High-risk gastrointestinal stromal tumour (GIST) and synovial sarcoma display similar angiogenic profiles: a nude mice xenograft study
Francisco Giner1, Isidro Machado3, Jose Antonio Lopez-Guerrero2, Empar Mayordomo-Aranda1 and Antonio Llombart-Bosch1
1Department of Pathology, Universitat de València Estudi General (UVEG), València 46010, Spain
2Laboratory of Molecular Biology, Fundación Instituto Valenciano de Oncología (FIVO), Valencia 46009, Spain
3Department of Pathology, Fundación Instituto Valenciano de Oncología (FIVO), Valencia 46009, Spain.
Correspondence to: Francisco Giner. E-mail: francescginer@hotmail.com
Abstract
Background: Gastrointestinal stromal tumour (GIST) is the most common primary mesenchymal tumour of the gastrointestinal tract. Spindle cell monophasic synovial sarcoma (SS) can be morphologically similar. Angiogenesis is a major factor for tumour growth and metastasis. Our aim was to compare the angiogenic expression profiles of high-risk GIST and spindle cell monophasic SS by histological, immunohistochemical and molecular characterisation of the neovascularisation established between xenotransplanted tumours and the host during the initial phases of growth in nude mice.
Methods: The angiogenic profile of two xenotransplanted human soft-tissue tumours were evaluated in 15 passages in nude mice using tissue microarrays (TMA). Tumour pieces were also implanted subcutaneously on the backs of 14 athymic Balb-c nude mice. The animals were sacrificed at 24, 48, and 96 h; and 7, 14, 21, and 28 days after implantation to perform histological, immunohistochemical, and molecular studies (neovascularisation experiments).
Results: Morphological similarities were apparent in the early stages of neoplastic growth of these two soft-tissue tumours throughout the passages in nude mice and in the two neovascularisation experiments. Immunohistochemistry demonstrated overexpression of pro-angiogenic factors between 24 h and 96 h after xenotransplantation in both tumours. Additionally, neoplastic cells coexpressed chemokines (CXCL9, CXCL10, GRO, and CXCL12) and their receptors in both tumours. Molecular studies showed two expression profiles, revealing an early and a late phase in the angiogenic process.
Conclusion: This model could provide information on the early stages of the angiogenic process in monophasic spindle cell SS and high-risk GIST and offers an excellent way to study possible tumour response to antiangiogenic drugs.
Keywords: GIST, synovial sarcoma, angiogenesis, nude mice xenograft, chemokines
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (
Published: 09/03/2017; Received: 20/10/2016
Introduction
High-grade sarcomas can be implanted into immunodeficient mice, where they grow as xenografts supported by the murine stroma blood supply [1]. In general, transplantations of low-grade tumours fail to establish, with successful xenografts deriving mainly from aggressive neoplasms. Although some properties of the original tumours may not be fully represented, xenografts have become very useful models for preclinical experiments in cancer research [2–5].
In recent years, much research has focused on the role of angiogenesis in tumour development, growth, invasion, and metastasis [6, 7]. It has become clear that tumour angiogenesis is the result of an imbalance between proangiogenic and antiangiogenic factors, the threshold of change in favour of proangiogenesis is considered to be the angiogenic switch. Several angiogenic factors and chemokines related with angiogenic mechanisms have been studied in different tumour types [6, 8, 9]. We recently communicated these findings in a nude mice osteosarcoma model [10] as well as in two high-grade chondrosarcomas (in press) [11]. The angiogenic process presents two different phases of tumour growth. An initial induction phase, in which new unstable vessels are built, followed by a remodelling phase, in which blood vessels are stabilised [12]. At this point, hypoxia occurs and the angiogenic process is activated through the well-known hypoxia-inducible transcription factors (HIF) that induce the expression of several tumour-derived cytokines, such as vascular endothelial growth factors (VEGF) or fibroblast growth factors (FGF) [6] and some chemokines (GRO, CXCL9, and CXCL10) with their respective receptors (CXCR2 and CXCR3) [8, 13, 14]. More recently, the CXCL12/CXCR4 axis was reported to be involved in mediating tumour cell invasion and proliferation and to play an important role in tumour angiogenesis, progression, and metastasis [15, 16]. Moreover, CXCR4 expression has been associated with poor survival in bone and soft-tissue sarcomas [17, 18] and many types of carcinomas [19]. Consequently, considerable interest has been generated in the therapeutic potential of targeting the growth of new vessels (antiangiogenesis) and the capacity to control those that have already been formed (vascular targeting) [13, 20].
Soft-tissue sarcomas are an infrequent group of mesenchymal tumours, they may be high grade and display poor survival [21]. Gastrointestinal stromal tumour (GIST) is the most common primary mesenchymal tumour of the gastrointestinal tract and spans a clinical spectrum from benign to malignant; most cases contain KIT- or PDGFRA-activating mutations [22]. Mutations in different genes may also be present, [23] although the main prognostic factors are tumour size, mitotic activity, location and capsular invasion [24, 25]. Targeted therapy with imatinib is indicated for high-risk cases and in advanced disease [23].
