Appearance of malignant melanoma after a non-cutaneous cancer diagnosis
Ugo Bottoni1, Rita Clerico2, Giovanni Paolino2, Marina Ambrifi2, Cecilia Luci2, Paola Corsetti2 and Stefano Calvieri2
1 University Magna Graecia, V.le Europa, 88100 Catanzaro, Italy
2 Clinica Dermatologica, Dipartimento di Medicina Interna e Specialità Mediche, University of Rome, La Sapienza, Viale del Policlinico, Rome, Italy
Correspondence to: Giovanni Paolino. Email: paolgio@libero.it
Abstract
Background: The aim of this study is to find the associations between malignant melanoma (MM) and other non-cutaneous malignancies and to see whether there are possible correlations between them.
Methods: We analysed a sample of 1720 patients collected by our melanoma database, to identify patients with both MM and non-cutaneous primary cancer (NCC). The incidence rate (IR) included in our database was calculated as the ratio between the observed patients with NCC and those with MM.
Results: A total of 74 patients, with both NCC and MM, were included in our analysis, corresponding to 4.30% of patients with MM present in our melanoma database. After breast cancer (24.3%; IR = 1:4), the most common malignancies were lymphomas (14.8%; IR = 1:4), renal cell carcinoma (13.5%; IR = 1:7), thyroid cancer (9.4%; IR = 1:11), and prostatic carcinoma (8.1%; IR = 1:12), followed by other cancers. Among patients with lymphomas, most patients (72.7%) had a non-Hodgkin lymphoma. Our study shows a high coexistence of multiple malignancies in patients with MM.
Conclusion: Although we cannot definitively confirm a true association between non-skin cancers and MM, we believe that there are sufficient links for further investigation in order to identify new aetiological factors and therapeutic targets for these cancers.
Keywords: melanoma; renal cell carcinoma; non-cutaneous malignancies; thyroid cancer; multiple primary malignancies
Introduction
The occurrence of multiple primary malignancies in the same patient is a well-recognized phenomenon. The increased incidence of subsequent non-cutaneous primary cancers (NCCs) in patients with cutaneous malignant melanoma (MM) is well documented [1].
Although in the literature a significant excess of NCC after an MM has been reported, an excess of MM after an NCC is much less frequently highlighted. Currently, there have been no clinicopathological reviews of such cases [2, 3].
We report the results obtained from an analysis of 1720 patients affected by MM over a period of 15 years.
Materials and methods
We analysed a sample of 1720 patients from our melanoma database, to identify patients with both MM and NCCs.
All NCCs, diagnosed during the initial MM staging, were considered synchronous. The clinical presentations were defined as asymptomatic for tumours that were discovered at any radiologic examination. The median follow-up of the entire cohort was 84 months.
The incidence rate (IR) was calculated as the ratio between the observed patients with NCC and with MM, and was included in our database. A 95% confidence interval was also performed for the entire cohort.
Results
Our analysis of 74 patients, with both NCC and MM, totaled 4.30% of patients with MM present in our melanoma database. Epidemiological analyses have reported a higher prevalence of female patients (n = 45) than male patients (n = 29). The median age of the entire cohort was of 56.5 years (ranging from 26 to 76) for female patients and 58 years (ranging from 25 to 74) for male patients (Table 1).
Table 1: Gender and age-adjusted incidence of non-cutaneous cancers (NCCs) in our cohort.
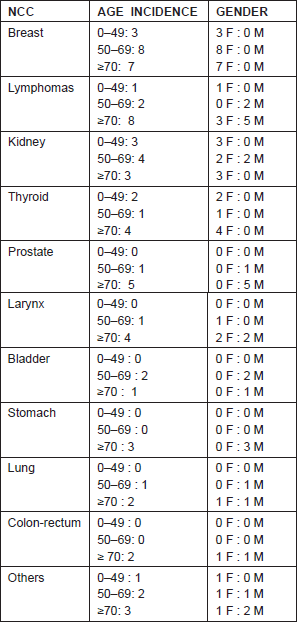
Regarding the primary melanoma thickness, 35 patients removed lesions ≤1.00 mm, while 29 patients ≥1.01 mm (ranging between 0.12 and 9.5 mm). According to the seventh American Joint Committee on Cancer (AJCC) for melanoma [4], 44 patients were in stage I, 16 in stage II, and nine in stage III. Two patients presented an MM with unknown primary. While, regarding NCC, 26 patients (35.13%) were in stage I at diagnosis, 30 patients (40.54%) were in stage II, 15 patients (20.3%) in stage III, and only three patients (4.1%) in stage IV.
After breast cancer (24.3%; IR = 1: 4), the most common malignancies were lymphomas (14.8%; IR = 1:4), renal cell carcinoma (13.5%; IR = 1:7), thyroid cancer (9.4%; IR = 1:11), and prostatic carcinoma (8.1%; IR = 1:12), followed by other cancers as reported in Table 2.
