Healthy living and cancer: evidence from UK Biobank
Peter C Elwood1, Alex Whitmarsh1, John Gallacher2, Anthony Bayer1, Richard Adams3, Luke Heslop1, Janet Pickering1, Gareth Morgan1, Julieta Galante4, Sunil Dolwani1, Marcus Longley5 and Zoe E Roberts1
1Division of Population Medicine, Cardiff University, Cardiff CF10 3AT, UK
2Dementia Platform, Department of Psychiatry, University of Oxford, Oxford OX1 2JD, UK
3Velindre Cancer Centre, Cardiff CF14 2TL, UK
4Department of Psychiatry, University of Cambridge, Cambridge CB2 1TN, UK
5University of South Wales, Cardiff CF24 2FN, UK
6Centre for Medical Education, Cardiff University School of Medicine, Cardiff CF10 3AT, UK
Correspondence to: Peter Elwood. Email: ElwoodPC@cf.ac.uk
Abstract
Context: UK Biobank is a prospective study of half a million subjects, almost all aged 40–69 years, identified in 22 centres across the UK during 2006–2010.
Objective: A healthy lifestyle has been described as ‘better than any pill, and no side effects [5]. We therefore examined the relationships between healthy behaviours: low alcohol intake, non-smoking, healthy BMI, physical activity and a healthy diet, and the risk of all cancers, colon, breast and prostate cancers in a large dataset.
Method: Data on lifestyle behaviours were provided by 343,150 subjects, and height and weight were measured at recruitment. 14,285 subjects were diagnosed with cancer during a median of 5.1 years of follow-up.
Results: Compared with subjects who followed none or a single healthy behaviour, a healthy lifestyle based on all five behaviours was associated with a reduction of about one-third in incident cancer (hazard ratio [HR] 0.68; 95% confidence intervals [CI] 0.63–0.74). Colorectal cancer was reduced in subjects following the five behaviours by about one-quarter (HR 0.75; 95% CI 0.58–0.97), and breast cancer by about one-third (HR 0.65; 95% CI 0.52–0.83). The association between a healthy lifestyle and prostate cancer suggested a significant increase in risk, but this can be attributed to bias consequent on inequalities in the uptake of the prostate specific antigen screening test.
Conclusions: Taken together with reported reductions in diabetes, vascular disease and dementia, it is clearly important that every effort is taken to promote healthy lifestyles throughout the population, and it is pointed out that cancer and other screening clinics afford ‘teachable moments’ for the promotion of a healthy lifestyle.
Keywords: prospective study, healthy behaviours, cancer incidence
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 04/01/2018; Received: 25/08/2017
Introduction
The risk of cancer in the UK population has increased from about 37% for people born in 1930 to around 50% for people born in 1960 [1]. The increase is largely due to the increasing age of the population, but even after standardisation for age, cancer rates have risen over the past ten years by 3% in men and 8% in women [1].
In the UK male population, prostate cancer accounted for 26% and colorectal cancer 13% of all cancers, and in the UK female population, breast cancer accounted for 31% and colorectal cancer 10%. Together, these cancers accounted for over half the malignancies in the total population [2].
There is a large body of literature describing beneficial relationships between a healthy lifestyle, usually defined as non-smoking, a low body weight, regular exercise, a healthy diet and a low alcohol intake and an accompanying risk reduction in a variety of diseases, including cancer. Khaw et al [3] described how four healthy behaviours combined predict a four-fold difference in the total mortality in men and women, with an estimated impact equivalent to an additional survival of about fourteen years. Ford et al [4] reported that the following of four healthy behaviours at baseline predicted a 78% (95% CI, 72–83%) lower risk of developing a chronic disease (diabetes, heart disease, stroke or cancer) than subjects neglecting the behaviours. Elwood et al [5] confirmed all the above and added evidence showing, in a 30-year follow-up, a reduced impairment of cognitive function and a 60% reduction in dementia, attributable to the following of a healthy lifestyle.
In this report, we examine the associations between a healthy lifestyle and incident cancer within the UK Biobank cohort [6].
Methods
UK Biobank is a prospective study of 502,642 participants aged between 40 and 69 years, identified in 22 centres across the UK during 2006–2010 [6].
