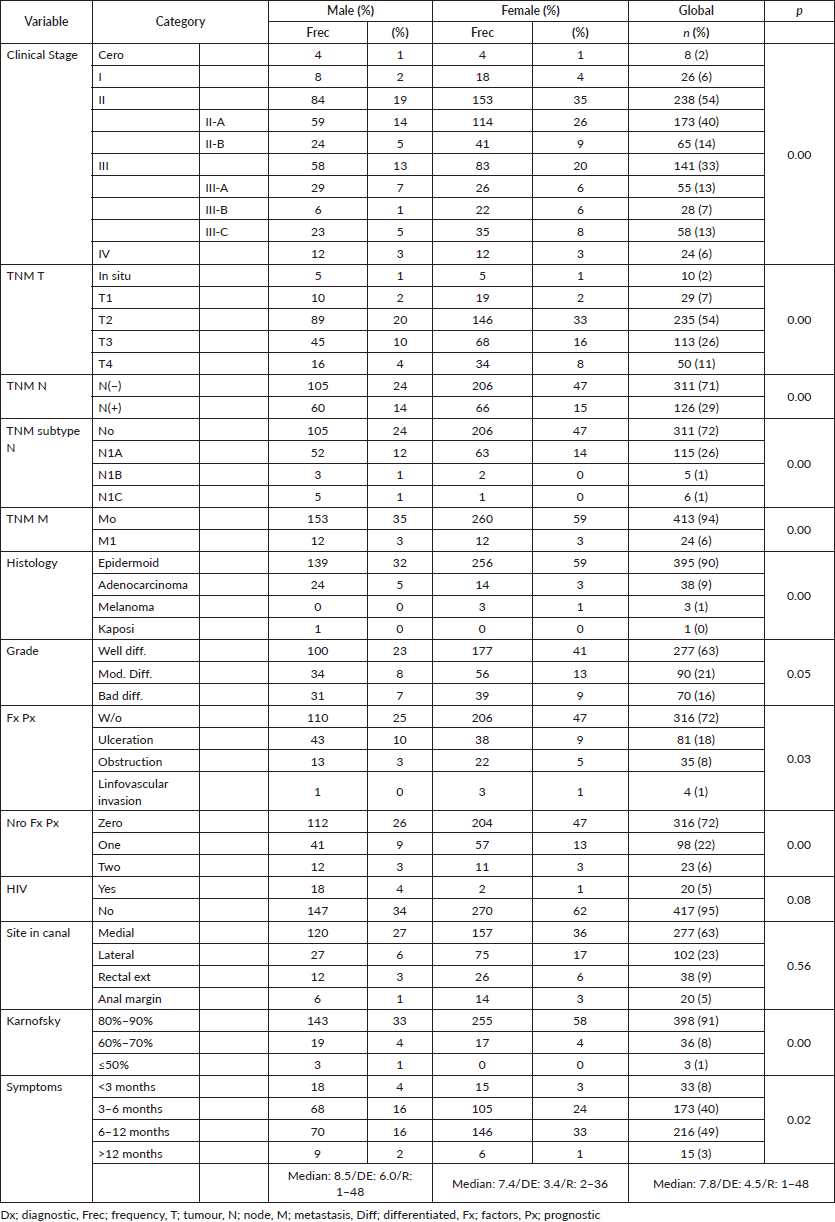
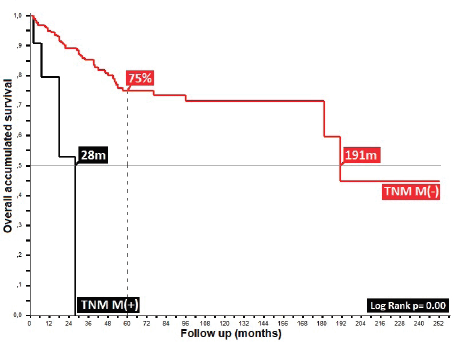
Figure 4. Distribution according to TNM (M) state in patients with anal cancer.
Table 3. Intervention characteristics.
