Gastric schwannoma with atypical manifestations in an adult patient: case report and literature review
Yanet Isabel Carrasco Rojas1,2, Albert Gabriel Turpo Peqqueña2,3,4, Natalia Camila Zenteno Tejada2 Victoria Elena Quispe Pastor2, Juan Augusto Martínez San Martín1,2, and Evelyn Maria Chambilla Huellcacure5
1Surgical Oncology Service, Carlos Alberto Seguín Escobedo National Hospital (HNCASE), EsSalud, Peral - El Filtro, S/N, Arequipa 04001, Peru
2Faculty of Human Medicine, Center for Research and Medical Studies (CIEM), Catholic University of Santa María, Urb San José s/n, Umacollo, Arequipa 04013, Peru
3Faculty of Biology, National University of San Agustin, Av Alcides Carrión s/n, Arequipa 04001, Peru
4Molecular Engineering Research Center (CIIM), Catholic University of Santa María, Urb San José s/n, Umacollo, Arequipa 04013, Peru
5Pathological Anatomy Service, Carlos Alberto Seguín Escobedo National Hospital (HNCASE), EsSalud, Peral - El Filtro, s/n, Arequipa 04001, Peru
ahttps://orcid.org/0009-0003-1975-5796
bhttps://orcid.org/0000-0002-9020-7659
chttps://orcid.org/0009-0008-9331-7408
dhttps://orcid.org/0000-0002-0061-127X
ehttps://orcid.org/0009-0003-5246-8342
fhttps://orcid.org/0009-0001-3717-8457
Abstract
Gastric schwannomas (GS) are rare tumours originating from Schwann cells that affect the gastrointestinal tract, posing a diagnostic challenge due to their nonspecific symptoms. We report the case of a 61-year-old female presented with gastric fullness and occasional episodes of abdominal pain. Computed tomography revealed a solid mass in the lesser curvature of the stomach, initially suspected to be a gastrointestinal stromal tumours. During surgery, an exophytic lesion was identified and confirmed histopathologically and immunohistochemically as a gastric schwannoma. GS should be considered in the differential diagnosis of gastric subepithelial tumours. Its diagnosis relies on immunohistochemistry, and surgical resection ensures effective treatment.
Keywords: neurilemoma, gastric neoplasms, tumours of the gastrointestinal wall, surgical resection
Correspondence to: Albert Gabriel Turpo Peqqueña
Email: albert.turpo@ucsm.edu.pe
Published: 27/05/2025
Received: 13/01/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (
Introduction
Gastric schwannomas (GS) are rare tumours originating from Schwann cells and represent about 2.0%–7.0% of benign neoplasms of the gastrointestinal tract [1, 2]. Histologically, GS are spindle cell tumours expressing the S-100 protein, characterised by a microtrabecular pattern, a peripheral lymphoid rim and occasionally germinal centers [3]. Furthermore, GS usually affects women between the fourth and sixth decades of life [1, 4]. Most cases of GS are asymptomatic because they are diagnosed incidentally on imaging studies performed for other indications [5]. However, in rare cases, they may manifest with nonspecific symptoms such as abdominal pain, gastrointestinal bleeding or palpable mass, which poses a diagnostic challenge [6]. Clinically, it is important to differentiate GS from other subepithelial neoplasms, especially gastrointestinal stromal tumours (GISTs), leiomyomas (LM) and leiomyosarcomas [7], since they can be easily confused when visualised in an upper gastrointestinal endoscopy [5]. Diagnostic confirmation is based on postoperative pathology and immunohistochemistry [8], with positive staining for S-100 protein and negative staining for c-kit, CD34, desmin or smooth muscle actin, which are distinctive features of schwannomas [9]. This report describes the case of an adult patient with an atypical gastric schwannoma, which complicated his initial diagnosis. Informed consent was obtained from the patient for the publication of this report for academic purposes and the confidentiality of the medical information used was maintained.