Table 2: Non-cutaneous cancers (NCCs) and relative incidence rate (IR) present in our cohort.
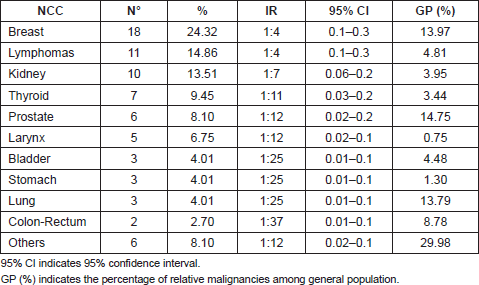
Among patients with lymphomas, most patients (72.7%) had a non-Hodgkin lymphoma. Among the non-Hodgkin lymphomas, four patients (50%) presented a follicular lymphoma, two patients (25%) a large B-cell lymphoma, one patient (12.5%) a small lymphocytic lymphoma, and one patient (12.5%) a mantle cell lymphoma.
Three female patients and two male patients showed a positive history for immunological disease such as rheumatoid arthritis, psoriatic arthritis, and autoimmune thyroiditis.
In the 66 patients analysed, an MM occurred after an NCC diagnosis, while in seven patients, an NCC was found after an MM diagnosis. In one patient, a renal cell carcinoma was found during MM staging and as a result it was considered synchronous.
No patients in our cohort showed a positive family history for MM or other syndromes that could justify the onset of multiple malignancies. B-RAF mutation was positive in one patient (1.4%) with asynchronous renal cell carcinoma.
For treatment a radiotherapy regimen was performed in six patients, a combined chemo and radiotherapy treatment was performed in 19 patients, while a chemotherapy treatment alone was performed in 45 patients. For the remaining patients, treatment was not performed because it was not provided or because the patients refused any treatment.
The most common MM risk factors observed in our cohort were the same for the general population (I and II Fitzpatrick’s photo type, high number of nevi, intermittent and intense sun exposure and sunburn at a young age), as well as an eventual immune system deficiency history (as reported in nine patients that had also had a bone marrow transplant). In fact, seven patients affected by lymphomas showed a white blood cell count of ≤4.500/μl at time of first visit.
The overall survival rate of the entire cohort was of 54 months (ranging from six to 144 months); in 77.7% of cases, this was connected to NCC, while in only 22.3%, it was connected to MM.
Discussion
The coexistence of multiple cancers in the same patient has been widely documented [2, 3, 5–7]. It is well established that patients with MM are at higher risk than the general population for developing a secondary primary tumour. However, the development of MM subsequently to NCCs is less documented.
The 4.3% of patients in our MM database also presented with an NCC. The most common cancers were breast cancer, lymphomas, and renal cell carcinoma. However, considering the high incidence of breast and prostatic cancers [8] in the general population, and the increased risk of developing a secondary malignancy in patients with haematologic disorders who received immunosuppressive therapies [6], the percentage (13.5%) of renal cell carcinoma and thyroid carcinoma out of our cohort can be emphasized.
The absence of familial MM in our cohort can also be explained by the simultaneous absence of pancreatic carcinoma. In the literature, the presence of pancreatic cancer in MM prone families has been consistently associated with an increased frequency of CDKN2A mutations [9, 10].
Like MM, the incidence of renal cell carcinoma has increased in the last few decades and different studies have found an association between these two malignancies [3–5]. In the literature, an excess of renal cell carcinoma and NCC after an MM diagnosis is often reported. However, in our analysis and in contrast with recent studies, 66 (89.18%) patients developed an NCC (including renal cell carcinoma) before an MM diagnosis [1, 3, 5–7].
Although the simultaneous occurrence of MM and NCC may be coincidental, there are several plausible links. In particular, recent studies support a genetic predisposition of coexisting MM and kidney cancer in the same patient, such as microphthalmia-associated transcription factor, CDKN2A and B-RAF [11–15].
Recently, B-RAF mutations have also been identified in thyroid carcinoma [16]; in this regard, the high number of thyroid carcinomas (9.4%) in our sample should be mentioned (Table 2).
Furthermore, in our cohort, there was a high prevalence of multiple malignancies (60.8%) in female patients. Considering the higher incidence of immunological diseases in female than in male patients [17], it can be hypothesized that immunological mechanisms predispose these patients to multiple malignancies. In our cohort, five patients (6.75%) showed a positive history for immunological diseases with previous immunomodulating treatments.
Finally the pre-existence of a cancer can alter the human immune system and facilitate the possible occurrence of a secondary malignancy; this can be related to the disease’s course or to the therapies.
Considering recent studies that have highlighted a possible role of virus-like particles in MM cells and also oncolytic viruses in several malignancies [18–20], a possible viral role in the onset of NCC associated with MM cannot be ruled out.
