Paediatric palliative care in cancer
Julia Downing1,2,3, Alexandra Daniels1,2, Michael J McNeil4, Mariam Ndagire5, Gayatri Palat1,2,6,7, Rima S Rassam8 and Justin N Baker1,2,4,9
1International Children’s Palliative Care Network (ICPCN), BS1 2NT Bristol, UK
2International Children’s Palliative Care Network (ICPCN), Durban 3624, South Africa
3Makerere/Mulago Palliative Care Unit (MMPCU), Kampala, Uganda
4St. Jude Global Palliative Care Program, St. Jude Children’s Research Hospital, Memphis, TN 38105, USA
5Uganda Cancer Institute, Kampala, Uganda
6MNJ Institute of Oncology and Regional Cancer Centre, Hyderabad 500004, Andhra Pradesh, India
7Two Worlds Cancer Collaboration, North Vancouver, BC V7H 2Y8, Canada
8St. Jude Global Nursing, St. Jude Children’s Research Hospital, Memphis, TN 38105, USA
9Division of Quality of Life and Pediatric Palliative Care, Lucille Packard/Stanford University, Palo Alto, CA 94305, USA
Abstract
More than 21 million children globally need access to palliative care (PC) – including children with cancer. Providing Paediatric Palliative Care (PPC) for children with cancer is an ethical imperative with pain relief being recognised as a human right and an important public health consideration, with PPC being essential for reducing suffering in children and families. PPC addresses children’s symptoms and aims to provide comfort even if a cure is not possible. PC for children with cancer is about ensuring that the child and family have the best possible quality of life starting at diagnosis and throughout the disease trajectory regardless of cancer treatment outcomes. Many principles of PPC for children with cancer are similar to those for children with other serious health conditions. These include the following: promotion of quality of life; provision of PC care across the continuum of care (from diagnosis through to bereavement); pain and symptom management; emotional support; social care; spiritual care; good communication with children and family; advance care planning; end-of-life care; and bereavement care. PPC should be provided across the range of care settings, wherever the child and their family need care, by an inter-disciplinary team providing support to the child, their families (including siblings) and other significant others, and consider the financial impact of having a child with cancer. It should not be a last resort, but an essential component of care. In this paper, we provide a brief overview of the integration of PPC into paediatric cancer care through the review of challenges in providing PPC in paediatric oncology, global examples of clinical provision of PPC in paediatric cancer care, a review of global research priorities in this area and examples of global education programmes aimed at improving PPC in paediatric cancer care.
Keywords: palliative care, paediatrics, cancer, education, oncology, research, medicines, service provision, Ghana, Lebanon, USA, India, Uganda
Correspondence to: Julia Downing
Email: julia.downing@icpcn.org
Published: 12/12/2024
Received: 17/10/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
More than 21 million children globally need access to palliative care (PC) [1] including children with cancer. More than 1,000 children (0–19 years) are diagnosed with cancer daily [2] and an estimated 400,000 children globally each year [3, 4], with approximately 90% living in low-and middle-income countries (LMICs) [5]. Cancer is curable in most children, more so when good diagnostics, therapeutics and supportive care services are available. Whilst more than 80% of children with cancer in high-income countries (HICs) survive, this is as low as 15%–45% in children in LMICs demonstrating great disparity and inequity globally [3, 6, 7]. Most common childhood cancers differ by region, with a higher incidence of lymphomas, retinoblastomas and renal tumours in African regions [8]. The consequences of a higher burden of cancer are avoidable deaths, social and economic hardship. When providing PC to children with cancer we ‘cloak’ their symptoms and aim to provide comfort even if cure is not possible thus ensuring that the child and family have the best possible quality of life regardless of disease prognosis. Many principles of paediatric palliative care (PPC) for children with cancer are similar to those for children with other conditions whilst recognising the specific impact of cancer on the child and their families.
PC in paediatric cancer care
The impact of cancer on children and their families is devastating both in HICs and LMIC, in terms of short and long-term psychosocial, emotional, society and economic consequences secondary to cancer and its treatment. This is compounded in LMICs where an estimated 80% of children present with advanced disease [9] resulting in increased suffering and reduced quality of life with limited access to treatment, medicines, services and trained personnel, and where treatment is available it is often unaffordable or of poor quality [7]. Thus, providing PPC for children with cancer is an ethical imperative, significant and important public health consideration, with pain management being recognised as a human right [10], and essential for both preventing and reducing suffering in children and families.