Synovial sarcoma (SS) is a mesenchymal tumour with a variable degree of epithelial differentiation and a specific chromosomal translocation t(X;18)(p11;q11) that leads to the formation of an SS18–SSX fusion gene. The differential diagnosis with a high-grade GIST may be difficult [26, 27]. Both tumour types cause metastasis and display an aggressive behaviour, suggesting that molecular reorganisations such as of SYT–SSX gene translocation and KIT mutations might be similarly essential for the growth of angiogenic factors. A recent publication described an intra-abdominal monophasic spindle cell SS that mimicked the morphology and immunohistochemistry of a high-risk spindle cell GIST [27].
Several animal models have been used in the study of tumour angiogenesis [12, 14, 28]. Studying angiogenesis through a xenograft model in high-grade sarcomas such as high-risk GIST and synovial sarcomas (SS) may provide a better understanding of this process and increase information regarding potential candidates for effective targeted therapy.
We developed a xenograft nude mice model to clarify the presence of angiogenic factors within the neoformed peritumoral stroma and in the internal tumour blood supply, during the early stages of tumour growth after the transfer into the subcutaneous tissue of the host. To this end, we used two previously established xenotransplanted tumour cell lines of human sarcomas: a high-risk spindle cell GIST and a monophasic spindle cell SS [22, 29].
Our aim was to characterise the markers associated with vasculogenesis using histology, immunohistochemistry, and molecular techniques and to search for similarities that may exist between the two tumours.
Materials and methods
Samples
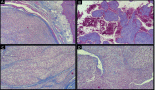
Samples were collected from patients treated at the Hospital Clínic Universitari de Valencia. The GIST came from a 63-year-old male with a gastric mass of approximately 26 × 20 × 35 cm diagnosed as a high-risk spindle cell tumour (Figure 2A). Firstly, the GIST was treated with imatinib (400 mg/day) for six months. The tumour responded partially to targeted therapy and finally resection of the mass was decided upon seven months after diagnosis. No metastasis was seen at the moment of diagnosis, but the patient died of various surgical complications after resection.
The SS came from a 32-year-old male who attended our hospital with a relapse in the right thigh and multiple lung metastases after chemo-radiotherapy. The tumour was approximately 10 × 8 × 8 cm and was diagnosed as monophasic spindle-cell SS, the patient died of tumour progression several months after diagnosis.
Molecular biology studies revealed genomic alterations in both tumours. The GIST had the KIT gene mutation and the SS had the typical translocation t(X,18)(SYT-SSX).
The tumours were collected for histopathological, ultrastructural, and genetic characterisation at our Pathology Department. The original tumours were transferred subcutaneously on the backs of nude mice (Nu407 and Nu335) and maintained for several generations (passages). In both tumours, we divided the passages into three time periods, early passages (from 1st to 5th passage), middle passages (from 6th to 10th passage) and late passages (from 11th to 15th passage). We calculated the average speed of tumour growth in both nude mice passages (Nu335 and Nu407) according to the formula (15 mm/days to next passage), 15 mm being the approximate tumour size when mice were sacrificed.
Tumour pieces 3–4 mm in size from the early passages of Nu407 and Nu335 were also xenografted subcutaneously on the backs of two sets of athymic Balb-c nude mice (n = 14 each). The animals were sacrificed at 24, 48, and 96 h; and 7, 14, 21, and 28 days after implantation (neovascularisation experiments). Tissue samples were fixed in 10% formaldehyde, paraffin-embedded, and haematoxylin and eosin (H&E) staining was performed for histological analysis. Moreover non-fixed samples were collected for molecular analysis. Approval for animal experimentation was obtained from the Ethics Committee of the Universitat de València Estudi General (UVEG).
Assembly of TMAs
Tissue microarrays were constructed using a Manual Tissue Arrayer (Beecher Instruments, Sun Praire, WI). Two cores (1 mm thick) of each sample were included, with additional cores in cases with diverse morphologic areas. The TMA contained normal tissue controls, original tumour, and the corresponding xenograft passages. The cores were grouped into early transfers 1–5, middle passages 6–10, and late passages 11–15. After assembly, an initial section from each TMA was stained with haematoxylin–eosin to evaluate the viability of the samples. Several 5-mm sections were also prepared for immunohistochemical (IHC) staining. Table 1 summarises the antibodies used for the IHC.
Immunohistochemistry
IHC was carried out by an indirect peroxidase method on paraffin sections following the same methodology, as we discussed in our previous papers [10] and [11].
Molecular biology
RNA was extracted from 50 to 200 mg of tumour samples obtained from the NU335 and Nu407 series.
The whole methodology and studied genes (Table 1S) are also discussed in our previous papers [10] and [11].
Results
Histological, immunohistochemical and ultrastructural characterisation
Comparing speed of growth in murine passages, we observed a similar average growth speed in SS (0.142 mm/day) and in GIST (0.103 mm/day)(Figure 1).
Table 1. Antibodies used in the experiences.
