Diagnostic validity of fine-needle capillary cytology in palpable tumours at the Oncology Institute of Peru
Milagros Abad-Licham1,2,3, Jose Galvez-Olortegui1,3, Juan Astigueta1,3,4 and Juan Díaz-Plasencia1
1Graduate School, Antenor Orrego Private University, Trujillo 13007, Peru
2Pathology Department, Regional Institute of Neoplastic Diseases, Trujillo 13601, Peru
3Scientia Clinical and Epidemiological Research Institute, Trujillo 13007, Peru
4Urology Department, Regional Institute of Neoplastic Diseases, Trujillo 13601, Peru
Correspondence to: Milagros Ana Amparo Abad Licham. Email: milagrosabadlicham@gmail.com
Abstract
Objective: To evaluate the diagnostic validity of fine-needle capillary cytology (FNCC) in palpable tumours.
Material and methods: A retrospective, single-tray, cross-sectional diagnostic test study was carried out. We reviewed the cytological reports of the case files of the Cytology Unit of the Northern Regional Institute of Neoplastic Diseases (IREN) from January 2012 to December 2016.
Results: A total of 332 patients were selected, with an average age of 54.77 years (range 13–90 years); 61.4% of patients were female. The most frequent anatomical sites were lymph nodes (49.7%), thyroid (13.3%), breast (12.3%) and soft tissues (11.4%). Twenty-five cytologies did not have a histological correlation and six showed an atypical result. In the lymph node study, the most frequent pathology was metastatic carcinoma (49.7%), followed by lymphoma (13.3%). The FNCC had a sensitivity of 99.55%, a specificity of 98.77%, a positive predictive value of 99.55% and a negative predictive value of 98.77%. The positive likelihood ratio was 80.63%.
Conclusions: FNCC is a useful, safe, reliable and economical ambulatory technique with minimal complications and high diagnostic accuracy.
Keywords: fine-needle biopsy, cytology, capillarity
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 01/02/2018; Received: 05/07/2017
Introduction
The origins of fine-needle aspiration (FNA) can be traced back to Arabic medicine [1, 2]. Centuries later, Kun (1847) described the technique and its benefits for diagnosing diseases, and this utility was confirmed by other authors in neoplastic and infectious pathology [2–5]. During the second decade of the 20th century, Dudgeon and Patrick in the United Kingdom and Martin and Ellis in the United States began the use of punctures for the rapid diagnosis of tumours [3, 6]. The first series of cases from the New York Memorial Hospital, in which an 18-gauge needle was used with good results [3, 7, 8], were published in the 1930s. At the same time in Europe, researchers such as Söderström and Franzen (Sweden), Lopes Cardozo (Holland) and Zajdela (France) termed the technique ‘fine-needle aspiration cytology’ (FNAC). Zajicek and Franzen at the Karolinska Hospital were the first pathologists to evaluate the diagnostic precision of the technique [3], popularising its use and coining the term ‘FNA biopsy’ when they successfully introduced 25–22 gauge needles and the aspiration gun [1, 7, 8]. In 1981, Zajdela modified the technique using the capillarity principle, creating the ‘aspirationless fine-needle’ variant (FNCC), in which sampling is performed using only a very thin needle and no syringe. Satisfactory results were achieved [2, 6, 9] and have been replicated in other series [7, 8].
FNCC is based on the capillary pressure exerted in a very slim tube and which is sufficient to keep cells attached to their lumen [3, 9]. Several studies have shown its usefulness in thyroid, lymph nodes, vascular tissues and some solid organs, except in breast and soft tissue tumours in which FNAC has proven slightly better [3]. FNCC offers multiple advantages, including being minimally invasive and ambulatory, fast results, low cost, high precision (when performed by trained and experienced staff), low risk of complications, less technology than surgical biopsy, sampling of multiple lesions in one session, and sampling of superficial and deep lesions with the support of imaging techniques [3]. Complications in the procedure are infrequent, with minimal haemorrhaging and spontaneous haematoma resolution [10]. There are exceptional reports of haemorrhaging, septicaemia, biliary peritonitis, pneumothorax and other complications [3, 10, 11] in deep lesions. Additionally, some aspects described in the past, but which are not currently considered, included the possibility of cancer cell dissemination, local tissue alteration with the consequent difficulty of histological diagnosis (capsular pseudoinvasion and pseudomalignant reparative reactions) [3], all highly unlikely effects given the fineness of the puncture needle.