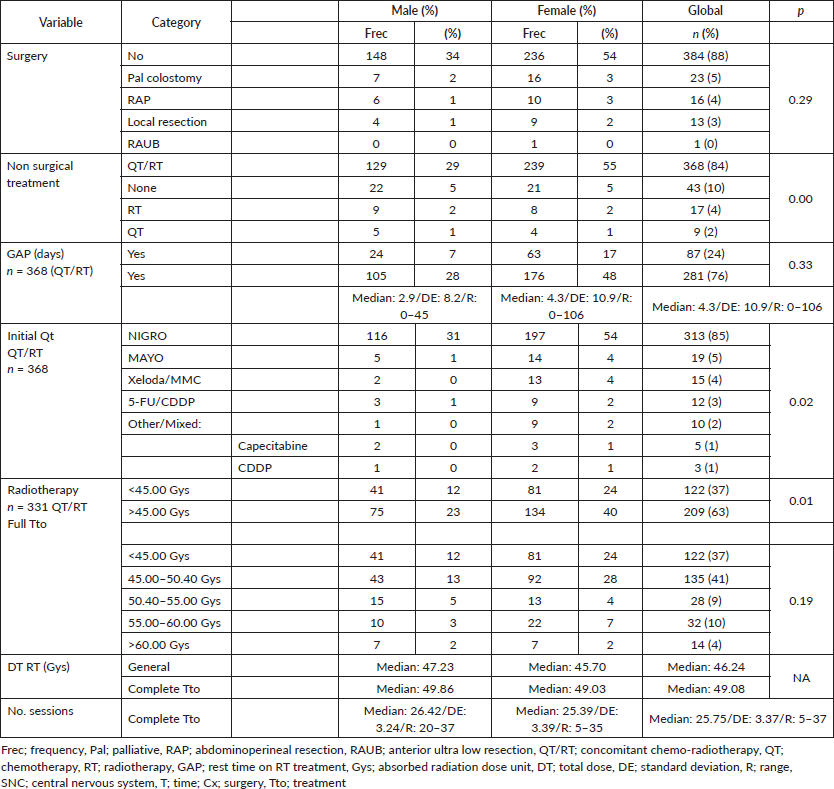
Table 4. Intervention characteristics.
Discussion
Anal cancer is a rare pathology that is on the rise, reflected by the trend found starting at 10% in the first 5-year period (2000–2004) and reaching 36% in the final 5-year period (2015–2019), similar to that found by other authors in Europe [5, 19], the United States [1, 2], as well as South America [6–11]. Survival and frequency increased over the analysis period, beginning at 54% survival in the 5-year period from 2010 to 2014 and reaching 74% for the 5-year period from 2015 to 2019 (p = 0.07), and maintaining a ratio of male:female favouring women during the different 5-year periods, the ratio being 1.65 generally with survival favouring same (5OS: 68% versus 63%, p = 0.31) and (mOS: 190 versus 182 months, p = 0.32), similar to others [6, 7, 19–22], and a greater incidence of presentation in the population in their sixth decade [2, 6, 19].
The localised stage, described as presenting with lesions less than 5 cm, was found in 6%, with sub-type II-A being the most frequent (40%), similar to others [2, 18, 20, 22–24]. Metastatic disease originally presented in 6%, which accords with other authors who report frequencies of less than 10% [1, 2, 18], with statistically significant results regarding the tumour (TNM T, p = 0.01), ganglia compromise (TMN N, p = 0.00), as well as metastasis (TNM M, p = 0.00), corroborated by other authors, wherein the clinical stage as defined by tumour size, extension, ganglion compromise, and particularly the ganglion level, are adverse prognostic factors [20, 21, 25] (Table 5).
Table 5. Intervention and survival results.
Similar to the literature world-wide, the epidermoid or squamous cell variety represented 90% [2, 15, 23], with 9% adenocarcinoma histology [7, 26–28], with the difference favouring the epidermoid type (5OS: 71% versus 24%, and mOS: 190 versus 28 months, p = 0.00), converting, as has been mentioned by others, to adenocarcinoma in an adverse prognostic histology [23, 26, 28, 29].