Participants were not specifically involved in setting the research questions or the outcome measures, nor were they involved in developing plans for recruitment, design or implementation of the present study. They were consulted over these issues through public meetings and through membership of the independent Ethics and Governance Council. They also remain involved with the study through newsletters, public meetings and consultation. However, neither the funding source nor the participants were asked to advise on the interpretation or writing up of any of the results from the study.
Extensive data were collected from those who agreed to participate, using computerised questionnaires, interviews, physical measurements and biological sample testing. Identification data were obtained to enable long-term follow-up of the subjects through linkage to national datasets on cancers and deaths, and agreement for this was obtained from each participant. Information on incident cancers and deaths was coded according to the 10th revision of the International Classification of Diseases (ICD-10) [7].
At recruitment, participants completed a computerised detailed questionnaire on social issues, lifestyle, smoking, physical activity, diet and alcohol. The answer ‘prefer not to answer’ was available for each question on the behaviours. Height and weight were measured by the clinic staff.
The answers to the questionnaires were used to define the following behaviours.
Smoking: Each subject was asked ‘do you smoke tobacco now?’ 429 who preferred ‘not to answer’ were excluded. Participants who responded ‘No’ were judged to fulfil this healthy behaviour. Participants who responded ‘Yes, on most or all days’, or ‘only occasionally’ were judged to fail this behaviour.
Height and Weight: Height and weight were measured on 499,537 participants. Those who had a BMI (weight/height2) of under 25 and over 18.5 were coded as ‘healthy’ for this behaviour. Height and weight were measured at recruitment.
Physical Activity: Participants were asked to report the number of days each week, and for how long, they did moderate, and/or vigorous physical activity. The criteria used were those defined by the NHS [8], namely 150 minutes or more of moderate activity per week or 75 minutes of vigorous activity per week, or an equivalent combination. 71,365 subjects were excluded for not responding to at least one of the questions.
Diet: Participants completed a questionnaire on the computer, which asked questions about the number of portions of cooked or raw vegetables, salad, fresh and dried fruit they consumed each day. No account was taken of other food items. 8,001 subjects were excluded for not responding to at least one of the questions. Subjects who consumed at least five portions of fruit or vegetables each day were defined as ‘healthy’.
Alcohol: Participants were asked to report their usual intake of different alcoholic beverages (per week or per month, depending on how frequently they reported drinking alcohol): red wine, white wine, beer/cider, spirits, fortified wine and other drinks. 73,061 subjects who should have answered the monthly questions had missing data and could not be included, because they had answered a previous version of the questionnaire. A further 8,948 participants were excluded for non-response. Healthy behaviour was defined as drinking within the NHS recommended guidelines, namely, 14 or less units per week [9].
Analyses: The number of healthy behaviours was modelled as a categorical variable. The reference group was participants with ≤1 healthy behaviour since there were few participants following no healthy behaviour. A test for trend was calculated by treating this variable as a continuous variable. The number of healthy behaviours was also modelled as an ordinal variable in order to estimate the average effect on cancer risk of adopting one additional healthy behaviour.
Cox proportional hazards models were used to analyse the association between lifestyle behaviours and cancer risk. Age was used as the underlying time variable. Participants were followed up from the date of the baseline assessment until the date of any cancer diagnosis, except non-melanoma skin cancer, the date of death or 31 March 2014 (end of follow-up), whichever came first.
All the analyses presented were adjusted for the Townsend deprivation index, an area-based measure of social class [10]. The Townsend scores were calculated for the national census output areas and the subjects were assigned Townsend scores based on the output area that their postcode was located in. Analyses were also adjusted for height.
Results
Following exclusions, 343,150 (169,715 men and 173,435 women) participants in UK Biobank were included in the present analyses. 26,857 (2%) subjects had to be excluded because of prevalent cancer at baseline, together with 12 subjects with uncertainties in the diagnostic data. Other limitations included the fact that each of the behaviours was used in the analysis as a simple dichotomy, defining ‘healthy’ and ‘unhealthy’ and no attempt was made to use quantitative data.
During a median 5.1 years of follow-up (range 3.5–8.1 years), 14,285 participants (7,944 men and 6,341 women) were diagnosed with cancer, other than non-melanoma skin cancer which was omitted (ICD-10 C00–C97 except C44). 1,651 subjects were diagnosed with colorectal cancer (1,035 men and 617 women) (ICD-10 C18–C20); 2,769 women were diagnosed with breast cancer (ICD-10 C50) and 3,220 men with prostate cancer (ICD-10 C61).