In conclusion, our study shows a high coexistence of multiple malignancies in patients with MM. Although we cannot definitively confirm a true association between NCC and MM, we believe that there are sufficient links for further investigation in order to identify new aetiological factors and therapeutic targets for these cancers.
References
1. Matin RN, Szlosarek P and McGregor JM (2013) Synchronous melanoma and renal carcinoma: a clinicopathological study of five cases Clin Exp Dermatol 38 47–9 DOI: 10.1111/j.1365-2230.2012.04399.x
2. Schmid-Wendtner MH, Baumert J and Wendtner CM (2001) Risk of second primary malignancies inpatients with cutaneous melanoma Br J Dermatol 145 981–5 DOI: 10.1046/j.1365-2133.2001.04507.x
3. Maubec E, Chaudru V, Mohamdi H, et al (2010) Characteristics of the coexistence of melanoma and renal cell carcinoma Cancer 116 5716–24 DOI: 10.1002/cncr.25562
4. Balch JM, Gershenwald JE, Soong SJ, et al (2009) Final version of 2009 AJCC melanoma staging and classification J Clin Oncol 27 6199–206 DOI: 10.1200/JCO.2009.23.4799 PMID: 19917835 PMCID: 2793035
5. Bhatia S, Estrada-Batres L, Maryon T, et al (1999) Second primary tumors in patients with cutaneous malignant melanoma Cancer 86 2014–20 DOI: 10.1002/(SICI)1097-0142(19991115)86:10<;2014::AID-CNCR19>;3.0.CO;2-4 PMID: 10570426
6. Wassberg C, Thorn M, Yuen J, et al (1996) Second primary cancers in patients with cutaneous malignant melanoma: a population-based study in Sweden Br J Cancer 73 255–9 DOI: 10.1038/bjc.1996.45 PMID: 8546916
7. Bradford PT, Freedman DM, Goldstein AM, et al (2010) Increased risk of second primary cancers after a diagnosis of melanoma Arch Dermatol 146 265–72 DOI: 10.1001/archdermatol.2010.2 PMID: 20231496
8. American Cancer Society: Cancer Facts and Figures (2012) Atlanta, GA: American Cancer Society http://www.cancer.org
9. Whelan AJ, Bartsch D and Goodfellow PJ (1995) Brief report: a familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene N Engl J Med 333 975–7 DOI: 10.1056/NEJM199510123331505 PMID: 7666917
10. Yang XR, Jessop L, Myers T, et al (2011) Lack of germline PALB2 mutations in melanoma-prone families with CDKN2A mutations and pancreatic cancer Fam Cancer 10 545–8 DOI: 10.1007/s10689-011-9447-9 PMID: 21614589 PMCID: 3244023
11. Liu H, Sundquist J and Hemminki K (2011) Familial renal cell carcinoma from the Swedish Family-Cancer Database Eur Urol 60 987–93 DOI: 10.1016/j.eururo.2011.05.031 PMID: 21621909
12. Brown ER, Charles KA, Hoare SA, et al (2008) A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer Ann Oncol 19 1340–6 DOI: 10.1093/annonc/mdn054 PMID: 18325912
13. Bertolotto C, Lesueur F and Bressac de Paillerets B (2012) MITF: a genetic key to melanoma and renal cell carcinoma? Med Sci 28 258–61 DOI: 10.1051/medsci/2012283010 PMID: 22480646
14. Gattenlöhner S, Etschmann B, Riedmiller H, et al (2009) Lack of KRAS and BRAF mutation in renal cell carcinoma Eur Urol 55 1490–1 DOI: 10.1016/j.eururo.2009.02.024 PMID: 19282104
15. Ghiorzo P, Pastorino L, Queirolo P, et al (2012) Prevalence of the E318K MITF germline mutation in Italian melanoma patients: associations with histological subtypes and family cancer history Pigment Cell Melanoma Res [Epub ahead of print] PMCID: 3490389
16. Li X, Abdel-Mageed AB and Kandil E (2012) BRAF mutation in papillary thyroid carcinoma Int J Clin Exp Med 5 310–5 PMID: 22993650 PMCID: 3443896
17. McCombe PA, Greer JM and Mackay IR (2009) Sexual dimorphism in autoimmune disease Curr Mol Med 9 1058–79 DOI: 10.2174/156652409789839116 PMID: 19747114
18. Patel P, Hanson DL, Sullivan PS, et al (2008) Incidence of type of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003 Ann Intern Med 148 728–36 PMID: 18490686
19. Muster T, Waltenberger A, Grassauer A, et al (2003) An endogenous retrovirus derived from human melanoma cells Cancer Res 63 8735–41 PMID: 14695188
20. Prestwich RJ, Errington F, Llet EJ, Morgan RS, Scott KJ, Kottke T et al (2008) Tumor infection by oncolytic reoviruses adaptive antitumor immunity Clin Cancer Res 14 7358–66 DOI: 10.1158/1078-0432.CCR-08-0831 PMID: 19010851