With respect to prognostic factors found in 28%, it can be concluded that positive ganglia, ulceration, adenocarcinoma-type histology, tumours ≥ 5 cm, TNM T4, obstruction, metastatic disease, and male gender, are considered, similarly to other authors, as factors that negatively affect survival [19–22, 30, 31], as was demonstrated by comparing negative or positive presence of ganglia (5OS: 72% versus 51%, p = 0.00) and the TNM N0 versus N1a sub-types in 20% (p = 0.00) [19–23, 25].
Initial presentation with TNM T as an adverse factor, particularly in tumours larger than 5 cm (T3), and T4 sub-classification (any size with vaginal, urethral or bladder compromise), corroborated by others, proved statistically significant (p = 0.00), generating results of 5OS of 95%, 71%, 53% and 43% for T1, T2, T3 and T4, respectively, with differences between stages with smaller than 5 cm (T1 and T2) and larger (T3 and T4) of 10% in 5OS of 100 months in mOS (p = 0.00), and regarding T3–T4, differences of 10% in 5OS and 29 months in mOS among them, comporting with adverse factors in a non-statistically significant manner (p = 0.8215), possibly because T4 also included therein tumours ≥ 5 cm, as defined in T3 [19, 20, 22].
Regarding the most common clinical stage, II, presenting in 54%, differences are found in 5OS of 12% between the II-A and II-B sub-types (p = 0.02), as also found by others [1–3], showing that the differences were essentially in tumour size (smaller or larger than 5 cm), defined in the 2017 AJCC and establishing the TNM II-B sub-type for future analysis as a risk factor for localised stages [20, 22, 24]. Data for 5OS for stage Zero-I was 100%, with 54%, 38% and 48% for stages III-A, III-B and III-C, with mOS of 78, 42, 50 and 18 months for stages III-A, III-B, III-C and IV, respectively, and, finally, not reaching mOS in localised stages (Zero, I, II-A and II-B) (p = 0.00), showing once again the TNM T4 sub-type to be an adverse prognostic factor in comparison to ganglion compromise in tumours of smaller size II-A (T1–T2 with positive ganglia) [20, 32].
Ulceration presented as an adverse factor in 5OS in 56%, similar to that reported by others, and constituting an adverse factor for consideration in future investigations, alongside node status (p = 0.00) and male gender (0.01) [33].
In the 6% of cases representing initial metastatic disease, the liver was the most affected site at 50%, similar to others, with data showing between 5% and 10% for metastatic disease [7, 19, 23, 29, 34] constituting an independent adverse prognostic factor with maximum survival of 28% in comparison to 75% for the group without metastasis, and mOS of 28 versus 191 months (p = 0.00), respectively, with differences resulting from the comparison of local disease with locally advanced/metastatic disease, such as the majority of the series wherein a regimen other than the Nigro protocol was proposed [35–38].
Upon histological analysis, differences are found both in frequency (90% versus 9%) and in results favouring the epidermoid (5OS 71% versus 24% and mOS: 162 months, p = 0.00) [15, 16, 23, 28, 29].
Regarding HIV and its role in the development of anal cancer, 1% of women and 28% of men with anal cancer have an underlying infection, with this neoplasm occurring more in this population in comparison with the general population. In 4.6%, HIV was found to be present; a low number, but maintaining the relationship to male gender, similar to others [28, 29, 39, 40], with marked numerical differences between HIV ( ) and HIV (−) populations, favouring the negative (5OS: 9% versus 66%, p = 0.08), without the ability to compare the larger series in which they were excluded [30, 32, 33, 41], and with contradictory results in different studies depending upon whether they were included in the analysed population [14, 42].
Similar to other authors, functional status is shown as a prognostic factor with differences in mOS between patients with Karnofsky ≤ 40%, 50%–70% and ≥80% of 8 and 180 months (10, 18 and 190 months, p = 0.00), respectively (Figure 5) [19, 41].