Case report
A 61-year-old female patient with no history of hypertension, diabetes mellitus, smoking or allergies except to sulfonamides. Since 2019, the patient has reported a persistent feeling of gastric fullness and occasional episodes of abdominal pain. During follow-up, in January 2024, upper gastrointestinal endoscopy showed an elevated, lobulated lesion with preserved mucosa of approximately 50 mm. Subsequently, endoscopic ultrasound in February 2024 confirmed a hypoechoic image of 56 × 51 mm dependent on the muscularis propria, without internal calcifications or ductal dilatations, compatible with a subepithelial tumour. Initially, these findings suggested a GIST. Routine laboratory tests were within normal ranges, and tumour markers (Table 1) were negative. In addition, a computed tomography (CT) scan was performed, which identified a solid lesion measuring 64 × 49 mm in the lesser curvature of the stomach (Figure 1).
Table 1. Auxiliary examinations of the patient during hospitalization.
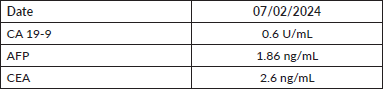
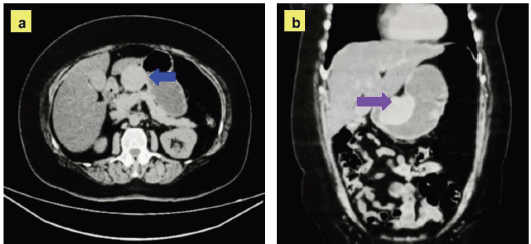
Figure 1. Contrast-enhanced CT with axial and coronal sections. (a): Axial section showing a solid lesion with well-defined borders, located in the lesser curvature of the stomach (blue arrow). The lesion measures approximately 64 × 49 mm, has homogeneous density and no invasion of adjacent structures. (b): Coronal section confirming the location of the lesion in the lesser curvature of the stomach (purple arrow).
Based on the clinical data and imaging evidence, it was decided to proceed with surgery. During the intervention, an 8 × 4 × 3 cm exophytic tumour was observed in the lesser curvature of the stomach, located 4 cm from the pylorus, with a hard consistency and a whitish surface. With these findings and a high suspicion of benign pathology, it was decided to perform a function-sparing gastrectomy: pylorus-preserving gastrectomy, preserving neurovascular structures and having a gastro-gastric anastomosis (Figure 2). The definitive diagnosis was confirmed by a histopathological and immunohistochemical study (Figure 3), reporting a gastric schwannoma (Table 2). Postoperative recovery was satisfactory, with stable vital signs and no immediate complications.
Discussion
GS are rare submucosal mesenchymal tumours, accounting for 0.1%–3% of all gastrointestinal tract neoplasias and 1%–2% of alimentary tract mesenchymal tumours. GS arise from Schwann cells of the gastric plexus and are usually benign, unlike GISTs or LM [4, 10], which have malignant potential. Furthermore, a notable female predominance has been reported, with a female:male ratio of 4:1, in ages ranging from 40 to 60 years [1, 4, 11], and although our patient was 61 years old, her epidemiological profile coincides with the typical features of GS.
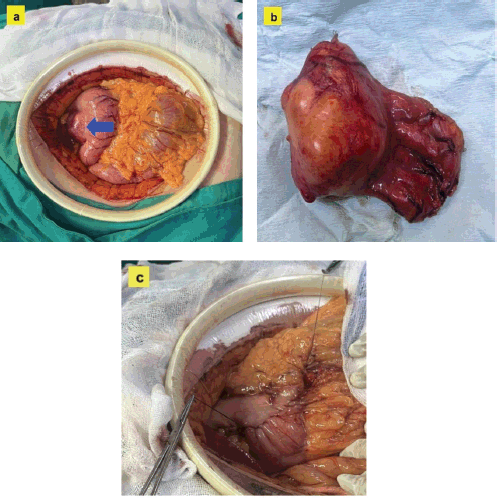
Figure 2. Images of the surgical procedure. (a): Intraoperative view showing the lesion in situ, displaying an exophytic tumour located in the lesser curvature of the stomach (blue arrow). (b): Image of the surgical specimen after surgical resection, showing a smooth, whitish surface with typical characteristics of a gastric schwannoma. (c): Intraoperative image illustrating the reconstruction performed by gastro-gastric anastomosis.