The fine-needle biopsy appeared in Peru in the 1990s. Columbie and Somocurcio (Edgardo Rebagliati Martins Hospital, Lima) were the first to use the aspirative version in the study of thyroid tumours, publishing their results in 1996 at the National Congress of Pathological Anatomy [12]. Years later, the National Institute of Neoplastic Diseases began to use the technique irregularly in assisting surgeons, but more recently in 2007 Abad, Takahashi and Dyer formally began to use cyto-interventionism in oncology, the first cases being in the lymph node and thyroid gland. To date, other hospitals have implemented this technique but they essentially use FNAC [13–15]. FNCC came to Peru in 2011, with the contribution of Ricardo Bardales in the newly created cytology unit of IREN Norte, the reference oncology centre of northern Peru, whose scope of action includes approximately 9 million inhabitants [16], and which became the first hospital to use the technique successfully in different organs and initiated its dissemination nationwide [17].
The present study evaluates the diagnostic validity of FNCC in palpable tumours and is the first Peruvian article in this regard.
Materials and methods
A retrospective, single-tray, cross-sectional diagnostic test study was carried out. The population consisted of patients with palpable tumours treated at the Northern IREN, Peru, in the period from January 2012 to December 2016. The inclusion criteria were patients with palpable tumours who were referred to the Northern IREN Cytology Unit; FNCC performed by the cytopathologist; compliance with the necessary criteria for performing the procedure (description and explanation of the technique, acceptance of the procedure and informed consent). Exclusion criteria were tumours on which the fine-needle biopsy was not performed in the Cytology Unit; lack of histological correlation in the palpable tumours that were punctured; severe coagulation disorders; and patients younger than 11 years old.
The technique: FNCC, which is physically based on the principle of capillarity [3], was performed using 27–23 gauge needles, a 10 cc. syringe for discharging the material, microscope slides and coverslips, and 96% ethanol as fixative. The procedure involves holding the needle directly with the fingers, and inserting it into the target tissue, moving it back and forth in various directions for several seconds (depending on the tissue cellularity and vascularity) and then, with the help of a syringe, discharging the material onto a microscope slide and spreading it with another slide. Fixation was immediate in all cases, the adequacy of the sample being assessed with toluidine blue. The average number of passes was three. PAP and H&E staining were used for the final cytodiagnosis. A cell block was not obtained.
Diagnostic test: The test to be assessed was FNCC cytological diagnosis, defined as the diagnosis resulting from studying the cells of a tumour lesion obtained using FNCC. The gold standard was histological diagnosis, defined as the product of the study on organ tissues punctured with capillary action or on the primary lesion (in the case of metastasis). ‘Insufficient’ was defined as the category in which cytological findings included haematic material and few inflammatory elements. ‘Atypical’ was defined as the category in which the cytomorphological findings did not permit a classification as benign or malignant (grey area).
Data acquisition process: The cytological reports from January 2012 to December 2016 from the Northern IREN Cytology Unit files were reviewed, and the medical record number, epidemiological data and cytodiagnosis were obtained from these. To obtain technique performance consistency, a pathologist (MAL) from the unit, who was trained and experienced in the procedure, collected and carried out the cytodiagnosis. Subsequently, the medical records were reviewed and the histological diagnosis was performed and recorded by the unit’s pathologists, who did not know the cytological result. On this basis, the eligible population matching the inclusion and exclusion criteria was selected. Data were collected by an expert researcher and co-author (JGO), who is not a pathologist. These data were stored in a Microsoft Excel spreadsheet, and processed using SPSS version 23 and Epidat 3.1.
Results
The average age of the 332 patients was 54.7 years, with a range between 13 and 90 years. The female/male ratio was 1.59. 61.4% of the patients were female with an average age of 52 years. The average age of males was 59 years old. In line with international standards, the cytological result was grouped into four categories: positive (68.7%), negative (28%), atypical (1.8%) and insufficient (1.5%). The histological diagnosis to assess the diagnostic accuracy of the technique was grouped into four categories: positive (66.9%), negative (25.3%), atypical (0.3%) and without histology (7.5%). The most frequent anatomical sites were lymph node (49.7%), thyroid (13.3%), breast (12.3%) and soft tissue (11.4%) (Table 1).