Providing PC is recognised as: a core component of cancer care [11]: an essential service in the World Health Assembly cancer resolution [12]; and a core part of both Universal Health Coverage [13] and Primary Health Care [14]. It is also an important component of Sustainable Development Goal 3 on health [15], where investing in childhood cancer supports the attainment of multiple health-related targets, and identified as key service through the WHO Global Initiative for Childhood Cancer (GICC) [7]. The GICC recognises global inequalities and seeks to reach at least 60% survival probability at 5 years for children with cancer by 2030, while reducing suffering for all children with cancer [7]. The CureAll approach of the GICC calls for preparing health systems to respond to the needs of children along the entire pathway through co-ordinated multi-disciplinary care including early detection; diagnosis; treatment and PC; and survivorship [7].
The following principles of PPC apply to children with cancer: promotion of quality of life; provision of PC care across the continuum of care (from diagnosis through to bereavement); pain and symptom management; emotional support; social care; spiritual care; good communication with children and family; advance care planning; end-of-life care; and bereavement care. PPC should be provided across a range of care settings, wherever the child and their family need care, by an inter-disciplinary team providing support to the child, their families (including siblings) and significant others, and considers the financial impact of having a child with cancer. It should not be a last resort, but an essential component of care.
By providing PPC we aim to help children and their families achieve the best possible quality of life, however long that life might be; to relieve suffering, of body, mind and spirit, and care for them with competence and compassion. Supporting families throughout their journey is essential and early integration of PPC regardless of prognosis or treatment is considered best practice. This ideal timing of PPC integration is often challenged by cultural issues or a lack of resources [16]. Lacerda et al [16] discuss possible models of PPC delivery in children’s cancer including: 1) Basic care, where paediatric oncologists provide primary PC after basic education they may have received during their residency or fellowship 2) Paediatric palliative oncology where paediatric oncology clinicians have ‘embedded expertise’ gained from combined training in both oncology and PPC; 3) an Integrated care model, where oncology and PC teams work together for diagnosis, utilising the combined expertise to provide comprehensive care throughout the cancer disease trajectory (Figure 1).
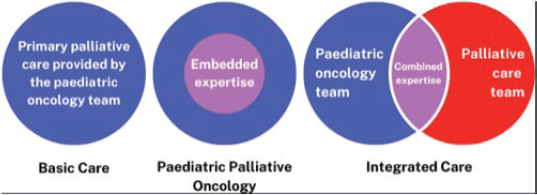
Figure 1. Possible models of PPC delivery in paediatric cancer care [16].
Challenges for the provision of PPC in cancer care
Access to PPC remains a significant challenge, both in HICs such as in the USA [17] and in LMICs [9, 18]. In the USA, challenges identified include the funding mechanisms, a lack of PPC programmes, difficulties in integrating PPC into existing paediatric oncology models and a lack of knowledge about PPC, discomfort at talking about death and cultural issues [17]. In LMICs there are a wide range of challenges that include a lack of:
- Policies on PPC;
- The recognition of the need for PPC and what it offers;
- Integration of PPC into the health system;
- Access to education;
- Accede to medicines, in particular to opioids; and
- Resources, such as financial [9].
Effective communication is critical in providing PPC [19]. However, many health professionals still find it challenging; finding the right words to explain life-threatening conditions or end-of-life options to children or their parents is tricky. If communication is poor, it may lead to impaired quality of life, inadequate management and mismanagement of the child’s and families care [19].
Delivering PPC can often give rise to ethical dilemmas [20]. Making decisions, such as when to stop treatment and discontinue invasive procedures can be challenging, especially when children may have views or desires that differ from their primary caregivers. Cultural beliefs also impact on acceptability of PC, along with accessing treatment and therefore late diagnosis.
PPC can be emotionally taxing and draining for healthcare providers as well who face daily suffering and death of their patients leading to distress and burnout [21]. PPC providers may also project their emotions into their private lives and relationships [22].