The number of healthy lifestyles followed by participants was inversely associated with the risk of cancer (Table 1). The HR comparing subjects, who followed five healthy behaviours with those who followed none or one healthy behaviour, was 0.68 (95% CI 0.63–0.74). The results for men and women were 0.74 (95% CI 0.66–0.84) and 0.55 (95% CI 0.48–0.64), respectively. Following a healthy lifestyle was also associated with a lower risk of colorectal cancer for men and women (HR 0.75; 95% CI 0.58–0.97) and breast cancer for women (HR 0.65; 95% CI 0.52–0.83). However, in contrast to the protective effect of healthy lifestyle behaviours on all cancers, a healthy lifestyle was associated with an increased risk of prostate cancer for men (HR 1.40; 95% CI 1.16–1.69).
Modelling the number of healthy behaviours as a continuous variable, the HR (95% CI) for all cancers was (HR 0.92 CI 0.91–0.94), i.e., each additional healthy behaviour was independently associated with an average of 8% reduction in risk for all cancers. The HR (95% CI) for colorectal cancer for men and women associated with an extra healthy behaviour was 0.91 (0.87–0.95), and 0.91 (0.87–0.94) for breast cancer for women. For prostate cancer, each additional healthy behaviour increased the risk on average by 6% (HR 1.06; 95% CI 1.03–1.10).
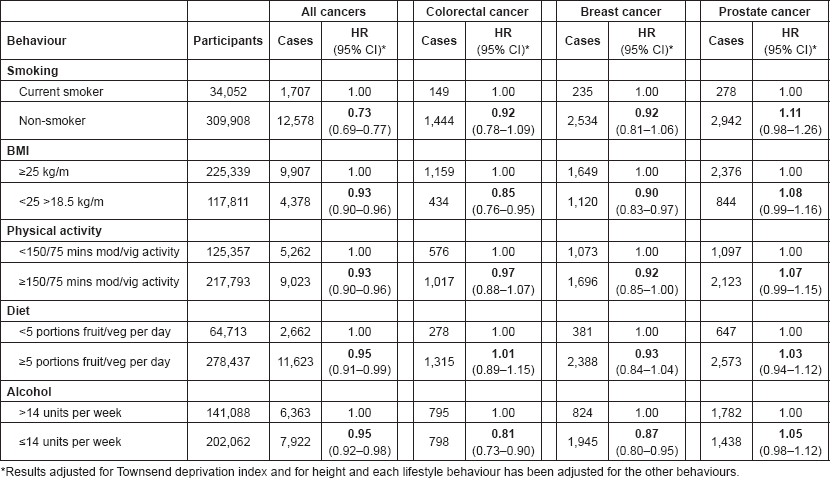
Table 2 shows the separate associations between each healthy behaviour and cancer. Each behaviour was associated with a decreased risk of all cancers, compared to not following the healthy behaviour. Being a non-smoker, compared to current smoking, showed the greatest reduction in risk (HR 0.73; 95% CI 0.69–0.77). A healthy BMI and low alcohol intake were both associated with a lower risk of colorectal cancer and breast cancer, and physical activity also seemed to be associated with a lower risk of breast cancer. For prostate cancer, each healthy behaviour seems to suggest an increased risk of prostate cancer.
Table 1. Association between number of healthy behaviours and the risk of all cancers, colorectal cancer, breast cancer and prostate cancer in UK Biobank.

Discussion
With almost half a million subjects, UK Biobank is one of the largest and most detailed studies ever set up [6, 11]. Extensive health and social data were obtained and agreement was obtained from all the subjects for their medical records to be followed indefinitely. The population response to UK Biobank was low [12], and although valid estimates of the prevalence of the behaviours, or the incidence of disease cannot be made with confidence, estimates of association between these can be made with a reasonable degree of confidence [13].
In addition to a low response rate, other limitations include the fact that the definitions of some of the behaviours was limited, and each behaviour was used in the analysis as a simple dichotomy, defining ‘healthy’ and ‘unhealthy’ with no attempt to use quantitative data. Furthermore, ‘smoking’ took no account of ‘ex-smoking’, and ‘diet’ ignored the consumption of foods other than fruit and vegetables.