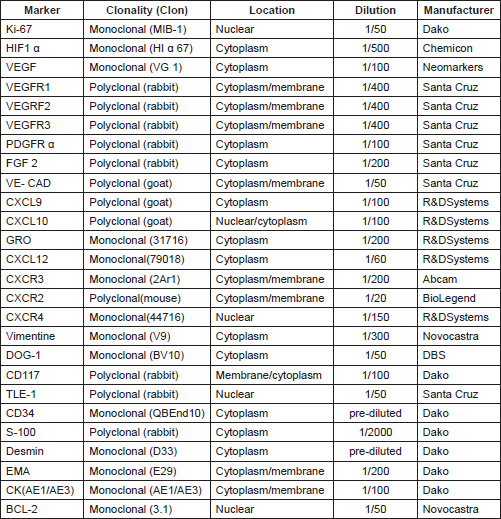
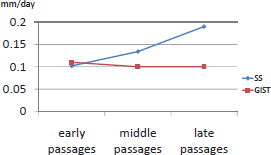
Figure 1. Graphics of speed of growth in both tumours throughout the passages in nude mice.
TMAs
No morphological changes were observed between passages in GIST; however, a high number of mitoses were clearly observed in the passages in both tumours (Figures 2B and 2F). The IHC study of GIST showed intense expression of vimentin, CD117, DOG1, desmin, and CD34 (Figures 2C and 2D) and was negative for PDGFRα and S-100. Ki67 was expressed in 15% of tumour cells in all cores (Figure 2E).
The IHC study of SS showed positivity for EMA (Figure 2G), cytokeratin (AE1/AE3) and bcl-2. Intense positivity was also revealed for vimentin and weak expression for TLE1 in all passages. Ki67 increased slightly over the passages, being positive in 20% of tumour cells in the last passages (Figure 2H), whereas with GIST it was more constant.
Double-immunofluorescence staining demonstrated that chemokine ligand expression in general was slightly higher in the xenograft passages than in the original tumour (Figure 3). There were very few differences between the two sarcomas with regard to chemokine expression profile. CXCL10 was constantly high in both tumours and GRO was mildly expressed in all passages (Figures 4A, 4D, and 4E). CXCL9 increased in both tumours over the passages (Figure 4B). Their receptors CXCR2 and CXCR3 were constantly expressed in all passages, with CXCR2 presenting a higher expression in SS. Finally, the CXCL12/CXCR4 axis was constantly overexpressed in all passages in both tumours (Figures 4C and 4F).
Neovascularisation experiments
In our neovascularisation experiments, during the first hours after xenografting, peritumoral haemorrhagic areas with inflammatory infiltration compounded by lymphocytes, plasma cells, neutrophils, karyorrhectic, and apoptotic figures were observed in SS and GIST. Small capillaries surrounded the xenograft associated with mesenchymal angioblastic and non-angioblastic cells included in a loose matrix.
Patchy hypoxic necrosis in SS appeared within the first 96 h after implantation, reaching a peak extension in the third week. The SS presented characteristic adipose tissue infiltrate and peritumoral skeletal muscle mouse fibres, but without the pseudocapsule observed in GIST. In GIST, the massive necrosis appeared during the first week, earlier than in the SS. After the fourth week, the histological picture of the GIST was re-established, with features similar to those of the human control, re-establishing also the amount of mitoses and the remission of necrosis, which became patchy and scant. During the third week after xenografting, the inflammatory component decreased. At this time, newly formed capillary vessels were remodelled and penetrated or sprouted into the tumour.
Areas of massive necrosis were associated with a lower proliferative index in both tumours. Ki67 was lower in the early stages after tumour xenografting in both tumours. In the last weeks, the increase in Ki67 expression was also inversely correlated with HIF1α in both neoplasms.
Angiogenic factors represented by the VEGF family and their receptors presented a different expression profile in the two tumours. In SS, maximum VEGF positivity presented 24 h after implantation and was also expressed in the extracellular matrix, while VEGF positivity was lower in GIST and appeared 96 h after xenografting (Figure 2I). VEGFR2 presented a similar expression profile to its ligand, and VEGFR3 was the most positive receptor in both tumours. HIF1α expression was slightly higher and more constitutively expressed in SS than GIST
Double-immunofluorescence staining showed chemokine expression (CXCL9, CXCL10 and GRO) in the tumour cell cytoplasm/nucleus and deposited in the extracellular matrix. This was also the case for their receptors (CXCR3 and CXCR2) (Figures 4G and 4H). Chemokine ligand expression was higher during the first 48 h in GIST; however, it appeared later in SS where peak expression occurred during the first week. The CXCL12/CXCR4 axis showed an intense coexpression in both sarcomas at all times throughout the experience. Interestingly, we observed that murine peritumoral stroma expressed CXCR4 but not CXCL12 in the two tumour xenografts (Figure 4I). It is worth mentioning that the chemokine receptors were expressed more constantly at all times in comparison with their ligands.

Figure 2. (A) GIST with spindle-cell pattern (H&E, 20X). (B) High-risk GIST with some mitotic figures (H&E, 40X). (C) Intense positivity of DOG-1 in GIST, 40X. (D) Intense positivity of CD34 in GIST, 40X. (E) High proliferative Ki67 index in late passages of GIST, 40X. (F) Monomorphic spindle-cell pattern in SS (H&E, 40X). (G) Mild EMA positivity in SS, 40X. (H) High proliferative Ki67 index in late passages of SS, 40X. (I) Intense VEGF expression in SS 24 h after tumour implantation, 40X.