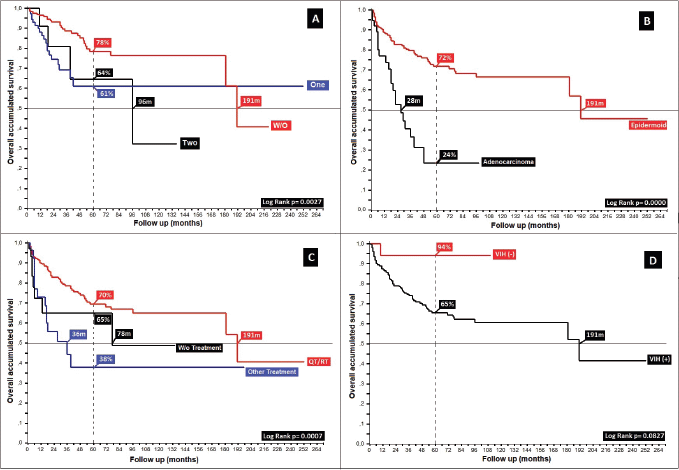
Figure 5. Distribution of patients with anal cancer according to different variables. (a): Distribution according to the presence of prognostic adverse factors. (b): Anal cancer behaviour according to histology. (c): Distribution according to the non-surgical treatment used. (d): Anal cancer behaviour based on HIV.
89% completed treatment based on concomitant chemotherapy/radiation therapy, of whom 87% received the Nigro protocol [35], generating differences upon grouping them as chemotherapy/radiation therapy, other treatments (surgery, radiation therapy, chemotherapy) and no treatment (p = 0.00). In the group who completed treatment, the differences between patients treated with Nigro (87%) compared to 5-FU/Cisplatin and others were 5OS: 72%, 62% and 56% (p = 0.10). This further confirms that expressed by multiple authors since the Nigro protocol was first presented at the Meeting of the American Proctologic
Society in Detroit in June of 1973, and published in 1974 [35]. The improvement with the Nigro protocol, which has become the standard treatment, shows minimal differences in comparison to 5FU/CDDP according to reports by others [29, 31, 37, 38], and corroborated by our results from the Nigro protocol versus 5-FU/CDDP (5OS 71% versus 48%, p = 0.04) and a difference of 151 months in mOS favouring the Nigro protocol (mOS 191 versus 40 months) [31, 32, 37, 38].
Analysing the other protocols employed, 5% received the Mayo protocol, with the difference (5OS: 55% versus 71%, p = 0.06) in favour of Nigro, and 4% received an alternative protocol, Capecitabine/MMC, with results showing slight benefit to the alternative protocol (5OS: 91% versus 75%, p = 0.58), with no statistically significant difference in concordance with different authors based on non-inferior results, such as alternative therapies in patients who were not candidates for Nigro [23, 29, 43, 44] for medical reasons, co-morbidities or lack of availability of infusion pumps to administer the Nigro protocol.
Radiation therapy, specifically for the group who completed treatment (76%), gave differences in favour of a higher dose, 45.00 Gys (5OS: 68% versus 77%, p = 0.01). Analysis of different applied doses shows, similar to others, that better survival is not reflective of increased dose [45], as is demonstrated by </> 60.00 Gys, with the difference favouring the group receiving the lower dose (5OS: 38% and mOS: 140 months, p = 0.57), defining the optimal dose for localised stages as between 45.00 and 50.40 Gys, and possibly for more advanced stages according to TNM T and N( ), a larger dose is required, up to 54.00–60.00 Gys [19]. With respect to a gap during radiation therapy, this presented in 24%, leading to differences of 2% in 5OS and 11 months in mOS, favouring the use of a gap in a non-statistically significant manner (p = 0.33), contrary to others who conclude that poorer outcomes are reflective of a gap [34, 46] (Figure 6).
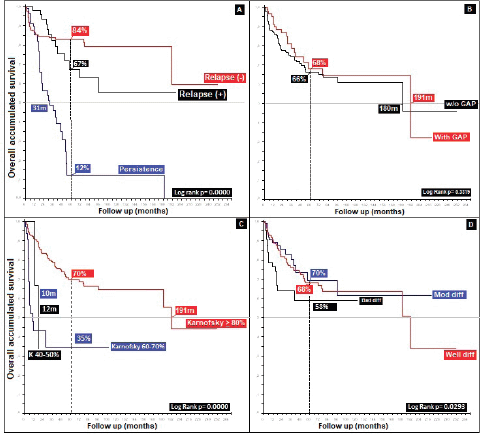
Figure 6. Distribution of patients with anal cancer according to different variables. (a): Distribution according to the presence of relapse. (b): Anal cancer behaviour according to the radiation GAP. (c): Distribution according to Karnofsky status. (d): Anal cancer behaviour according to histological grade.