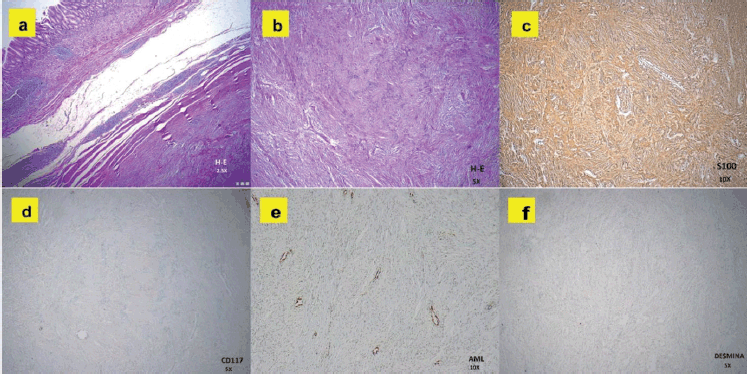
Figure 3. Histopathological images of the gastric tumour. (a): H&E at 2.5×: Subepithelial lesion with spindle cells in a fascicular pattern, without nuclear atypia. (b): H&E at 5×: Compact spindle cells with elongated nuclei, consistent with mesenchymal tumour. (c): S-100 at 10×: Diffuse and strong positivity, confirming neural differentiation. (d): CD117 (c-KIT) at 5×: Negative, ruling out GIST. (e): AML at 10×: Negative, excluding a lesion of smooth muscle origin. (f): Desmin at 5×: Negative, indicating the absence of myogenic differentiation.
Table 2. Histopathological and immunohistochemical examination.
Although most of the GS are asymptomatic and are discovered incidentally during diagnostic procedures for other pathologies, with a rate ranging between 30% and 33% [4]. Pu et al [12], Lamb et al [13] and Cordera et al [14] reported diagnoses of GS incidentally during imaging studies for other reasons. The most common symptoms of GS include upper gastrointestinal bleeding, abdominal pain, diffuse discomfort, nausea and gastric outlet obstruction [15]. Li and Liu [16], Khan et al [17] and Oudad et al [18] reported clinical presentations with marked symptoms, such as severe abdominal pain and gastrointestinal bleeding. In our case, the patient presented nonspecific symptoms of gastric fullness and abdominal contractures, which complicated the initial diagnosis. In Zheng’s study, out of 29 cases, 5% (1 patient) reported gastric fullness as a symptom [19]. Also, in the Miettinen report, which included 1,241 patients, 1.45% (18 patients) presented gastric fullness, while 0.24% (3 patients) presented pulsatile gastric contractions [20]. Although pulsatile gastric contractions are not the same as abdominal contractures, it is relevant to note that they are present in GS.