Table 2. Association between each healthy behaviour and the risk of all cancers, colorectal cancer, breast cancer and prostate cancer in UK Biobank.
The data on incident cancer, which are summarised here, have accumulated over a period of only five years yet they present a powerful message on the benefits of a healthy lifestyle. Overall, the analyses give unequivocal evidence of a substantial reduction in cancer associated with the following of a healthy lifestyle. The overall risk of cancer is reduced in the subjects who claimed to be living a healthy lifestyle by about one-third (HR 0.68; 95% CI 0.63–0.74) and all the five healthy behaviours appear to contribute to this overall reduction.
This study and these data therefore enrich the known benefits of a healthy lifestyle and increase the responsibility with which healthcare professionals have to communicate the benefits of healthy behaviours widely, and with conviction [14, 15]. The ability of clinicians to ‘follow the science’, and of patients to exercise ‘rational’ choice is, of course, circumscribed by the contexts – social, cultural, economic and others – in which both live, and usually requires a variety of appropriate ‘nudges’ and support [16].
Our findings concur with those of many other studies. The EPIC research team defined a healthy lifestyle as non-smoking, regular physical activity, moderate alcohol intake and they used as a surrogate for a healthy diet, plasma vitamin C level at baseline. Within the total cohort of one-third of a million subjects in nine European countries, they reported that compared with subjects who followed no healthy behaviour, those who followed all four behaviours had a relative risk of death of 0.25 (95% CI 0.18–0.34), and a relative risk of cancer of 0.27 (95% CI 0.17–0.43) [17]. In the 35-year follow-up of the Caerphilly cohort, healthy living, based on the consistent following of four or five of the behaviours, was associated with a reduction in death (HR 0.40; 95% CI 0.24–0.67), a one-third reduction and a six-year delay in the development of cancer [5].
We also report a 25% reduction in colorectal cancer (HR 0.75; 95% CI 0.58–0.97) and a 35% reduction in breast cancer (HR 0.65; 95% CI 0.52–0.83) (Table 2), and these results are in reasonable conformity with other published data. Thus, Romaguera et al [17] reported a reduction of 27% (HR 0.73; 95% CI 0.65–0.81) for colorectal cancer, and about a 15% reduction for breast cancer (HR 0.84 CI 0.78–0.90). In a 24-year follow-up of 81,000 women in the US Nurses’ Health Study, women who followed a healthy lifestyle had an HR for colon cancer of 0.55 (95% CI 0.35–0.87) [18]. Kabat et al [19] reported reductions of approximately 35–50% for colon and rectal cancer for men and women and approximately 20% for breast (HR 0.81; 95% CI 0.76–0.87) in a cohort of half a million subjects, and Makarem [20] reported non-significant reductions in colorectal cancer associated with healthy living (HR 0.80; CI 0.64–1.01), and breast cancer (HR 0.87; 95% CI 0.74–1.03).
Our results for the association of healthy living with prostate cancer differ greatly from the results for the other cancers examined, showing a significant increase of about 40% (Table 1). Similar inconsistencies appear to be shown within much of the published literature and although the associations are generally small and non-significant, many suggest a possible increase in risk for prostate, but not other cancers [17, 19, 20]. A few authors, however, report reductions associated with healthy living, but their studies are based on fatal prostate cancer alone [21], or selected subjects with aggressive [22] or advanced cancer [23], or in studies conducted before the prostate specific antibody (PSA) test was introduced [24, 25].
A likely explanation of the increase in prostate cancer in subjects following the healthy behaviours appears to be an effect of screening for prostate cancer. All health screening checks are taken up inequitably, a wide range of beliefs, social, health and lifestyle characteristics being associated with differential service use [26]. The PSA test is likely to be especially sensitive to selection bias from these factors, because it has been developed relatively recently, its value is disputed [27] and it has not been promoted within the UK.