The WHO Conceptual Model for PC Development [23] identifies six key components to developing PC, including PPC, which can help to overcome these challenges: 1) Empower people and communities, 2) Health policies; 3) Research; 4) Essential medicines; 5) Education and training and 6) Integration of PPC into health services. This paper discusses examples of PPC provision in cancer care; research on PPC in cancer care; and examples of PPC education in cancer care.
Examples of PPC provision in cancer care
Four examples of PPC are discussed from a low-income country (Uganda), two Middle-income countries from different regions (India and Lebanon) and a HIC (United States of America).
Uganda
Few African countries have any form of PC programmes [18, 24]. In Uganda, the high prevalence of HIV/AIDS and cancer in the 1980s and early 1990s highlighted the need for PC. Thus, the first service – Hospice Africa Uganda started in 1993, establishing a systematic approach to PC in Uganda [25, 26], which became a model for the region.
In Uganda, PC for all hospitals was included in the National Health Sector Strategic Plan (HSSP) 2000/01–2004/05 [27, 28]. In 2004, the National Drug Policy and Authority Statute 1993 was amended so specially trained PC providers could prescribe morphine to improve access to opioids [28, 29]. The HSSP II, 2006–2011, expanded PC to all Hospitals and Health Centre IVs [26].
PPC in Uganda ranges from home-based care to hospital/facility-based care and integrated care. Several organisations provide PPC, e.g., The Uganda Cancer Institute, Hospice Africa Uganda, Mulago/Makerere PC Unit, Jinja Hospice, Kitovu Mobile Hospice, Joy Hospice, Kawempe Home Care and Mildmay Uganda, amongst others. Both Mildmay Uganda and Hospice Africa Uganda also train PPC providers at certificate, diploma and degree levels. It is important to note that Uganda has more than 50 tribes with distinct languages, cultures, norms and traditions, all influencing how they accept the concept of PPC [24]. Nevertheless, as the number of children with cancer rises, all stakeholders including government, civil society and child activists must take deliberate actions to increase access to appropriate PPC.
Lebanon
PPC in Lebanon is emerging as an important approach to the treatment of childhood cancer, despite poor resources. There are a few major paediatric cancer centres in Lebanon with most located in urban areas, primarily in Lebanon’s Capital, Beirut, which offer childhood cancer treatment for Lebanon and surrounding regions using state-of-art cancer treatment within a multidisciplinary approach [30]. They provide disease-directed therapy, striving to integrate PPC within care through optimising symptom management, decision-making and advanced care-planning.
Given limited resources, PC services are limited to a few institutions and are often used in adult oncology settings typically at terminal stages. In one major paediatric cancer centre, an adult PC team collaborates to offer PPC services [30].
In the community, one non-governmental organisation provides home-based PC covering the capital with the possibility of telehealth for geographically far locations. However, demand exceeds bandwidth to optimise reach. This lack of accessibility along with cultural factors (such as the family involvement in the care during illness) accentuate parents’ role in delivering PPC, especially at home. A recent policy brief stipulates the provision of PC at patients’ residence as a ‘viable option’, valuing family ties [31]. Unpublished data on PPC tasks highlighted that parents in Lebanon engage in various PPC activities including managing treatment side effects, meeting emotional needs and praying with the child.
The Ministry of Public Health recently launched the national cancer plan with a chapter fully dedicated to PC, overarching both adults and paediatrics [32] which is a welcome development.
India
According to a recent report on the National Cancer Registry Programme, childhood cancers account for 4.0% of all cancers in India [33]. Availability of PC becomes imperative given poor awareness, delayed diagnosis and treatment abandonment. However, less than 1% of those needing it, i.e., less than 42,500 children and their families have access to PC services in India.