Of the 332 FNCC cases, 25 did not have histological correlation and six showed an atypical result. Consequently, the diagnostic test was carried out on 301 cases (Figure 1): 1.8% (5 cases) of the cytological findings were insufficient, corresponding to 1 thyroid sample, 2 inguinal node samples, 1 supraclavicular node sample and 1 axillary node sample (Table 2).
In the atypical results, there were three lymph nodes (retroauricular and cervical), one orbit, one thyroid and one soft tissue (cervical region). Of these, three showed positive malignant histology (non-Hodgkin’s lymphoma, histiocyte-rich Hodgkin’s and insular carcinoma), two were negative (inflammatory pseudotumour and chronic, non-specific lymphadenitis) and one retained the histomorphological characteristics of atypical disease (Table 3). Comparing the type of tumour, the cytological diagnosis of adenocarcinoma presented a discordant histological result (carcinoma), while the cytological diagnosis of carcinoma showed three different histological findings (adenocarcinoma, lymphoma and melanoma). The cytological diagnosis of poorly differentiated sarcoma had a different histological correlation from poorly differentiated carcinoma (Figures 2 and 3).
In the enlarged lymph nodes studied, the most frequent diagnosis was metastatic carcinoma (49.7%), followed by lymphoma (13.3%) and metastatic adenocarcinoma (12.7%). Of the 114 cases of metastatic lymph node disease, the most common primary cases were breast, stomach, prostate, thyroid and skin.
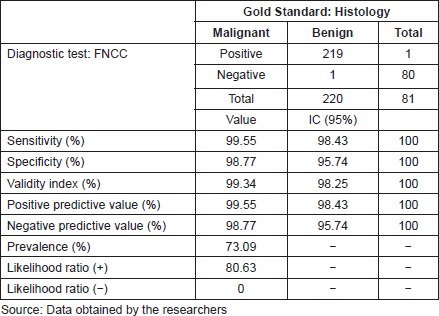
For the diagnostic test analysis, 301 cytological samples fulfilling the eligibility criteria were selected (Table 4), excluding the insufficient cytological results that did not have histological correlation. The false positive result was a round-cell malignant tumour, whose histology reported lymphoid hyperplasia, whereas the false negative result was an inflammatory cytology whose histology reported a lymphoma. FNCC had a sensitivity of 99.55%, a specificity of 98.77%, a positive predictive value of 99.55% and a negative predictive value of 98.77%. The positive likelihood ratio was 80.63%.
Table 1. FNCC: epidemiological characteristics.
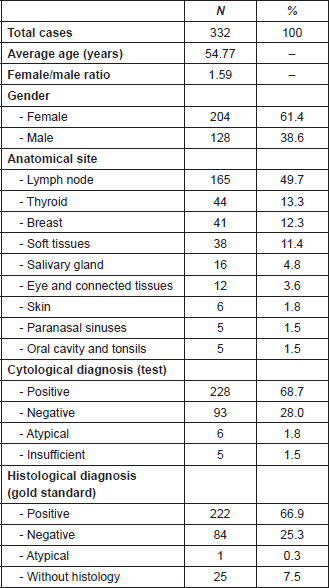
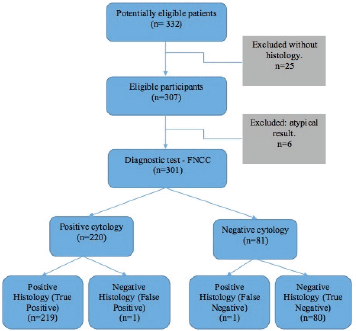
Figure 1. FNCC flow chart.
Table 2. Fine-needle capillary cytology: cytohistological correlation.

Table 3. FNCC: diagnostic correlation of the cytological and histological results.