In fact, the issue of possible biases arising from an unequal uptake of the PSA test has been examined within UK Biobank, and it has been shown that a number of socio-demographic, lifestyle and health characteristics, which are predictive for prostate cancer, also define men likely to seek PSA testing [28]. In another study, it has been shown that PSA testing advances a diagnosis of prostate cancer by an average of about 11 years [29]. Selection bias is therefore likely with those who seek PSA testing being those with better lifestyles because they generally care about their health, and PSA testing increasing the likelihood of being diagnosed with a cancer that would otherwise go undetected for on average another 11 years.
It seems likely therefore that the relatively recent availability of PSA testing has distorted what is most probably a reduction in prostate cancer by healthy behaviours, in line with the relationships of the other cancers. While a similar bias from screening is likely to occur in colon and breast cancer, the relevant screening tests were introduced very much earlier and are vigorously promoted in the UK, making selective uptake of the tests much less likely than is the case with the PSA. On the other hand, it could simply be that prostate cancer is less sensitive to lifestyle behaviours, but we are not aware of any data supportive of this explanation.
A similar occurrence of this bias was reported from US Physicians, in whom aspirin-taking was associated with a reduction in prostate cancer (HR 0.59; 95% CI 0.43–0.81) ‘before the PSA era’, while ‘during the PSA era’ the association with aspirin was lost (HR 1.01; 95% CI 0.60–1.69) [24]. A further example was reported by Islami et al [24], who found smoking to be associated with an increase in prostate cancer, but only in studies published ‘before the prostate-specific antigen screening era’.
In an evaluation of healthy living, it is essential to keep in mind that there are large reductions in diseases other than cancer, including diabetes [5, 30], vascular disease [5, 31, 32] and dementia [5, 33]. The bottom line on healthy behaviours is that they are of enormous relevance to the overall preservation of health and to survival, and not just to the prevention of cancer or any other single disease.
The magnitude of these benefits of healthy living make a healthy lifestyle extremely attractive, but the responsibility for its maintenance lies ultimately with the subjects themselves [15], and the evidence is that relatively few people follow the healthy behaviours consistently. Thus in 1979, within a cohort of middle-aged men in the UK, only 5% were judged to follow a healthy lifestyle while 40% neglected all or most of the healthy behaviours [5], yet 30 years later these proportions had hardly changed [34].
Perhaps, advice to take up one additional healthy behaviour is the most acceptable message for most subjects. In the present study, each additional healthy behaviour was associated with a reduction of about 8% in cancer, independendent of the effects of the other behaviours. Kirkegaard et al [35] reported that for each additional recommendation met, there was a lower risk of colorectal cancer of about 13% (95% CI 4–22%). In another study, a one-point increment in the healthy living score was a 5% (95% CI 3–7%) reduction in total cancer [18]. The authors of the long-term study in Caerphilly reported that had the 2,500 men in their cohort each been urged at baseline to adopt one additional healthy behaviour (any one), and if only half them had complied, then during the following 35 years there would have been a 13% reduction in dementia, a 12% drop in diabetes, 6% less vascular disease and a 5% reduction in total mortality [5].
Elsewhere it is urged that health practitioners should ‘make every contact count’ [36] and screening procedures for cancer provide a most suitable ‘teachable moment’ to urge, encourage, nudge and challenge subjects to consider their lifestyle [16, 37, 38]. In a randomised trial, advice on the healthy behaviours given to subjects within a colorectal screening project led to an increased uptake of a healthy diet and an increase in physical activity [39].
Conclusions
This study adds powerful evidence to the literature showing large health benefits from healthy behaviours (non-smoking, a low BMI, regular physical activity, a healthy diet and a low alcohol intake). These all contribute to a total reduction of about one-third in cancer risk and possibly a greater reduction in cancer mortality.
The reduction in cancer risk, and cancer mortality, taken together with the reductions in diabetes, vascular disease and dementia, which are not presented in detail here, indicate that healthy living is, as the authors of one paper put it: ‘Better than any pill – and no side effects’![5]
Conflicts of interest
All the authors declare that they have no conflict of interest.
Author’s contributions
All of the authors contributed to the conception and design of the study and the interpretation of the data. AW analysed the data, and PCE and GM drafted the manuscript. All the authors gave the final approval for the manuscript to be published.
Funding
No special funding was sought for the work described in this paper.
Acknowledgments
We are grateful to the organisers of UK Biobank for access to the data for this study.