Hyderabad runs one of the largest PPC programmes in the country through a well-integrated programme in a public cancer hospital, MNJ Institute of Oncology (MNJIO) and in Niloufer Children’s hospital, forming linkages in the community through a non-governmental organisation called ‘Pain Relief and PC Society’ (PRPCS). In MNJIO, every child admitted for cancer treatment is routinely screened for pain and offered PC support early in the course of illness. If the child relapses or has recurrent disease, there is a seamless transition to palliative and end-of-life care. The care continuum is ensured through a paediatric home-based programme and a ten bed children’s hospice – ‘Mandara’ (Hibiscus) – run by PRPCS. Many of these children come from remote districts of Telangana for treatment and are referred to Government-led district-based PC centres closest to home. Every service is provided with the help of a dedicated and trained multidisciplinary team and essential medications, such as oral morphine, are available even in most remote districts of the state. On average, 30 new children are referred for PC every month in MNJIO. Additionally, about 20 children are admitted to the hospice every month and on average 150 children are registered under the home care programme at any time point. The Hyderabad model of PPC is an example of providing comprehensive, cost effective and equitable coverage of care which benefits children and their families and is easily replicable.
United States
Cancer remains the leading cause of death by disease among children in the United States, with approximately one in five children dying from their illness [34]. In the US, different entities including the American Society of Clinical Oncology, American Academy of Pediatrics, the Institute of Medicine, Association of Pediatric Hematology/Oncology Nurses and the National Consensus Project support the integration of PPC into health care. Specialist PPC is offered by PPC teams at 75% of childhood cancer centres [35]. Various models for the delivery of PC have been utilised and demonstrated to improve a variety of outcomes [36].
Different PPC programmes have varying inpatient, outpatient, community, virtual and home PPC services [35]. Multiple models of PC integration in oncology in the United States have been implemented, ranging from traditional consult-based models to oncologist-delivered primary PC, to referral for specialty PC in a consultative model, to systems-based trigger-prompted involvement, to embedded and fully integrated models of care [37]. Within the integrated model, care is led by the oncologist but they work closely with the PC team who attend weekly team meetings and visit the inpatient units on a daily basis. Such proximity is likely to help successful integration with education, relationship building and collaboration, as well as prompt identification of children who may benefit from PC [38]. A model combining this approach with systems-based triggers that prompts primary oncology teams to initiate PC consults is utilised at multiple US-based paediatric cancer centres. This model both combines the role of primary oncology teams and provides guidelines for when PC consultation may be beneficial.
Fully integrated, embedded or universal consultation models in paediatric oncology at a basic level with triggers for escalating levels of involvement, which effectively delivers individualised titrated PC services to patients is another approach. Thus, PC teams aim to reach the majority of high-risk patients early in their course of treatment but reserve full team resources for those who would benefit most. Combined training in paediatric haematology/oncology and hospice and palliative medicine (paediatric palliative oncology) can help facilitate this model [39]. A fully integrated model wherein PC consultation is embedded in teams such as the Haematopoietic Stem Cell Transplantation team and provides full consultative services to all patients is one that has great potential benefits but requires significant dedicated resources [40]. A recent report on fully integrated paediatric palliative oncology models includes embedding PPC in oncology in the following ways: (1) the floating clinic model where PPC experts float between inpatient, outpatient and home-based care, (2) a disease-specific embedded PPC expert where a PPC physician or nurse practitioner serves as a fully integrated member of the primary oncology team, (3) trigger-based or (4) consultation-based clinics where PPC consultations are triggered by pre-defined list of criteria or by oncology team and (5) telehealth PPC clinics [41].
Examples of research in PPC provision in cancer care
There is a lack of robust evidence for PPC in cancer care [42]. Much of this evidence comes from high-income settings like UK, Europe, North America, Australia and New Zealand. Three examples of PPC research for children with cancer will be discussed from a range of settings: the ‘Assessing Doctors’ Attitudes on Palliative Treatment’ (ADAPT) study; review of literature on healthcare workers, parents and communities’ perspectives to PPC, and needs assessment and situational analysis for PPC in Ghana.
ADAPT studies
Multiple barriers including lack of trained healthcare professionals (HCPs) and financial limitations exist in the integration of PPC. Additionally, underlying perceptions of PC and patient/parent refusal have also been identified as significant barriers to earlier integration of PC for children with cancer [43–47]. Historically, most research has been performed in high-income contexts, with little known about perceived barriers to PC for children in low- and middle-income contexts.
The ‘ADAPT’ survey was developed to assess physician perceptions of PC including physician comfort in providing PC, knowledge of PC principles and identification of perceived barriers to earlier integration [48–52]. The survey was originally developed for countries in Eastern Europe and Central Asia and has subsequently been translated and distributed in over 50 countries worldwide.