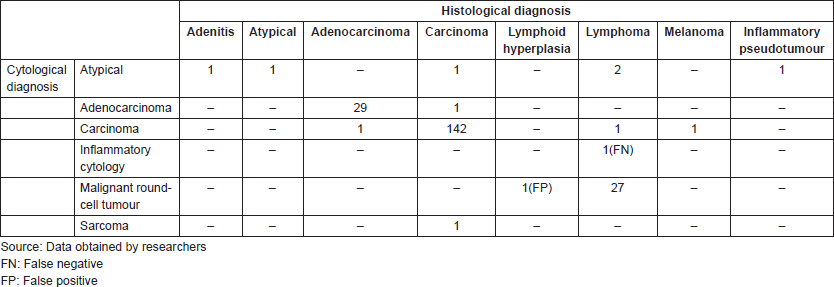
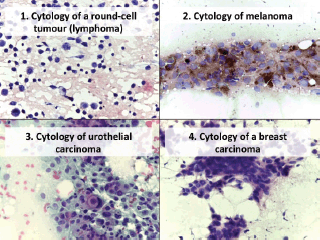
Figure 2. Cytology microphotographs obtained by FNCC.
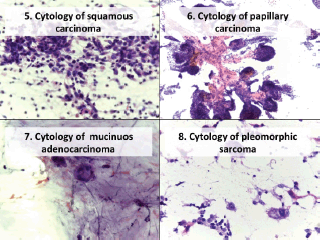
Figure 3. Cytology microphotographs obtained by FNCC.
Table 4. FNCC: diagnostic test results.
Discussion
Fine-needle cytology is a simple technique with high diagnostic value and growing interest worldwide [11, 18]. The FNCC variant is a safe, simple and quick, minimally invasive technique that can be performed on outpatients. It is economical and reproducible and has a high diagnostic accuracy. Its use has been demonstrated in the treatment of various diseases, particularly cancers [19–21]. The use of the technique in Peru is recent and not widespread and therefore no reports have been published about it. In this study, 332 patients with palpable tumours permitting the use of FNCC were assessed. The average age was 54.55 years, slightly under the age at which patients present with oncological diseases.
The largest number of lesions was in lymph nodes, demonstrating the usefulness of FNCC in lymphadenopathy and its advantages over FNAC, as reported by Sajeev et al [22] and Farooq et al [23]. The high number of lymph node tumours is probably due to the fact that IREN North is a national reference centre in oncology, and therefore patients come not only for diagnosis but also for follow-up. Metastatic lymph node disease represented the highest percentage, similar to what was reported by Zhou et al [24] and Anila et al [25], breast cancer being the most common. In our region, cancer among women is a serious public health problem, because the vast majority of women only come to the hospital once they are in the advanced stages. Second, we find the primary nodal disease, reported cytologically as a round-cell malignant tumour, which, in the histological and immunohistochemical study, corresponded mainly to diffuse large B-cell lymphoma. In our findings, we should mention the group of benign diseases, in which granulomatous lymphadenitis stands out, similar to what was reported by Bharathi et al [7, 26]. These results are of epidemiological interest, for consideration in the differential diagnostic approach (pseudotumour pathology).
Thyroid disease was the second most frequent disease assessed using FNCC, with good results. On this point, the American Thyroid Association states that FNA biopsy is a very important method in the assessment of thyroid nodules and recommends its use in nodules larger than 1.0, 1.5 and 2.0cm, depending on whether the ultrasound malignancy risk is intermediate, low or very low, respectively [27]. This document mentions FNA, however, in literature there are several works that validate the utility of FNCC, with results similar to our study [20, 28–31]. The work of Zhou et al suggests that the size of the lesion could be used as a reference for choosing which technique to use [32]. In recent years, in Peru, the use of FNCC in the thyroid has increased, due to the reliability of the procedure.
Other assessed diseases were tumours of the head and neck including tumours of the eye and conjoining tissue, with satisfactory results, similar to those reported by Hamaker et al [33], Braun et al [34] and Deshpande et al [35]. Hamaker’s study on masses in the head and neck reports that, although FNCC produces lower-volume samples, the quality is good and the cell architecture is preserved, ensuring a high value diagnosis [33]. Two years later, Braun’s group compared 166 lesions in the same anatomical region and found that FNCC was more effective in the thyroid, lymph nodes, thyroglossal cysts and salivary glands, with the exception of the submaxillary salivary glands [34].