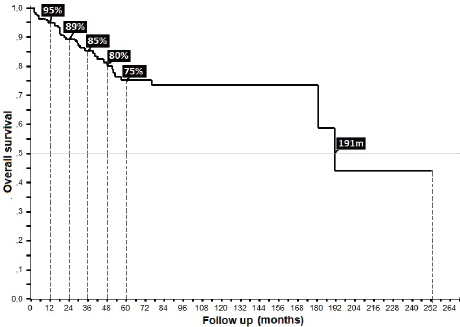
Figure 7. Overall survival of anal cancer.
Recurrence was noted in 11% at an average of 39.83 months (standard deviation 35, 39); 55% at a local level and 10% in the liver, similar to what others have noted [47, 48]. Persistence in 21% generated differences in 5OS between the presence of persistence, recurrence and no recurrence of 12%, 67% and 84%, respectively, (p = 0.00), and, upon grouping the data as recurrence or no recurrence, the differences remain (40% versus 84%, p = 0.00). Regarding rescue therapy, similar to others, the practice of only making a therapeutic attempt leads to differences of 30% in 5OS and 140 months in mOS (p = 0.13), in comparison with not attempting it. Finally, with respect to rescue chemotherapy, the protocols are based on 5-FU/CDDP (F-FU/Cisplatin), followed by Nigro (5-FU/Mitomycin) the overall population, favouring 5-FU/CDDP as the rescue. In modern series with few patients, triconjugate protocols are employed; others are based on taxanes, platins, and, finally, based on targeted and immunomodulatory therapy, without reaching an ideal rescue protocol to replace 5-FU/CDDP [36, 37, 43, 47–49], as shown in (Figure 7), with 82% of the patients categorised as completing treatment and classifying them on the basis of whether or not recurrence occurred, 32% presented with this event, with 53% receiving rescue treatment and completing it in 96% of cases.
Conclusion
Anal cancer is increasing in the Colombian Coffee Area, with localised disease comprising the majority of cases, and the highest probabilities of cure ultimately defined by an epidemiological profile of women between 50 and 70 years of age, homemakers, from urban areas, initial stage of II-A, being T2, without ganglia and without initial metastasis. In the case of presenting ganglia of type N1a, with well-differentiated epidermoid histology and ulceration, 5% with HIV, the localised lesion found in the medial portion of the anal canal, a good functional state represented by Karnofsky ≥ 80% and symptoms over 6–12 months, who could receive treatment with chemotherapy/radiation therapy on the Nigro protocol with no gap, with a radiation therapy dose greater than 45.00 Gys. Despite the foregoing, recurrence at the locoregional and/or hepatic level occurred in 32% after 3 years of monitoring, and was principally treated with surgery, radiation therapy or chemotherapy/radiation therapy using the 5-FU/CDDP protocol reaching an average monitoring period of 39.42 months to ultimately generate an overall survival of 95%, 89%, 85%, 81% and 69% from the first through fifth years of follow-up. For the aforementioned, it was considered that the work complied with the objectives formed from the start to show the behaviour of this neoplasm in the coffee region.
Conflicts of interest
The authors declare no conflicts of interest of any kind in the collection, analysis of information or the results from an academic, labour or economic point of view.
Funding statement
The concepts presented herein are the sole responsibility of the authors, and the work was entirely financed by the investigators’ own resources without the intervention of any company or entity.
Acknowledgments
To the patients for their trust and patience; to the employees of Oncologos del Occidente SAS for their daily efforts to benefit patients, and especially to a very valuable brother who left a great mark on all the authors and who eventually died of this disease.