Some common global themes have emerged including a persistent misperception amongst physicians worldwide that PC is synonymous with end-of-life care [48, 50, 52]. Also there appears to be differences between what is viewed as ideal timing for PPC involvement and what happens in practice. Common barriers to integration exist including lack of home-based services, physician knowledge, access to PPC specialists, perceived family resistance and physician discomfort [49, 51, 52].
Identifying these barriers are important to provide interventions that can be similar worldwide, while also tailoring unique interventions, with the eventual goal of directing educational and advocacy efforts that are global in scope while also adaptable to specific regional/country level needs.
Healthcare workers, parents and communities, perspectives about PPC
Existing literature regarding knowledge, attitudes and beliefs toward PPC amongst HCPs, parents and communities was recently summarised [53] to explore barriers challenging early PPC integration including lack of knowledge, negative attitudes and beliefs toward PPC. Sixty articles were included in the analysis, of which 82% (n = 49) were derived from HICs. Publications from LMICs were modest in number. Perspectives of HCPs were more extensively explored versus parents and community. The reports described confusion between PPC and end-of-life care. Factors associated with knowledge and attitudes included respondents’ demographic characteristics and patients’ clinical information. Although poor knowledge and negative attitudes toward PC were recurrent among HCP, patients, their parents and community, a positive attitude prevailed whenever respondents possessed accurate information about such care. Findings underscored a pressing need for prompt interventions among professionals and for timely awareness among non-professionals to alleviate children’s suffering.
Needs assessment and situational analysis in Ghana
To understand the need for PPC in Ghana, a needs assessment and situational analysis was undertaken through World Child Cancer (WCC) by a collaborative team from International Children’s PC Network (ICPCN), WCC, the Ministry of Health, Ghana Health Service, Korle-Bu Teaching Hospital, Komfo Anokye Teaching Hospital and Greater Accra Teaching Hospital. PPC in Ghana is relatively new, with isolated and limited provision of services [54]. There are five trained paediatric oncologists in the country and access to healthcare services is limited, thus delaying care. Two focus group discussions with 21 participants and 17 in-depth interviews were conducted, with three overarching themes emerging: a conceptual model for PC, challenges to PPC and how to advance PPC in Ghana. Recommendations from this study are being reviewed, and a paper and policy brief being developed for PPC development in Ghana. Recommendations were aimed at the Ministry of Health and Ghana Health Services, for hospitals and service providers, education providers, researchers, donors and other stakeholders [55].
Examples of education in PPC provision in cancer care
To provide PPC, we need essential resources and well-trained, competent personnel to provide such services. There are a wide range of education programmes available globally, including virtual, face-to-face and hybrid programmes, along with both generalist and specialist training. Three examples of education programmes spanning many countries are The Education in Palliative and End-of-Life Care (EPEC) – Paediatrics Programme; The ICPCN e-learning and webinars; and a 1-year Clinical Fellowship in Paediatric Palliative Medicine in South and South-East Asia.
The EPEC – paediatrics programme
The EPEC-Paediatrics programme was funded through the US National Institutes of Health to support the delivery of primary PPC within the haematology/oncology setting. It was developed in response to a lack of advanced training in the prevention and treatment of pain and other distressing symptoms, along with the need for more widespread PC training for the paediatric haematology/oncology interdisciplinary team [56]. The curriculum is delivered through a combination of online and face-to-face sessions, and the training comprises of 24 core PC modules. EPEC-Paediatrics has been utilised as an end-user curriculum to improve HCPs knowledge of PPC in order that they can deliver primary PC. Additionally, the course is delivered in-person by ‘Master Facilitators’ at ‘Train-the-Trainer’ (TtT) conference sessions where EPEC-Paediatric training approach emphasises teaching participants advanced teaching skills such as how to utilise EPEC-Paediatrics to disseminate PPC concepts to others. The TtT Conferences focuses on delivering PPC material in engaging ways, fostering acquisition of attitudes, knowledge and skills (AKSs) that in turn help achieve desired clinical outcomes. Trainers (Paediatric clinicians) are provided with 22 adaptable PowerPoint presentations, all training videos and a Trainer’s handbook for each module to teach interdisciplinary teams across a range of settings.