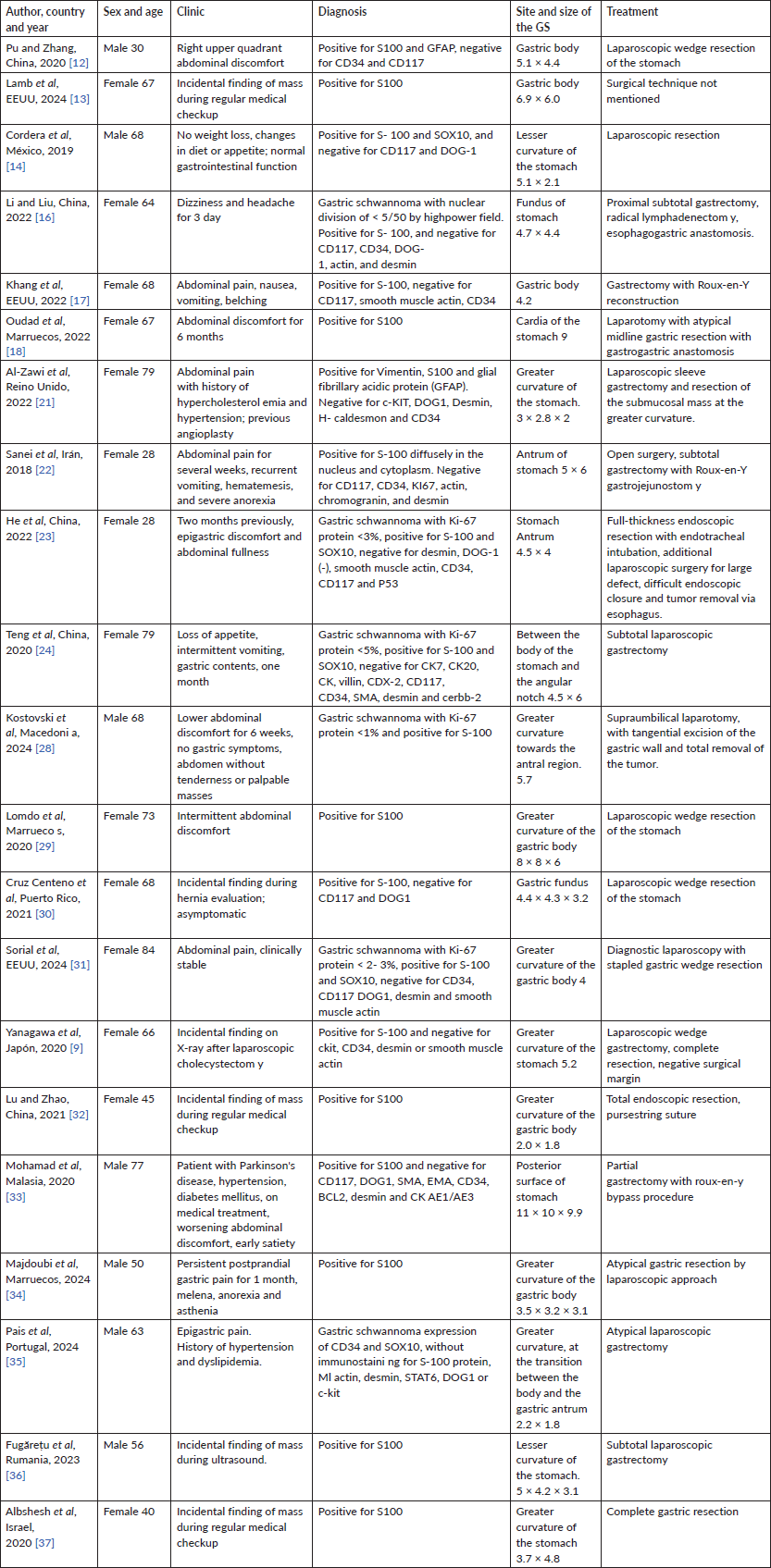
The stomach is the most common site for GS, accounting for 60% to 70% of cases, and they usually originate mainly in the gastric body (50%), followed by the antrum (32%) and fundus (18%) [9]. GS typically presents as solitary lesions less than 5 cm in size. In Peng’s study, of 78 reported cases, 72.2% (56 patients) were located in the gastric body, specifically in the lesser curvature and middle third of the stomach [4, 21]. In this report, we found that the tumour mass was also located in the lesser curvature, coinciding with several authors (Table 3).
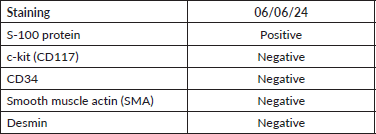
Endoscopy with ultrasound, as highlighted by Sanei et al [22], He et al [23] and Tang et al [24], is a key tool for the characterisation of subepithelial lesions. However, the diagnosis of GS is based on pathological and immunohistochemical findings, and several authors highlight S100 as a specific marker with 97.6%100% positivity (Table 3) [4]. GS is also positive for vimentin, but negative for c-kit, CD34 and CD117, which contrasts with GIST, and are also negative for desmin and smooth muscle actin, unlike LM [15, 25, 26]. In our case, the diagnosis was confirmed by immunohistochemistry, with positivity for S-100 and negativity for c-kit, CD34, desmin and smooth muscle actin (Table 2).