References
1. Siegel RL, Miller KD, and Jemal A (2020) Cancer statistics CA Cancer J Clin 70 7 https://doi.org/10.3322/caac.21590 PMID: 31912902
2. National Comprehensive Cancer Network (NCCN) NCCN clinical practice guidelines in oncology [https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf] Date accessed: 2/05/20
3. Shiels MS, Kreimer AR, and Coghill AE, et al (2015) Anal Cancer Incidence in the United States, 1977–2011: distinct patterns by histology and behavior Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 24(10) 1548–1556 https://doi.org/10.1158/1055-9965.EPI-15-0044
4. Rogers JE and Eng C (2017) Pharmacotherapy of anal cancer Drugs 77(14) 1519–1530 https://doi.org/10.1007/s40265-017-0792-3 PMID: 28770514
5. Cancer today [Internet] [http://gco.iarc.fr/today/home] Date accessed: 2/05/20
6. Parra R, Brito A, and Rocha J, Féres O (2007) Retrospective analysis of patients with anal tumors diagnosed at the school of medicine of Ribeirão Preto Hospital and clinics (HC-FMRP), between 1979 and 2004 and literature review Rev Bras Coloproctologia 27 119–129 https://doi.org/10.1590/S0101-98802007000200001
7. Vera SL, Lafee NU, and Colmenares OL, et al (2015) Carcinoma de ano tratamiento combinado radioterapia y quimioterapia 13 años de experiencia Rev Venezolana Oncol 27(2) 96–103
8. (PDF) Situación del cáncer en chile 2000 – 2010 [Internet] [https://www.researchgate.net/publication/307930008_Situacion_del_cancer_en_chile_2000_-_2010?enrichId=rgreq-0339a24473f661757eb6d219e2aeb97b-XXX&enrichSource=Y292ZXJQYWdlOzMwNzkzMDAwODtBUzo0MDQyNTk3MTY3ODAwMzJAMTQ3MzM5NDQzNTYwNg= =&el=1_x_2&_esc=publicationCoverPdf] Date accessed: 2/05/20
9. GLOBOCAN Colombia [Internet] [https://gco.iarc.fr/today/data/factsheets/populations/170-colombia-fact-sheets.pdf] Date accessed: 2/05/20
10. Colombia, Ministerio de Salud (2001) Guías de práctica clínica en enfermedades neoplásicas (Bogotá: Instituto Nacional de Cancerología)
11. Oliveros R, Rey-León C, and Olarte H, et al (1990) Carcinoma del ano experiencia con 82 pacientes Rev Col Cir 5(3) 139–146
12. Lopez GA (2012) Registro poblacional de cáncer de Manizales-Incidencia y mortalidad 2003–2007 Informe final de investigación Caldas ISSN 1794-9432 pp 26–100 editorial Capital Graphic; Dic/2012
13. Villegas CR, Chacón JA, and Cardona JP, et al (2012) Perfil clínico epidemiológico de los pacientes con cáncer tratados en una Institución de tercer nivel. Manizales, Colombia, 1995–2004 Colomb Med 43(1) 11–18 https://doi.org/10.25100/cm.v43i1.1054
14. Wang C-Ch J, Sparano J, and Palefsky JM (2017) Human immunodeficiency virus/aids, human papillomavirus, and anal cancer Surg Oncol Clin N Am 26(1) 17–31 https://doi.org/10.1016/j.soc.2016.07.010
15. Hoff PM, Coundry R, and Venchiarutti CM (2017) Pathology of anal cancer Surg Oncol Clin N Am 26 57–71 https://doi.org/10.1016/j.soc.2016.07.013
16. Symer MM and Yeo HL (2018) Recent advances in the management of anal cancer [version 1; peer review: 2 approved] F1000Res 7 (F1000 Faculty Rev) 1572 https://doi.org/10.12688/f1000research.14518.1
17. Ramos M-V AJ, Vázquez-Barquero JL, and Herrera Castanedo S (2002) CIE-10 (I): Introducción, historia y estructura general Pápeles Médicos 11(1) 24–35
18. Amin MB, Greene FL, and Edge SB, et al (2017) The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging CA Cancer J Clin 67 93–99 https://doi.org/10.3322/caac.21388 PMID: 28094848
19. Glynne-Jones R, Nilsson PJ, and Aschele C, et al (2014) Anal cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up Ann Oncol 25(Supplement 3) iii10–iii20 https://doi.org/10.1093/annonc/mdu159 PMID: 25001200