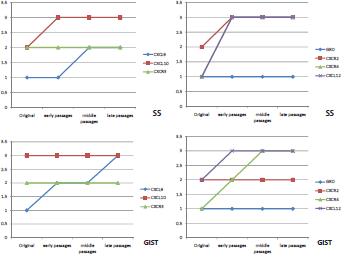
Figure 3. Graphics of chemokine ligand and receptor expression in both tumours throughout the passages in nude mice.
qRT-PCR low-density arrays of angiogenesis-related genes
Gene expression profiles (Figure 5) in GIST were similar at 24 h and 7 days but differed from those observed at 48 h, 14 and 21 days. However, SS expression profiles were similar at 24 h and 28 days, differing from those at 48 h and 14 days. In GIST, the early phase appeared 96 h after xenografting and was characterised by the overexpression of genes clearly involved in angiogenesis induction, including VEGF, PDGFA, PDGFB, VEGFC, and their receptors. In contrast, the earlier phase in SS occurred during the first week after xenografting. Finally, in GIST, the late phase of the angiogenic process (remodelling phase) appeared during the first week after xenografting, while in SS, this phase appeared later in the fourth week.
RNA samples corresponding to the third week of SS and to the fourth week of GIST were not viable for analysis.
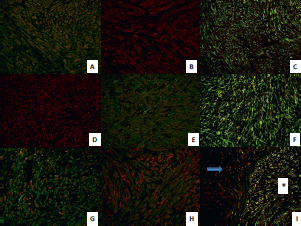
Figure 4. Immunofluorescent expression of chemokines and their receptors in GIST and SS throughout the early, middle, and late passages and in the neovascularisation experiment. (A) Double-immunofluorescence staining shows coexpression of chemokine ligand CXCL10 (red) and its receptor CXCR3 (green) in GIST tumour cells in early passages (40X). (B) Immunofluorescence staining shows expression of chemokine ligand CXCL9 (red) in GIST tumour cells in later passages (40X). (C) double-immunofluorescence staining shows coexpression of chemokine ligand CXCL12 (green) and its receptor CXCR4 (red) in GIST tumour cells in middle passages (40X). (D) Immunofluorescence staining shows expression of chemokine ligand GRO (red) in SS tumour cells in middle passages (40X). (E) double-immunofluorescence staining shows coexpression of chemokine ligand CXCL10 (red) and its receptor CXCR3 (green) in SS tumour cells in late passages (40X). (F) Double-immunofluorescence staining shows high coexpression of chemokine ligand CXCL12 (green) and its receptor CXCR4 (red) in SS tumour cells in late passages (40X). (G) Double-immunofluorescence staining shows coexpression of chemokine ligand GRO (red) and its receptor CXCR2 (green) in SS tumour cells 24 h after xenografting (40X). (H) Double-immunofluorescence staining shows coexpression of chemokine ligand GRO (red) and its receptor CXCR2 (green) in GIST tumour cells in the control tumour (40X). (I) Double-immunofluorescence staining shows coexpression of chemokine ligand CXCL12 (green) and its receptor CXCR4 (red) in GIST tumour cells two weeks after xenotransplantation (40X), observe the different expression between murine stroma (arrow) and tumour cells (asterisk).
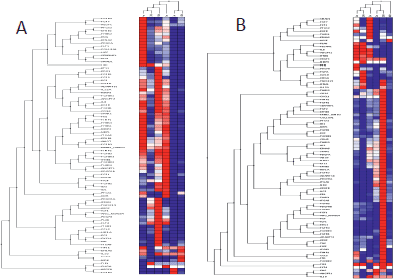
Figure 5. Cluster tree of genes related to angiogenesis by qRT-PCR obtained from the Nu335 (SS) (A) and Nu407 (GIST) (B) series using distance correlation and applying a linear correlation coefficient at different times. Overexpressed genes are shown in dark red, underexpressed genes in dark blue and no change in white. Samples from third week in SS, and fourth week in GIST were not available for analysis (Table 2S and 3S).
Table 1. Supplementary. Genes and assays included in the low-density array for the study of the expression of angiogenic factors by quantitative RT-PCR.
Table 2. Supplementary. 2-DDCt values corresponding to the Nu335 series.