The main treatment of GS is surgical resection, and it is important to ensure the complete removal of the tumour [27]. Function-sparing gastrectomies, such as segmental or subtotal, are the most commonly used approach for large tumours or those located in complex areas, as reported by Pu et al [12], Kostovski et al [28], He et al [23] and Lomdo et al [29]. This approach ensures negative margins and minimises recurrence. On the other hand, endoscopic resection is used for smaller tumours or those without suspicion of deep invasion, as reported by Cruz Centeno et al [30], Tang et al [24] and Sorial et al [31], due to its lower morbidity and rapid recovery.
Conclusion
Our case report highlights the importance of including GS in the differential diagnosis of gastric subepithelial tumours, particularly in patients with nonspecific symptoms. Definitive diagnosis depends on histopathological and immunohistochemical studies, with positivity for S-100 and negativity for c kit and CD34. Complete surgical removal, with a function-sparing approach, ensures effective treatment and favourable outcomes.
Table 3. Cases reported in the literature of gastric schwannoma, 2018–2024.
Acknowledgments
We thank the Carlos Alberto Seguín Escobedo National Hospital (HNCASE) and the Faculty of Medicine at the Catholic University of Santa María for their support in the preparation of this work.
Conflicts of interest
Our authors have no competing interests, and the contents of this manuscript have not been published elsewhere. There are no conflicts of interest to disclose.
Funding
No external funding or funding by any author has been received for this study.
Informed consent
Informed consent was obtained from the patient for the publication of this report for academic purposes and the confidentiality of the medical information used was maintained. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author contributions
The original manuscript was authored and approved by all contributing authors.
Yanet Isabel Carrasco Rojas: Review of intellectual content, data collection, preparation of photographs and approval of the version sent to the editorial process.
Albert Gabriel Turpo Peqqueña: Preparation of the document from its conception, design, complete writing and final modifications, acquisition of information, critical review of intellectual content and approval of the version sent to the editorial process.
Natalia Camila Zenteno Tejada: Bibliographic review, contributions to the intellectual content and approval of the version sent to the editorial process.
Victoria Elena Quispe Pastor: Bibliographic review, contributions to the intellectual content and approval of the version sent to the editorial process.
Augusto Martínez San Martín: Preparation of photographs, bibliographic review, contributions to the intellectual content and approval of the version sent to the editorial process.
Evelyn Chambilla Huellcacure: Preparation of photographs, bibliographic review, contributions to the intellectual content and approval of the version sent to the editorial process.
References
1. Singh A, Aggarwal M, and Chadalavada P, et al (2022) Natural history of gastrointestinal schwannomas Endosc Int Open 10(06) E801–E808 https://doi.org/10.1055/a-1784-0806 PMID: 35692918 PMCID: 9187404
2. Pattarapuntakul T (2018) Gastric schwannoma J Health Sci Med Res 36 157–164 https://doi.org/10.31584/jhsmr.v36i2.9
3. Levy AD, Quiles AM, and Miettinen M, et al (2005) Gastrointestinal schwannomas: CT features with clinicopathologic correlation Am J Roentgenol 184 797–802 https://doi.org/10.2214/ajr.184.3.01840797