20. Gunderson LL, Moughan J, and Ajani JA, et al (2013) Anal carcinoma: impact of TN category of disease on survival, disease relapse, and colostomy failure in US Gastrointestinal Intergroup RTOG 98-11 phase 3 trial Int J Radiat Oncol Biol Phys 87(4) 638–645 https://doi.org/10.1016/j.ijrobp.2013.07.035 PMID: 24035327 PMCID: 3938865
21. Ajani JA, Winter KA, and Gunderson LL, et al (2010) Prognostic factors derived from a prospective database dictate clinical biology of anal cancer the intergroup trial (RTOG 98-11) Cancer 4007–4013 https://doi.org/10.1002/cncr.25188
22. Gunderson LL, Jessup JM, and Sargent DJ, et al (2010) AJCC Hindgut Taskforce. Revised TN categorization for colon cancer based on national survival outcome data J Clin Oncol 28 264–271 https://doi.org/10.1200/JCO.2009.24.0952
23. Cummings BJ, Ajani JA, and Swallow CJ (2008) Cancer of the anal región Cancer: Principles & Practice of Oncology 8th edn, eds VT DeVita Jr., TS Lawrence, and SA Rosemberg, et al (Philadelphia, PA: Lippincott, Williams & Wilkins)
24. Goffredo P, Garancini M, and Robinson TJ, et al (2018) A National-Level Validation of the New American Joint Committee on Cancer 8th Edition Subclassification of Stage IIA and B Anal Squamous Cell Cancer Ann Surg Oncol 25(6) 1654–1660 https://doi.org/10.1245/s10434-018-6449-y PMID: 29572706
25. Lewis GD, Haque W, and Butler EB, et al (2019) Survival outcomes and patterns of management for anal adenocarcinoma Ann Surg Oncol 26(5) 1351 [Epub 2019 Feb 4] https://doi.org/10.1245/s10434-019-07202-4 PMID: 30719638
26. Anwar S, Welbourn H, and Hill J, et al (2013) Adenocarcinoma of the anal canal – a systematic review Colorectal Dis 15 1481–1488 https://doi.org/10.1111/codi.12325 PMID: 23809885
27. Marquez MF, Velasco AFJ, and Belda LR, et al (2013) Adenocarcinoma del canal anal Revision de conjunto 91(5) 281–286 https://doi.org/10.1016/j.ciresp.2013.01.002
28. MOC Brasil [Internet] [https://mocbrasil.com/es/moc-tumores-solidos/cancer-gastrointestinal/12-ano/] Date accessed:2/05/20
29. David P, Ryan DP, and Willett CG (2020) UpToDate: classification and epidemiology of anal cancer Anal Cancer [Internet] [Disponible en: https://www. https://www.uptodate.com/contents/classification-and-epidemiology-of-anal-cancer?search=adenocarcinoma del canal anal&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2] Date accessed:2/05/20
30. Northover J, Glynne-Jones R, and Sebag-Montefiore D, et al (2010) Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Br J Cancer 102 1123–1128 https://doi.org/10.1038/sj.bjc.6605605 PMID: 20354531 PMCID: 2853094
31. James RD, Glynne-Jones R, and Meadows HM, et al (2013) Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2×2 factorial trial Lancet Oncol 14(6) 516–524 https://doi.org/10.1016/S1470-2045(13)70086-X PMID: 23578724
32. Gunderson LL, Winter KA, and Ajani JA, et al (2012) Long-term update of US GI Intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin J Clin Oncol 30(35) 4344–4351 https://doi.org/10.1200/JCO.2012.43.8085 PMID: 23150707 PMCID: 3515768
33. Bartelink H, Roelofsen F, and Eschwege F, et al (1997) Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups J Clin Oncol 15(5) 2040–2049 https://doi.org/10.1200/JCO.1997.15.5.2040 PMID: 9164216
34. Ben-Josef E, Moughan J, and Ajani JA, et al (2010) Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of radiation therapy oncology group trials 87-04 and 98-11 J Clin Oncol 28 5061–5066 https://doi.org/10.1200/JCO.2010.29.1351 PMID: 20956625 PMCID: 3018356
35. Nigro ND, Vaitkevicius VK, and Considine B (1974) Combined therapy for cancer of the anal canal Dis Colon Rectum 17(3) 354–356 https://doi.org/10.1007/BF02586980 PMID: 4830803