Reference
1. Ahmad AS, Ormiston-Smith N, and Sasieni PD (2015) Trends in the lifetime risk of developing cancer in Great Britain: comparison of risk for those born from 1930 to 1960 Br J Cancer 112(5) 943–947 https://doi.org/10.1038/bjc.2014.606 PMID: 25647015 PMCID: 4453943
2. Office for National statistics (2013) Cancer Registration Statistics
3. Khaw KT, Wareham N, and Bingham S, et al (2008) Combined impact of health behaviours and mortality in men and women: the EPIC Norfolk Prospective population study PLOS Med 5(1) e12 https://doi.org/10.1371/journal.pmed.0050012 PMID: 18184033 PMCID: 2174962
4. Ford ES, Bergmann MM, and Kroger J, et al (2009) Healthy living is the best revenge: findings from the European prospective investigation into cancer and nutrition - Potsdam study Arch Intern Med 169 1355–1362 https://doi.org/10.1001/archinternmed.2009.237 PMID: 19667296
5. Elwood P, Galante J, and Pickering J, et al (2013) Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the Caerphilly cohort study PLOS ONE 8(12) e81877 https://doi.org/10.1371/journal.pone.0081877 PMID: 24349147 PMCID: 3857242
6. Sudlow C, Gallacher J, and Allen N, et al (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age PLoS Med 12(3) e10017797 https://doi.org/10.1371/journal.pmed.1001779
7. World Health Organization (1992) International Statistical Classification of Diseases and Related Health Problems 10th edn (Geneva: World Health Organization)
8. NHS Guidelines for Physical Activity http://www.nhs.uk/Livewell/fitness/Pages/physical-activity-guidelines-for-adults.aspx
9. NHS Guidelines for Alcohol Intake https://www.drinkaware.co.uk/alcohol-facts/alcoholic-drinks-units/latest-uk-alcohol-unit-guidance/
10. Mackenbach JP (1988) Health and Deprivation: Inequality and the North eds P Townsend, P Phillimore, and A Beattie (London: Croom Helm)
11. Allen N, Sudlow C, and Downey P, et al (2013) UK Biobank: current status and what it means for epidemiology Health Policy and Technology 1(3) 123–126 https://doi.org/10.1016/j.hlpt.2012.07.003
12. Watts G (2007) UK Biobank gets a 10% response rate as it starts recruiting volunteers BMJ 334 659 https://doi.org/10.1136/bmj.39167.407616.DB
13. Elwood JM (2013) Comment on ‘Why representativeness should be avoided’ Int J Epidemiol 42 1014–1015 https://doi.org/10.1093/ije/dyt101 PMID: 24062288
14. World Health Organisation (1986) The Ottawa Charter for Health Promotion. First International Conference on Health Promotion, Ottawa
15. Elwood PC and Longley M (2010) My health: whose responsibility? a jury decides J Epidemiol Community Health 64 761–764 https://doi.org/10.1136/jech.2009.087767
16. R. Thaler and C. Sunstein (2008) Nudge: Improving Decisions About Health, Wealth, and Happiness (Penguin Books)
17. Romaguera D, Vergnaud AC, and Peeters PH, et al (2012) Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? results from the EPIC study Am J Clin Nutr 96 150–163 https://doi.org/10.3945/ajcn.111.031674 PMID: 22592101
18. Erdrich J, Zhang X, and Giocannucci I, et al (2015) Proportion of colon cancer attributable to lifestyle in a cohort of US women Cancer Causes Control 26(9) 1271–1279 https://doi.org/10.1007/s10552-015-0619-z PMID: 26092381 PMCID: 4545459
19. Kabat GC, Matthews CE, and Kamensky V, et al (2015) Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study Am J Clin Nutr 101 558–569 https://doi.org/10.3945/ajcn.114.094854 PMID: 25733641 PMCID: 4340061
20. Makarem N, Lin Y, and Bandera EV, et al (2015) Concordance with World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines for cancer prevention and obesity-related cancer risk in the Framingham Offspring cohort (1991-2008) Cancer Causes Control 26(2) 277–286 https://doi.org/10.1007/s10552-014-0509-9 PMID: 25559553 PMCID: 5062601