Regarding diagnostic accuracy, using the described, internationally accepted categories, the technique achieves values that reflect high reliability and does not differ significantly whether or not ultrasound is used [36–38]. The use of capillary cytology in the context of suitable clinical and imaging context is an accurate diagnostic tool [33, 36]. Our results are similar to those described by other authors, ranging from 92% to 99% depending on the organ studied [19, 22, 25, 39]. Lower percentages of diagnostic accuracy have been described in the series by Tauro et al [20] and McElvanna et al [40].
In a separate comment, it is worthwhile comparing FNCC and FNAB, which both use a ‘fine needle’, a very thin needle that, regardless of the physical principle with which it is used, allows cells to be extracted from a given mass [41]. The diagnostic accuracy of both is operator-dependent and therefore requires proper training, sensitivity and high specificity for malignancy, not only for diagnostic purposes but also for therapeutic and prognostic purposes [11, 30, 42]. The main technical difference between FNCC and FNAC is that the former eliminates the handling of the device, using only the needle, thus making the technique simpler with equal or better diagnostic accuracy in certain pathologies [21, 31, 43, 44]. It has also been shown that with a smaller needle diameter, a better quality of cell material is obtained, while at the same time reducing patient discomfort and the risk of bleeding [45]. Another important aspect is the immediate fixation of the slide in 96% ethyl alcohol to prevent desiccation from affecting the cellular details and hindering the diagnosis [26, 40, 43, 45]. Furthermore, this procedure is readily accepted by the patient, who does not require anaesthesia, and can be repeated if the sample was inadequate (immediate evaluation) [26, 46, 47]. Madhuri et al (1998) used FNCC to assess 670 patients with superficial and deep lesions in various organs, using imaging for the deep lesions, and reported high diagnostic accuracy, highlighting the fact that this method allows greater technical control and a better perception of tumour consistency [19]. In the opinion of Tauro and De Carvalho, given that differences are not statistically significant between the two techniques, the choice of one over the other will depend on the operator [20, 39], who must be adequately trained [14, 42].
Among the limitations of the technique is the differentiation of some types of malignant lesions, such as poorly-differentiated tumours, which, even in a histological correlation, are not adequately typified without the help of an immunohistochemical study, as well as the definition of invasive or in situ tumours, which are determined by studying the tissue itself [26, 48]. On this point, we note that immunocytochemical tests have been carried out in recent years, not only to determine cell lineage but also for prognostic purposes, despite the poor amount of material that is obtained. Some alternatives such as thin-layer cytology and the use of the cell block have been suggested with promising results [48–50]. In the present study, we did not include this method when it was carried out in the surgical biopsy. Finally, FNCC is an effective alternative which, due to its benefits, should be considered in public health policies within the context of a diagnostic approach to a cancer patient, in view of the cost and waiting time that a histological diagnosis of a surgical biopsy entails in many hospitals.
Conclusions
FNCC is an ambulatory, fast-diagnosis, economical and reliable technique with minimal complications that must be carried out by trained staff.
The diagnostic accuracy of FNCC is high, and because of its advantages, should be promoted and implemented in the country and in other regions that have a similar situation to Peru.
Conflicts of interest
The authors have no conflict of interests to declare.
Authors’ contributions
The main idea came from MAL. JGO and MAL collected and analysed the data. MAL, JGO, JCA and JDP wrote the protocol and manuscript and approved the final version.
Acknowledgments
Dr. Ricardo Bardales (Outpatient Pathology Associates, Sacramento, California) and Dr. Ronald Balassanian (University of California, San Francisco), and molecular biologist Oriana Vasquez (IREN North).