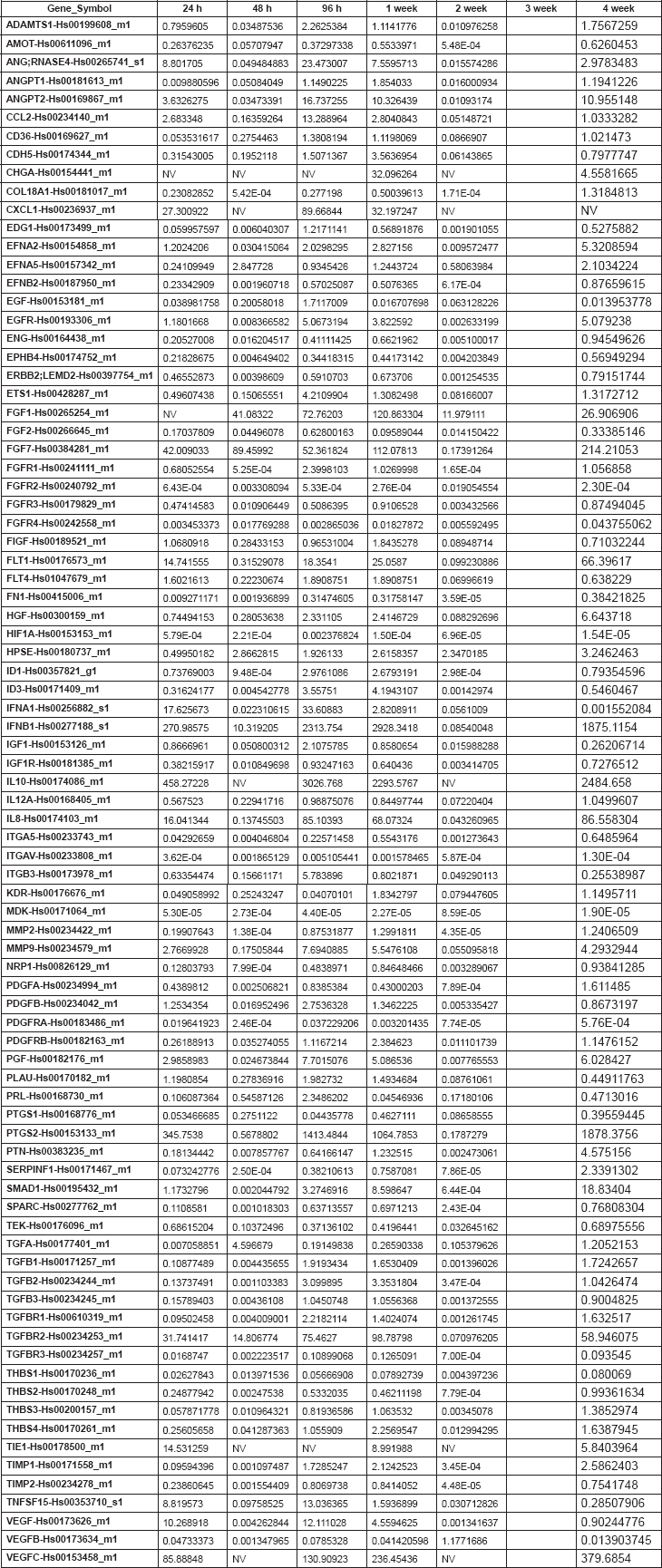
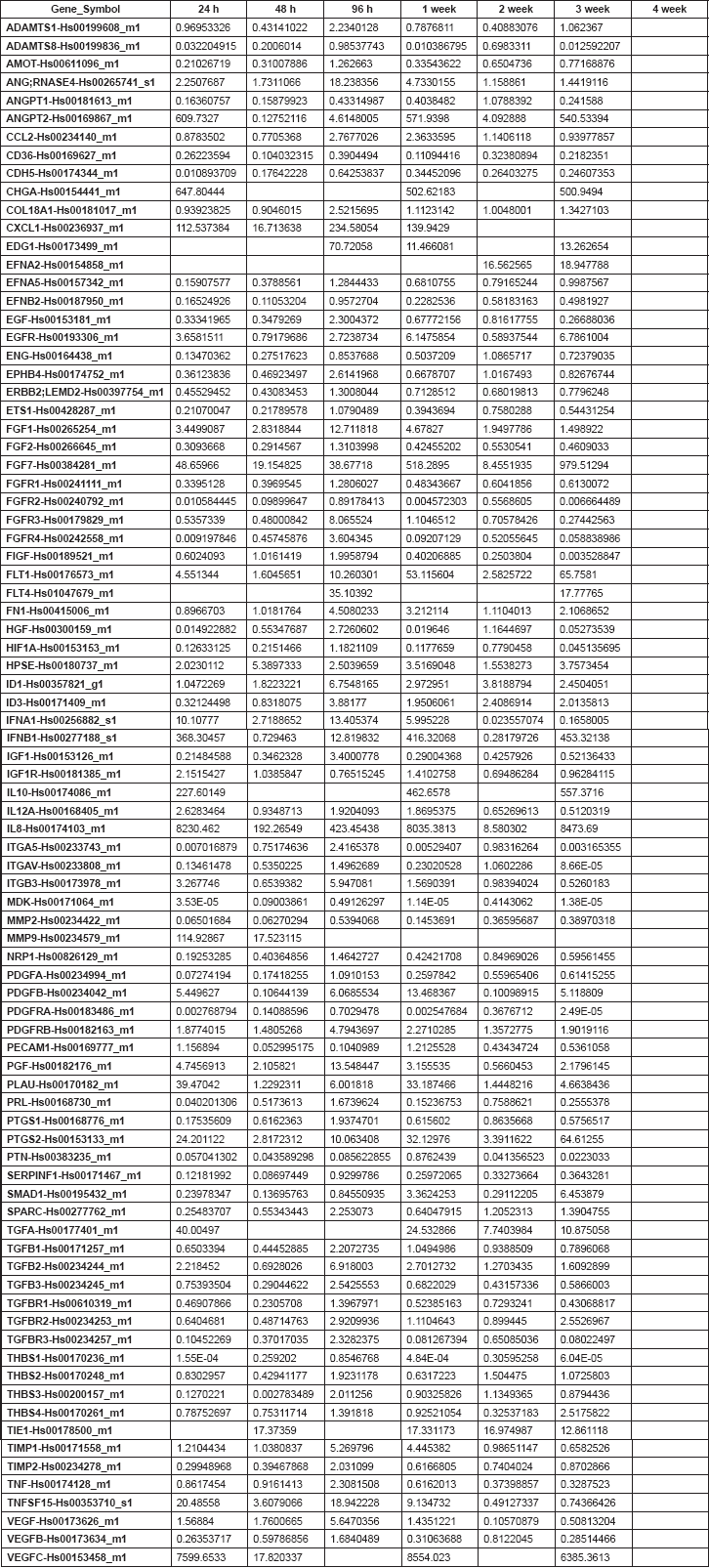
Table 3. Supplementary. 2-DDCt values corresponding to the Nu407 series.