36. Richard Kim, Byer J, and Fulp WJ, et al (2014) Carboplatin and paclitaxel treatment is effective in advanced anal cancer Oncology 87 125–132 https://doi.org/10.1159/000361051 PMID: 25012155
37. Olivatto LO, Cabral V, and Rosa A, et al (2011) Mitomycin-C– or cisplatin-based chemoradiotherapy for anal canal carcinoma: long-term results Int J Radiat Oncol Biol Phys 79(2), 490–495 https://doi.org/10.1016/j.ijrobp.2009.11.057
38. Gunderson LL, Winter K A, and Ajani JA, et al (2012) Long-term update of US GI Intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin J Clin Oncol 30(35) 4344–4351 https://doi.org/10.1200/JCO.2012.43.8085 PMID: 23150707 PMCID: 3515768
39. Cataño CJC (2004) Cáncer anal en la era del VIH: papel de la citología anal IATREIA 17(4) 396–403
40. Shiels MS, Pfeiffer RM, and Chaturvedi AK, et al (2012) Impact of the HIV epidemic on the incidence rates of anal cancer in the United States J Natl Cancer Inst 104(20) 1591–1598 https://doi.org/10.1093/jnci/djs371 PMID: 23042932 PMCID: 3611819
41. Tournier-Rangeard L, Mercier M, and Peiffert D, et al (2008) Radiochemotherapy of locally advanced anal canal carcinoma: Prospective assessment of early impact on the quality of life (randomized trial ACCORD 03) Radiother Oncol 87(3) 391–397 https://doi.org/10.1016/j.radonc.2007.12.004 PMID: 18191265
42. Grew D, Bitterman D, and Leichman CG, et al (2015) HIV infection is associated with por poor outcomes for patients with anal cancer in the highly active antiretroviral therapy era Dis Colon Rectum 58(12) 1130–1136 https://doi.org/10.1097/DCR.0000000000000476 PMID: 26544809
43. Glynne-Jones R, Meadows H, and Wan S, et al (2008) EXTRA—a multicenter phase II study of chemoradiation using a 5 day per week oral regimen of capecitabine and intravenous mitomycin C in anal cancer Int J Radiat Oncol Biol Phys 72(1) 119–126 https://doi.org/10.1016/j.ijrobp.2007.12.012 PMID: 18472366
44. Peixoto RD’A, Wan DD, and Schellenberg D, et al (2016) A comparison between 5-fluorouracil/mitomycin and capecitabine/ mitomycin in combination with radiation for anal cancer J Gastrointest Oncol 7(4) 665–672 [sci-hub.tw/10.21037/jgo.2016.06.04] https://doi.org/10.21037/jgo.2016.06.04 PMID: 29623585
45. Prasad RN, Elson J, and Kharofa J. (2018) The effect of dose escalation for large squamous cell carcinomas of the anal canal Clin Transl Oncol https://doi.org/10.1007/s12094-018-1863-y PMID: 29623585
46. Weber DC, Kurtz JM, and Allal AS (2001) The impact of gap duration on local control in anal canal carcinoma treated by split-course radiotherapy and concomitant chemotherapy Int J Radiat Oncol Biol Phys 50(3) 675–680 https://doi.org/10.1016/S0360-3016(01)01510-3 PMID: 11395235
47. Akbari RP, Paty PB, and Guillem JG, et al (2004) Oncologic outcomes of salvage surgery for epidermoid carcinoma of the anus initially managed with combined modality therapy Dis Colon Rectum 47(7) 1136–1144 https://doi.org/10.1007/s10350-004-0548-5 PMID: 15164245
48. Bentzen AG, Guren MG, and Wanderås EH, et al (2012) Chemoradiotherapy of anal carcinoma: survival and recurrence in an unselected national cohort Int J Radiat Oncol Biol Phys 83(2) e173–e18 https://doi.org/10.1016/j.ijrobp.2011.12.062 PMID: 22436791
49. Garg MK, Zhao F, and Sparano JA, et al (2017) Cetuximab plus chemoradiotherapy in immunocompetent patients with anal carcinoma: a phase II Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group Trial (E3205) J Clin Oncol 35(7) 718–726 https://doi.org/10.1200/JCO.2016.69.1667 PMID: 28068178 PMCID: 5455423