4. Peng H, Han L, and Tan Y, et al (2022) Clinicopathological characteristics of gastrointestinal schwannomas: a retrospective analysis of 78 cases Front Oncol 12 1003895 https://doi.org/10.3389/fonc.2022.1003895 PMCID: 9792477
5. Mekras A, Krenn V, and Perrakis A, et al (2018) Gastrointestinal schwannomas: a rare but important differential diagnosis of mesenchymal tumors of gastrointestinal tract BMC Surg 18 47 https://doi.org/10.1186/s12893-018-0379-2 PMID: 30045739 PMCID: 6060462
6. Hou YY, Tan YS, and Xu JF, et al (2006) Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases Histopathology 48 536–545 https://doi.org/10.1111/j.1365-2559.2006.02370.x PMID: 16623779
7. Daimaru Y, Kido H, and Hashimoto H, et al (1988) Benign schwannoma of the gastrointestinal tract: a gastrointestinal and immunohistochemical study Hum Pathol 19 257–264 https://doi.org/10.1016/S0046-8177(88)80518-5 PMID: 3126126
8. Wu X, Li B, and Zheng C, et al (2020) Clinical characteristics and surgical management of gastrointestinal schwannomas Biomed Res Int 2020 9606807 https://doi.org/10.1155/2020/9606807 PMID: 32685549 PMCID: 7327551
9. Yanagawa S, Kagemoto K, and Tanji H, et al (2020) A rare case of gastric schwannoma: a case report and literature review Case Rep Oncol 13(1) 330–335 https://doi.org/10.1159/000506450 PMID: 32308600 PMCID: 7154269
10. Wang Y, Chen Y, and Zhao R, et al (2022) Preoperative differentiation of gastric schwannomas and gastrointestinal stromal tumors based on computed tomography: a retrospective multicenter observational study Front Oncol 12 1344150 https://doi.org/10.3389/fonc.2022.1003895
11. Voltaggio L, Murray R, and Lasota J, et al (2012) Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature Hum Pathol 43 650–659 https://doi.org/10.1016/j.humpath.2011.07.006
12. Pu C and Zhang K (2020) Gastric schwannoma: a case report and literature review J Int Med Res 48(9) 300060520957828 https://doi.org/10.1177/0300060520957828 PMID: 32962485 PMCID: 7518005
13. Lamb K, Kayyali S, and Schulman M, et al (2024) Unraveling the enigma: a case study on gastric schwannoma and its clinical implications Am J Gastroenterol 119(10S) S2610–S2611 https://doi.org/10.14309/01.ajg.0001045484.68091.f5
14. Cordera F, Salazar-Vitale A, and Mejía-Sánchez E, et al (2019) Laparoscopic resection of gastric schwannoma: a case report Int J Surg Case Rep 65 271–274 https://doi.org/10.1016/j.ijscr.2019.10.037 PMID: 31743845 PMCID: 6864170
15. Miettinen M and Lasota J (2001) Gastrointestinal stromal tumors: definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis Virchows Arch 438 1–12 https://doi.org/10.1007/s004280000338 PMID: 11213830
16. Li QQ and Liu D (2022) Gastric schwannoma misdiagnosed as gastrointestinal stromal tumor by ultrasonography before surgery: a case report World J Clin Cases 10(5) 1667–1674 https://doi.org/10.12998/wjcc.v10.i5.1667 PMID: 35211607 PMCID: 8855257
17. Khan S, Honganur NS, and Kumar S, et al (2022) Gastric schwannoma: a case report and literature review Cureus 14(5) e24785 https://doi.org/10.7759/cureus.24785 PMID: 35673307 PMCID: 9165928
18. Oudad F, Benayad S, and Bouchbika Z, et al (2022) Gastric schwannoma, rare presentation of a gastric mass, with an excellent outcome Clin Med Rev Case Rep 9 407 https://doi.org/10.23937/2378-3656/1410407
19. Zheng L, Wu X, and Kreis ME, et al (2014) Clinicopathological and immunohistochemical characterisation of gastric schwannomas in 29 cases Gastroenterol Res Pract 2014 202960 https://doi.org/10.1155/2014/202960 PMID: 24688535 PMCID: 3942198
20. Miettinen M, Sobin LH, and Lasota J (2005) Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up Am J Surg Pathol 29 52–68 https://doi.org/10.1097/01.pas.0000146010.92933.de