21. Kenfield, S. A., Batista JL, and Jan JL, et al (2015) Development and application of a lifestyle score for prevention of lethal prostate cancer J Natl Cancer Inst 108(3) https://doi.org/10.1093/jnci/djv329 PMID: 26577654
22. Zu K and Giovannucci E (2009) Smoking and aggressive prostate cancer: a review of the epidemiologic evidence Cancer Causes Control 20(10) 1799–1810 https://doi.org/10.1007/s10552-009-9387-y PMID: 19562492
23. World Cancer Research Fund International: Continuous Update Project (2014) Diet, nutrition physical activity and prostate cancer
24. Islami F, Moreira DM, and Boffetta P, et al (2014) A systematic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies Eur Urol 66 1054–1064 https://doi.org/10.1016/j.eururo.2014.08.059 PMID: 25242554 PMCID: 4566150
25. Downer MK, Allard CB, and Preston MA, et al (2017) Regular aspirin use and the risk of lethal prostate cancer in the physicians health study Eur Urol 72(5) 821–827 https://doi.org/10.1016/j.eururo.2017.01.044 PMID: 28189429
26. Dryden R, Williams B, and McCowan C, et al (2012) What do we know about who does and does not attend general health checks? Findings from a narrative scoping review BMC Public Health 12 723 https://doi.org/10.1186/1471-2458-12-723 PMID: 22938046 PMCID: 3491052
27. Loughlin KR. How reliable is the prostate-specific antigen (PSA) test with it comes to detecting prostate cancer? http://www.harvardprostateknowledge.org/is-psa-reliable
28. Littlejohns TJ, Travis RC, and Key TJ, et al (2016) Lifestyle factors and prostate-specific antigen (PSA) testing in UK Biobank: implications for epidemiological research Cancer Epidemiol 45 40–46 https://doi.org/10.1016/j.canep.2016.09.010 PMID: 27693812 PMCID: 5147810
29. Tornblom M, Eriksson H, and Franzen S, et al (2004) Lead time associated with screening for prostate cancer Int J Cancer 108 122–129 https://doi.org/10.1002/ijc.11554
30. Hu FB, Manson JE, and Stampfer MD, et al (2001) Diet, lifestyle and the rise of type 2 diabetes in women N Engl J Med 345 790–797 https://doi.org/10.1056/NEJMoa010492 PMID: 11556298
31. Chiuve SE, McCullough ML, and Sacks FM, et al (2006) Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and non-users of lipid lowering and antihypertensive medications Circulation 114(2) 160–167 https://doi.org/10.1161/CIRCULATIONAHA.106.621417 PMID: 16818808
32. Kvaavik E, Batty D, and Ursin G, et al (2010) Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey Arch Intern Med 170 711–718 https://doi.org/10.1001/archinternmed.2010.76 PMID: 20421558
33. Sabia S, Nabi H, and Kivimaki M, et al (2009) Health behaviours from early to late mid life as predictors of cognitive function: the Whitehall II study Am J Epidemiol 170 428–437 https://doi.org/10.1093/aje/kwp161 PMID: 19574344 PMCID: 2727179
34. Anonymous (2010) Welsh Health Survey 2009 http://gov.wales/statistics-and-research/welsh-health-survey/?lang=en
35. Kirkegaard et al (2010) Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study BMJ 341 c5504 https://doi.org/10.1136/bmj.c5504 PMID: 20978063 PMCID: 2965150
36. NHS England http://www.makingeverycontactcount.co.uk/
37. Anderson AS, Mackison D, and Boath C, et al (2013) Promoting changes in diet and physical activity in breast and colorectal cancer screening settings: an unexpolored opportunity for endorsing healthy behaviours Cancer Prev Res 6 165–172 https://doi.org/10.1158/1940-6207.CAPR-12-0385
38. Ligibel JA, Alfano CM, and Courneya KS, et al (2014) American society of clinical oncology positive statement on obesity and cancer J Clin Oncol 32(31) 3568–3574 https://doi.org/10.1200/JCO.2014.58.4680 PMID: 25273035 PMCID: 4979237
39. Robb KA, Power E, and Kralj-Hans I, et al (2010) The impact of individually tailored lifestyle advice in the colorectal cancer screening context: a randomised pilot study in North West London Preventive Medicine 51 505–508 https://doi.org/10.1016/j.ypmed.2010.10.002