Discussion
Angiogenesis is critical for the growth and metastasis of tumours. Early in tumorigenesis, an angiogenic switch is activated by hypoxia, promoting the expression of pro-angiogenic growth factors, such as the VEGF family, its receptors, and HIF1α among others [7]. Recent publications have highlighted the difficulties in differentiating between GIST and monophasic intra-abdominal SS, where molecular mutations are sometimes the only distinguishing feature [27]. It has been suggested that it would be particularly informative to explore possible relationships between the presence of vasculogenic structures and the response to antiangiogenesis therapy [30, 31]. Furthermore, it is interesting to speculate that antiangiogenic therapy may result in a selective growth advantage for cells exhibiting vasculogenic mimicry and vascular co-option, promoting drug-induced resistance [30, 31].
HIF1α is a principal regulator of cellular and systemic homeostatic response to hypoxia as it activates many genes, including those involved in angiogenesis [6]. In our model, HIF1α was overexpressed in the early stages, indicating that the angiogenic process is constitutively active in the xenotransplanted tumour. HIF1α plays an important role in angiogenic induction and remodelling phases and in an increased VEGF expression [32]. Some recent studies of GIST and chondrosarcoma have shown a correlation between the expression of angiogenic markers, such as VEGF and microvessel density, and a worse prognosis [33, 34], suggesting that the development of antiangiogenic chemotherapy might be useful. However, in other tumours, such as osteosarcoma, microvessel density seems to be associated with a longer overall and relapse-free survival [34, 35]. Imatinib continues to be used as a first-line medical treatment for advanced GIST, although resistance and non-response sometimes appear. Sunitinib and regorafenib, antiangiogenic drugs, are used as second- and third-line therapies, respectively, and are given in imatinib-resistant GIST cases [23]. Volumetric growth and the development of metastases in cases of GIST appear to be related to the development of a new vascular network [36]. The importance of vascularisation in the context of GIST is the action mechanism of the second-generation drug sunitinib, which is based on the blockade of VEGF activity along with tyrosine kinase receptor blockade that has been used with success in some GIST patients [37, 38].
Anthracyclines and ifosfamide, either alone or in combination, are the gold standard treatments for advanced SS [39]. However, after failure of conventional first-line cytotoxic chemotherapy, available treatment options are severely limited because of a high risk-to-benefit ratio in terms of patient tolerability and survival. Recently, it has been demonstrated that pazopanib is a feasible option for patients who have been heavily pre-treated for metastatic SS [40].
Cluster analysis performed on our qRT-PCR expression studies revealed two additional groups of genes clearly separated into two stages, corresponding to early angiogenic induction where VEGF and PDGF family genes among others play an important role, and the later remodelling phases where other angiogenic genes are overexpressed. Apparently the high-risk GIST behaved biologically as high-grade sarcoma in the passages and neovascularisation experiments similar to our previous molecular results [10]. However, induction and remodelling phases of SS appeared later than GIST and other high-grade bone tumours [10]. This difference may be related to a different sensibility and response to antiangiogenic drugs, with GIST being more sensitive. Nevertheless, we cannot be sure that this difference will have any biological translation.
In addition to angiogenic factors, chemokines also play an important role during angiogenic induction. The coexpression of ligands and chemokine receptors in neoplastic cells and extracellular matrix suggests that autocrine and paracrine stimulation by the tumour cells results in production of angiogenic factors in response to hypoxia during the first stages of tumour growth, as reported in other neoplastic and non-neoplastic conditions [8, 12, 30, 41]. Moreover, we found a correlation between high chemokine ligand expression and hypoxic necrosis in both tumours. Few studies of chemokines in SS and GIST have been made [42, 43]. CXCR4 expression has been related with poor prognosis in patients with bone and soft-tissue sarcomas in a meta-analysis [17]. High expression of CXCL12/CXCR4 was observed in all passages of both tumours and in the neovascularisation experiment, this could be related with their aggressive clinical behaviour. The CXCL12/CXCR4 axis is related to mediating tumour cell invasion and proliferation and plays an important role in tumour angiogenesis, progression and metastasis [44]. CXCL12/CXCR4 is overexpressed by tumour cells, but not by murine stromal peritumoral cells which only produce CXCR4. Perhaps CXCL12 induces murine stromal cells to generate new vessels in a paracrine effect and may be a good objective for targeted therapy to reduce tumour growth.
Conclusions
This model provides information on the early stages of the angiogenic process in monophasic spindle-cell SS and high-risk GIST. We suggest that different angiogenic molecular profiles could predict different biological and clinical behaviour and determine the response to antiangiogenic treatment. We also demonstrate the importance of chemokine expression as a therapeutic target of tumour growth.