21. Al-Zawi ASA, Lahmadi S, and Jalilzadeh Afshari S, et al (2022) Gastric schwannoma as an important and infrequent differential diagnosis of gastric mesenchymal tumours: a case report and review of literature Cureus 14(12) e32112 https://doi.org/10.7759/cureus.32112
22. Sanei B, Kefayat A, and Samadi M, et al (2018) Gastric schwannoma: a case report and review of the literature for gastric submucosal masses distinction Case Rep Med 2018 1230285 https://doi.org/10.1155/2018/1230285 PMID: 29849652 PMCID: 5914132
23. He CH, Lin SH, and Chen Z, et al (2022) Laparoscopic-assisted endoscopic full-thickness resection of a large gastric schwannoma: a case report World J Gastrointest Surg 14(4) 362–369 https://doi.org/10.4240/wjgs.v14.i4.362 PMID: 35664360 PMCID: 9131838
24. Tang C, Pan Q, and Xu Z, et al (2020) Gastric schwannoma with giant ulcer and lymphadenopathy mimicking gastric cancer: a case report BMC Gastroenterol 20 36 https://doi.org/10.1186/s12876-020-011862 PMID: 32059647 PMCID: 7023701
25. Miettinen M, Virolainen M, and Maarit-Sarlomo-Rikala (1995) Gastrointestinal stromal tumors–value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas Am J Surg Pathol 19 207–216 https://doi.org/10.1097/00000478-199502000-00009 PMID: 7530409
26. Fletcher CDM, Berman JJ, and Corless C, et al (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach Hum Pathol 33(5) 459–465 https://doi.org/10.1053/hupa.2002.123545 PMID: 12094370
27. Euanorasetr C and Suwanthanma W (2011) Gastric schwannoma presenting with perforation and abscess formation: a case report and literature review J Med Assoc Thai 94(11) 1399–1404
28. Kostovski O, Trajkovski G, and Ristovski G, et al (2024) Gastric schwannoma: a case report J Surg Case Rep 2024(3) rjae181 https://doi.org/10.1093/jscr/rjae181 PMID: 38549725 PMCID: 10973393
29. Lomdo M, Setti K, and Oukabli M, et al (2020) Gastric schwannoma: a diagnosis that should be known in 2019 J Surg Case Rep 2020(1) rjz382 https://doi.org/10.1093/jscr/rjz382 PMID: 31976062 PMCID: 6963168
30. Cruz Centeno N, Suarez Dominguez A, and Mojica Mañosa P, et al (2021) Incidental finding of a gastric schwannoma: a case report J Surg Case Rep 2021(11) rjab509 https://doi.org/10.1093/jscr/rjab509 PMID: 34804489 PMCID: 8599024
31. Sorial V, Khan AS, and Welsh T, et al (2024) Gastric schwannoma in an octogenarian: a case report and review of the literature Cureus 16(4) e58857 doi: https://doi.org/10.7759/cureus.58857 PMID: 38800239 PMCID: 11116083
32. Lu ZY and Zhao DY (2021) Gastric schwannoma treated by endoscopic full-thickness resection and endoscopic purse-string suture: a case report World J Gastroenterol 27(25) 3940–3947 https://doi.org/10.3748/wjg.v27.i25.3940 PMID: 34321856 PMCID: 8291012
33. Mohamad MA, Jarmin R, and Md Pauzi SH (2020) Gastric schwannoma in an elderly man: a case report Malays J Pathol 42(3) 455–459 PMID: 33361729
34. Majdoubi A, El Achchi A, and El Hammouti M, et al (2024) Gastric schwannoma: the gastrointestinal tumor simulator-case report and review of the literature Int J Surg Case Rep 116 109389 https://doi.org/10.1016/j.ijscr.2024.109389
35. Pais DP, Andrade S, and Colaco IB, et al (2024) Gastric schwannoma: a rare cause of gastric bleeding J Med Cases 12(12) 371–375 https://doi.org/10.14740/jmc4312
36. Fugărețu C, Mișarca C, and Petcu L, et al (2023) Schwannoma: a rare case of submucosal gastric tumor Diagnostics 13(12) 2073 https://doi.org/10.3390/diagnostics13122073
37. Albshesh A, Kaufmann MI, and Levy I (2020) Gastric schwannoma Clin Gastroenterol Hepatol 18(12) e142–e143 https://doi.org/10.1016/j.cgh.2019.08.027