References
1. Diamantis A, Magiorkinis E, and Koutselini H (2009) Fine-needle aspiration (FNA) biopsy: historical aspects Folia Histochem Cytobiol 47(2) 191–197 https://doi.org/10.2478/v10042-009-0027-x PMID: 19995703
2. Das DK (2003) Fine-needle aspiration cytology: its origin, development, and present status with special reference to a developing country, India Diagn Cytopathol 28(6) 345–351 https://doi.org/10.1002/dc.10289 PMID: 12768643
3. Orell S and Sterret G (2012) Orell and Sterrett’s Fine Needle Aspiration Cytology 5th edn (Shangai: Churchill Livingstone) p 512
4. Greig ED and Gray AC (1904) Note on the Lymphatic glands in sleeping sickness Br Med J 1(2265) 1252 https://doi.org/10.1136/bmj.1.2265.1252-a PMID: 20761565 PMCID: 2353964
5. Guthrie C (1921) Gland puncture as a diagnostic measure Bull Johns Hopkins Hosp 32 266–269
6. Rosa M (2008) Fine-needle aspiration biopsy: a historical overview Diagn Cytopathol 36(11) 773–775 https://doi.org/10.1002/dc.20915 PMID: 18831026
7. Bharathi K, Anuradha S, and Khalique A, et al (2012) A prospective study to compare the aspiration and non-aspiration techniques in fine-needle cytology of Lymphnode and to evaluate the diagnostic accuracy of aspiration cytology in Lymphnode lumps Int J Biol Med Res 3(1) 2147–2152
8. Frable WJ (1989) Needle aspiration biopsy: past, present, and future Hum Pathol 20(6) 504–517 https://doi.org/10.1016/0046-8177(89)90242-6 PMID: 2656496
9. Zajdela A, Zillhardt P, and Voillemot N (1987) Cytological diagnosis by fine needle sampling without aspiration Cancer 59(6) 1201–1205 PMID: 3815294
10. Kocjan G (2006) Fine Needle Aspiration Cytology. Diagnostic Principles and Dilemmas (Leipzig: Springer)
11. Cibas E and Ducatman B (2009) Cytology - Diagnostic Principles and Clinical Correlates 3rd edn (Philadelphia: Saunders Elsevier)
12. Somocurcio Peralta J (2010) Biopsia Punción - Aspiración con Aguja Fina para el diagnóstico del Cáncer de Tiroides (Unidad de Tiroides del Hospital Edgardo Rebagliati Martins en el período del 01 de Enero del 2001 al 31 de Diciembre del 2005) [Tesis de especialidad] (Lima: Universidad Nacional Mayor de San Marcos)
13. Goicochea Vargas LM (2001) Biopsia aspiración con aguja fina en glándulas salivales en el período Marzo 1998-Marzo 2001 en el Hospital Edgardo Rebagliati Martins) [Tesis de especialidad] (Lima: Universidad Nacional Mayor de San Marcos)
14. Ng D, Ljung B-M, and Bardales R, et al (2016) Developing a breast fine needle aspiration biopsy service in Peru Ann Glob Heal 82(3) 546–547 https://doi.org/10.1016/j.aogh.2016.04.473
15. Contreras I and Anderson L (2015) The Woman Behind PATH’s Small But Mighty Program in Peru (Seattle: PATH) [http://blog.path.org/2015/11/the-woman-behind-paths-small-but-mighty-program-in-peru/ http://www.aeped.es/protocolos/dermatologia/index.htm] Date accessed: 07/02/17
16. Instituto Nacional de Estadística e Informática (2016) (Perú: Sintesis Estadistica, Gráfica Burgos SAC)
17. Abad-Licham M, Cusma T, and Salazar W, et al (2014) Validez Diagnóstica de la Biopsia Capilaridad con énfasis en la evaluación inmediata con azul de toluidina (método de menos de un minuto) Poster sesion presented at: XXII Congreso Peruano de Anatomía Patológica (Lima-Perú, Nov. 20–22 2014)
18. Ly A, Ono JC, and Hughes KS, et al (2016) Fine-needle aspiration biopsy of palpable breast masses: patterns of clinical use and patient experience J Natl Compr Canc Netw 14(5) 527–536 https://doi.org/10.6004/jnccn.2016.0061 PMID: 27160231
19. Madhuri K, Kamal M, and Bobhate S, et al (1998) Evaluation of fine needle capillary sampling in superficial and deep-seated lesions. an analysis of 670 cases Acta Cytol 42(3) 679–684 https://doi.org/10.1159/000331826