The fact that angiogenesis is a dynamic, changing and multistep process over time should be taken into consideration when developing future therapeutic strategies in soft-tissue tumours.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by grants of the 6th FP of the EC: Prognosis and Therapeutic Targets in the Ewing Family of Tumors (PROTHETS), Contract number: 503036; and EuroBoNeT Network, contract number: 018814, and from the Fundación Instituto Valenciano de Oncología (FIVO), Valencia, Spain.
References
1. Kresse SH, Meza-Zepeda LA and Machado I et al (2012) Preclinical xenograft models of human sarcoma show nonrandom loss of aberrations Cancer 118 558–570 DOI: 10.1002/cncr.26276
2. Morton CL and Houghton PJ (2007) Establishment of human tumor xenografts in immunodeficient mice Nat Protoc 2 247–250 DOI: 10.1038/nprot.2007.25 PMID: 17406581
3. Marangoni E, Vincent-Salomon A and Auger N et al (2007) A new model of patient tumor-derived breast cancer xenografts for preclinical assays Clin Cancer Res 13 3989–3998 DOI: 10.1158/1078-0432.CCR-07-0078 PMID: 17606733
4. Fiebig HH, Maier A and Burger AM (2004) Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery Eur J Cancer 40 802–820 DOI: 10.1016/j.ejca.2004.01.009 PMID: 15120036
5. Garber K (2009) From human to mouse and back: ‘tumorgraft’ models surge in popularity J Natl Cancer Inst 101 6–8 DOI: 10.1093/jnci/djn481 PMID: 19116380
6. Carmeliet P and Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis Nature 473 298–307 DOI: 10.1038/nature10144 PMID: 21593862 PMCID: 4049445
7. Hanahan D and Weinberg RA (2011) Hallmarks of cancer: the next generation Cell 144 646–674 DOI: 10.1016/j.cell.2011.02.013 PMID: 21376230
8. Berghuis D, Santos SJ and Baelde HJ et al (2012) Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8( ) T-lymphocyte infiltration and affect tumour progression J Pathol 223 347–357 DOI: 10.1002/path.2819
9. Pinto S, Martinez-Romero A and O’Connor JE et al (2014) Intracellular coexpression of CXC- and CC- chemokine receptors and their ligands in human melanoma cell lines and dynamic variations after xenotransplantation BMC Cancer 14 118 DOI: 10.1186/1471-2407-14-118 PMID: 24559071 PMCID: 3943502
10. Giner F, Lopez-Guerrero JA and Machado I et al (2015) The early stages of tumor angiogenesis in human osteosarcoma: a nude mice xenotransplant model Virchows Arch 467 193–201 DOI: 10.1007/s00428-015-1791-y PMID: 26055533
11. Giner F, López-Guerrero JA and Machado I et al Expression profiles of angiogenesis in two high grade chondrosarcomas: a xenotransplant experience in nude mice, Hist Histopathol, in press.
12. Llombart-Bosch A, Lopez-Guerrero JA and Carda Batalla C et al (2003) Structural basis of tumoral angiogenesis Adv Exp Med Biol 532 69–89 DOI: 10.1007/978-1-4615-0081-0_8 PMID: 12908551
13. Link MP, Goorin AM and Miser AW et al (1986) The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity N Engl J Med 314 1600–1606 DOI: 10.1056/NEJM198606193142502 PMID: 3520317
14. DuBois S and Demetri G (2007) Markers of angiogenesis and clinical features in patients with sarcoma Cancer 109 813–819 DOI: 10.1002/cncr.22455 PMID: 17265525
15. Yoshitake N, Fukui H and Yamagishi H et al (2008) Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer Br J Cancer 98 1682–1689 DOI: 10.1038/sj.bjc.6604363 PMID: 18443596 PMCID: 2391124
16. Wendt MK, Johanesen PA and Kang-Decker N et al (2006) Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis Oncogene 25 4986–4997 DOI: 10.1038/sj.onc.1209505 PMID: 16568088 PMCID: 4610155
17. Li YJ, Dai YL and Zhang WB et al (2015) Clinicopathological and prognostic significance of chemokine receptor CXCR4 in patients with bone and soft tissue sarcoma: a meta-analysis Clin Exp Med PMID: 26678086
18. Sand LG, Scotlandi K and Berghuis D et al (2015) CXCL14, CXCR7 expression and CXCR4 splice variant ratio associate with survival and metastases in Ewing sarcoma patients Eur J Cancer 51 2624–2633 DOI: 10.1016/j.ejca.2015.08.020 PMID: 26428435
19. Dahl O, Fluge O and Carlsen E et al (2009) Final results of a randomised phase III study on adjuvant chemotherapy with 5 FU and levamisol in colon and rectum cancer stage II and III by the Norwegian gastrointestinal cancer group Acta Oncol 48 368–376 DOI: 10.1080/02841860902755244 PMID: 19242829
20. Mantadakis E, Kim G and Reisch J et al (2001) Lack of prognostic significance of intratumoral angiogenesis in nonmetastatic osteosarcoma J Pediatr Hematol Oncol 23, 286–289 DOI: 10.1097/00043426-200106000-00010 PMID: 11464984
21. DM Fletcher, JA Bridge and PCW Hogendoorn et al (2013) WHO Classification of Tumours of Soft Tissue and Bone, 4th edn (Lyon).
22. Calabuig-Farinas S, Lopez-Guerrero JA and Llombart-Bosch A (2011) The GIST paradigm: how to establish diagnostic and prognostic criteria Arkh Patol 73 13–21 PMID: 22164425
23. Nishida T, Blay JY and Hirota S et al (2016) The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines Gastric Cancer 19 3–14 DOI: 10.1007/s10120-015-0526-8
24. Rubin BP (2006) Gastrointestinal stromal tumours: an update Histopathology 48 83–96 DOI: 10.1111/j.1365-2559.2005.02291.x
25. Joensuu H, Vehtari A and Riihimaki J et al (2012) Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 13 265–274 DOI: 10.1016/S1470-2045(11)70299-6
26. Fisher C, Folpe AL and Hashimoto H et al (2004) Intra-abdominal synovial sarcoma: a clinicopathological study Histopathology 45 245–253 DOI: 10.1111/j.1365-2559.2004.01950.x PMID: 15330802
27. Wong NA, Campbell F and Shepherd NA (2015) Abdominal monophasic synovial sarcoma is a morphological and immunohistochemical mimic of gastrointestinal stromal tumour Histopathology 66 974–981 DOI: 10.1111/his.12593
28. Machado I, Giner F and Mayordomo E et al (2008) Tissue microarrays analysis in chondrosarcomas: light microscopy, immunohistochemistry and xenograft study Diagn Pathol 3 Suppl. 1 25 DOI: 10.1186/1746-1596-3-S1-S25
29. Subramaniam MM, Noguera R and Piqueras M et al (2007) Evaluation of genetic stability of the SYT gene rearrangement by break-apart FISH in primary and xenotransplanted synovial sarcomas Cancer Genet Cytogenet 172 23–28 DOI: 10.1016/j.cancergencyto.2006.07.008
30. van der Schaft DW, Seftor RE and Seftor EA et al (2004) Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells J Natl Cancer Inst 96 1473–1477 DOI: 10.1093/jnci/djh267 PMID: 15467037
31. Wu MH, Huang CY and Lin JA et al (2014) Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells Oncogene 33 1725–1735 DOI: 10.1038/onc.2013.109
32. Lin C, McGough R and Aswad B et al (2004) Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes J Orthop Res 22 1175–1181 DOI: 10.1016/j.orthres.2004.03.002 PMID: 15475194
33. Basilio-de-Oliveira RP and Pannain VL (2015) Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell proliferation (Ki67) for gastrointestinal stromal tumors World J Gastroenterol 21 6924–6930 PMID: 26078569 PMCID: 4462733
34. Kubo T, Shimose S and Fujimori J et al (2013) Diversity of angiogenesis among malignant bone tumors Mol Clin Oncol 1 131–136 PMID: 24649135 PMCID: 3956242
35. Kreuter M, Bieker R and Bielack SS et al (2004) Prognostic relevance of increased angiogenesis in osteosarcoma Clin Cancer Res 10 8531–8537 DOI: 10.1158/1078-0432.CCR-04-0969 PMID: 15623635
36. Imamura M, Yamamoto H and Nakamura N et al (2007) Prognostic significance of angiogenesis in gastrointestinal stromal tumor Mod Pathol 20 529–537 DOI: 10.1038/modpathol.3800767 PMID: 17334345
37. Chen YY, Yeh CN and Cheng CT et al (2011) Sunitinib for Taiwanese patients with gastrointestinal stromal tumor after imatinib treatment failure or intolerance World J Gastroenterol 17 2113–2119 DOI: 10.3748/wjg.v17.i16.2113 PMID: 21547131 PMCID: 3084397
38. Reichardt P, Kang YK and Rutkowski P et al (2015) Clinical outcomes of patients with advanced gastrointestinal stromal tumors: safety and efficacy in a worldwide treatment-use trial of sunitinib Cancer 121 1405–1413 DOI: 10.1002/cncr.29220 PMID: 25641662 PMCID: 4442000
39. Judson I, Verweij J and Gelderblom H et al (2014) Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial Lancet Oncol 15 415–423 DOI: 10.1016/S1470-2045(14)70063-4 PMID: 24618336
40. Yoo KH, Kim HS and Lee SJ et al (2015) Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series BMC Cancer 15 154 DOI: 10.1186/s12885-015-1160-x PMID: 25885855 PMCID: 4438639
41. van der Schaft DW, Hillen F and Pauwels P et al (2005) Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia Cancer Res 65 11520–11528 DOI: 10.1158/0008-5472.CAN-05-2468 PMID: 16357161
42. Lombardi L, Tavano F and Morelli F et al (2013) Chemokine receptor CXCR4: role in gastrointestinal cancer. Crit Rev Oncol Hematol 88 696–705 DOI: 10.1016/j.critrevonc.2013.08.005 PMID: 24120239
43. Sugiyama R, Agematsu K and Migita K et al (2014) Defect of suppression of inflammasome-independent interleukin-8 secretion from SW982 synovial sarcoma cells by familial Mediterranean fever-derived pyrin mutations Mol Biol Rep 41 545–553 DOI: 10.1007/s11033-013-2890-y
44. Drury LJ, Wendt MK and Dwinell MB (2010) CXCL12 chemokine expression and secretion regulates colorectal carcinoma cell anoikis through Bim-mediated intrinsic apoptosis. PLoS One 5 e12895 DOI: 10.1371/journal.pone.0012895 PMID: 20877573 PMCID: 2943927