20. Tauro LF, Lobo GJ, and Fernandes H, et al (2012) A comparative study on fine needle aspiration cytology versus fine needle capillary cytology in thyroid nodules Oman Med J 27(2) 151–156 https://doi.org/10.5001/omj.2012.31 PMID: 22496942 PMCID: 3321336
21. Aydin C, Dellal F, and Tam A, et al (2017) Comparative analysis of diagnostic adequacy rates between aspiration and no aspiration techniques of fine needle of cytology Diagnostyc cytopath 45(10) 889–894 https://doi.org/10.1002/dc.23793
22. Sajeev S and Siddaraju N (2009) A comparative analysis of fine-needle capillary cytology vs. fine-needle aspiration cytology in superficial lymph node lesions Diagn Cytopathol 37(11) 787–791 https://doi.org/10.1002/dc.21109 PMID: 19526570
23. Farooq S, Singh K, and Amin J (2017) Comparasion between aspiration and non aspiration technique in fine neddle citology of lymph node J Evid Based Med 51(4) 3112–3115
24. Zhou J, Li F, and Meng L, et al (2016) Fine needle aspiration cytology for lymph nodes: a three-year study Br J Biomed Sci 73(1) 28–31 https://doi.org/10.1080/09674845.2016.1144947 PMID: 27182674
25. Anila KR, Nayak N, and George PS, et al (2015) Utility of fine needle aspiration cytology in evaluation of lymphadenopathy - an audit from a Cancer Centre in South India Gulf J Oncolog 1(19) 50–56 PMID: 26499831
26. Steel BL, Schwartz MR, and Ramzy I (1995) Fine needle aspiration biopsy in the diagnosis of lymphadenopathy in 1,103 patients. role, limitations and analysis of diagnostic pitfalls Acta Cytol 39(1) 76–81 PMID: 7847013
27. Haugen R, Alexander E, and Bible K, et al (2016) 2015 American thyroid association management. guidelines for adult patients with thyroid nodules and differentiated thyroid cancer Thyroid 26(1) 1–133 https://doi.org/10.1089/thy.2015.0020 PMCID: 4739132
28. Kamal MMM, Arjune DGD, and Kulkarni HHR (2011) Comparative study of fine needle aspiration and fine needle capillary sampling of thyroid lesions Acta Cytol 46(1) 30–34 https://doi.org/10.1159/000326712
29. Pinki P, Alok D, and Ranjan A, et al (2015) Fine needle aspiration cytology versus fine needle capillary sampling in cytological diagnosis of thyroid lesions Iran J Pathol 10(1) 47–53 PMID: 26516325 PMCID: 4539790
30. Song H, Wei C, and Li D, et al (2015) Comparison of fine needle aspiration and fine needle nonaspiration cytology of thyroid nodules: a meta-analysis Biomed Res Int 2015 19–21 https://doi.org/10.1155/2015/796120
31. Buzuduga CM, Galesanu C, and Vulpoi C, et al (2015) Thyroid fine-needle biopsy: aspiration versus capillary Rev Med Chir Soc Med Nat Iasi 119(1) 45–50
32. Zhou JQ, Zhang JW, and Zhan WW, et al (2014) Comparison of fine-needle aspiration and fine-needle capillary sampling of thyroid nodules Cancer Cytopathol 122(4) 266–273 https://doi.org/10.1002/cncy.21382
33. Hamaker RA, Moriarty AT, and Hamaker RC (1995) Fine-needle biopsy techniques of aspiration versus capillary in head and neck masses Laryngoscope 105(12 Pt 1) 1311–1314 https://doi.org/10.1288/00005537-199512000-00009 PMID: 8523983
34. Braun H, Walch C, and Beham A, et al (1997) Fine needle capillary cytology versus fine needle aspiration cytology--a comparison of quality between puncture techniques in the ENT area Laryngorhinootologie 76(6) 358–363 https://doi.org/10.1055/s-2007-997442 PMID: 9333280
35. Deshpande AH and Munshi MM (2003) Fine needle capillary sampling of eyelid masses. a study of 70 cases Acta Cytol 47 349–358 https://doi.org/10.1159/000326532 PMID: 12789913
36. Khalid AN, Quraishi SA, and Hollenbeak CS, et al (2008) Fine-needle aspiration biopsy versus ultrasound-guided fine-needle aspiration biopsy: cost-effectiveness as a frontline diagnostic modality for solitary thyroid nodules Head Neck 30(8) 1035–1039 https://doi.org/10.1002/hed.20829 PMID: 18442056
37. Tublin ME, Martin JA, and Rollin LJ, et al (2007) Ultrasound-guided fine-needle aspiration versus fine-needle capillary sampling biopsy of thyroid nodules: does technique matter? J Ultrasound Med 26(12) 1697–1701 https://doi.org/10.7863/jum.2007.26.12.1697 PMID: 18029921
38. El Haddad R, Barret M, and Beuvon F, et al (2016) The slow-pull capillary technique increases the quality of endoscopic ultrasound fine needle biopsy samples in solid pancreatic lesions Eur J Gastroenterol Hepatol 28(8) 911–916 https://doi.org/10.1097/MEG.0000000000000638 PMID: 27140228
39. De Carvalho GA, Paz-Filho G, and Cavalcanti TC, et al (2009) Adequacy and diagnostic accuracy of aspiration vs. capillary fine needle thyroid biopsies Endocr Pathol 20(4) 204–208 https://doi.org/10.1007/s12022-009-9092-0 PMID: 19757207
40. McElvanna K, Pyper P, and Miller KA (2009) Comparison of fine-needle aspiration versus non-aspiration cytology of thyroid nodules patients Internet J Surg 25(2) 1–6
41. Wright CA (2012) Fine-needle aspiration biopsy (FNAB), when performed by trained operators, and for the correct indications, is a safe and minimally invasive procedure, with an excellent diagnostic yield CME 30(2) 56–60
42. Tsu VD, Winkler JL, and Anderson BO, et al (2013) Coordinated training on early detection and diagnosis of breast cancer across different levels of health workers: an example from Peru Breast and Gynecological Cancers ed M En Shetty (New York: Springer) p 273–284 https://doi.org/10.1007/978-1-4614-1876-4_14
43. Vélez A, Monsalve YM, and López E, et al (2005) Comparación entre la técnica de capilaridad y la biopsia aspiración con aguja fina (BACAF) en nódulos de tiroides Med UPB 24(2) 165–170
44. Akhtar M, Ali MA, and Huq M, et al (1998) Fine-needle biopsy: comparison of cellular yield with and without aspiration Diagn Cytopathol 5(2) 162–165 https://doi.org/10.1002/dc.2840050210
45. Hopper KD, Grenko RT, and Fisher AI, et al (1996) Capillary versus aspiration biopsy: effect of needle size and length on the cytopathological specimen quality Cardiovasc Intervent Radiol 19(5) 341–344 https://doi.org/10.1007/BF02570187 PMID: 8781156
46. Nasuti JF, Yu G, and Boudousquie A, et al (2000) Diagnostic value of lymph node fine needle aspiration cytology: an institutional experience of 387 cases observed over a 5-year period Cytopathology 11(1) 18–31 https://doi.org/10.1046/j.1365-2303.2000.00208.x PMID: 10714372
47. Chau I, Kelleher MT, and Cunningham D, et al (2003) Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients Br J Cancer 88(3) 354–361 https://doi.org/10.1038/sj.bjc.6600738 PMID: 12569376 PMCID: 2747551
48. Geethamala K, Murthy VS, and Vani BR, et al (2017) Comparison of immunocytochemistry and immunohistochemistry on breast carcinoma: a boon or a bane? J Lab Physicians 9(1) 5–10 https://doi.org/10.4103/0974-2727.187915 PMID: 28042209 PMCID: 5015499
49. Wuertz FG, Kresnik E, and Malle P, et al (2016) Fine-needle aspiration with immunohistochemistry using a modified scrape cell block technique for the diagnosis of thyroid and parathyroid nodules Acta Cytologica 60, 118–130 https://doi.org/10.1159/000446466 PMID: 27231232
50. Rossi ED, Raffaelli M, and Mulè A, et al (2008) A relevance of immunocytochemistry on thin-layer cytology in thyroid lesions suspicious for medullary carcinoma: a case-control study Appl Immunohistochem Mol Morphol 16(6) 548–553 https://doi.org/10.1097/PAI.0b013e3181690ca3 PMID: 18685492