EPEC-Paediatrics remains the largest and most comprehensive PPC training curriculum and dissemination project worldwide and has been translated in many languages. It has shown improvement in teaching ability and improved PPCAKSs in two core domains of PPC: communication and pain and symptom management. Most attendees of EPEC-Paediatric conferences report improvements in the clinical care of children with serious illnesses at their own institutions [57].
Additionally, EPEC-Paediatrics has been utilised as an integrated knowledge translation project where regional teams of 3–6 health professionals based at 15 paediatric oncology programmes in Canada became EPEC-Paediatrics Trainers and taught health curriculum to health professionals and implemented quality improvement (QI) projects [58]. The curriculum also demonstrated success in its regional adaptation, including the novel delivery of course content via a virtual bilingual format. The Eurasia-based course resulted in significant improvement in participant attitudes, knowledge of PPC and an understanding of ideal timing of PC consultation and comfort in providing PPC to children with cancer [59]. The EPEC – Paediatric curriculum is constantly being updated, adapted and expanded to encourage interdisciplinary global participation.
ICPCN’s e-learning and webinars
Education of HCPs lies at the heart of a coordinated response [23] to address PC needs of more than 21 million children and families globally [1]. The ICPCN’s education programme provides opportunities for both face-to-face and online training.
The ICPCN’s free e-learning platform, in its 12th year of existence, continues to grow with >9,000 participants from countries representing all 6 WHO geographic regions enrolled on the site. Courses cover several PPC topics, some available in 14 languages. Key to the advancement of the platform is focussing on optimising ‘user’ experience and engagement by adopting new technology and through the development of high-quality resources addressing existing gaps in CPC education.
The COVID-19 Pandemic necessitated a shift to virtual learning experiences with new initiatives being undertaken:
a) The Global PC and COVID-19 series was developed in collaboration with ICPCN, the Worldwide Hospice PC Alliance, the International Association of Hospice and PC, and PC in Humanitarian Aid Situations and Emergencies. It included 124 experts from 27 countries who contributed to a series of webinars and accompanying briefing notes that provided globally relevant PC information and guidance within the context of the COVID-19 Pandemic.
b) A set of monthly webinars linked to the book, CPC: an International case-based manual, edited by ICPCN [27] was developed in collaboration with contributors to the book. These webinars ran for 14 months and >2,800 attendees from >110 countries attended.
c) Following the above webinars linked to the book, regular third Thursday monthly webinar meetings have continued with a range of topics identified and a diverse panel of speakers invited to share their expertise with a global audience.
Webinars are available to watch on ICPCN’s website and YouTube channel. Evaluation of ICPCN’s education programmes conducted in 2021 showed considerable change in knowledge, skills and attitudes with >80% saying it had improved their clinical practice. Importantly, online initiatives were seen as having a positive impact in terms of developing PPC service provision [60].
1-year clinical fellowship in paediatric palliative medicine for South and Southeast Asia
A 1-year fellowship programme was developed through an existing partnership between Two Worlds Cancer Collaboration, Canada and the Hyderabad Centre for PC, India, to train paediatricians as specialists and leaders in PPC in South and Southeast Asia. The format included an option of both a full time 1-year residential programme or a ‘Hybrid’ model.
The fellowship includes formal teaching, clinical rotations, mentorship, regular assessments of trainees and a scholarly project. Teaching includes 100 hours of weekly online classes, focusing on case-based learning and leadership skills. Mandatory 4 months of clinical rotations in PPC include 2 months in the regional center of PPC excellence in Hyderabad. A mentorship programme provides additional support, which continues beyond the fellowship through an early career mentorship group Trainees’ progression toward programme competencies is assessed through written and observed standardised clinical examinations. As part of research and QI training, fellows complete a scholarly project with support and supervision from experienced research mentors.
Since 2018, eight paediatricians (3 full time and 5 hybrid) have completed the fellowship, with three fellows currently in training. Graduated fellows have become regional and national leaders in PPC, developing new PPC programmes and implementing new PPC training in their home countries. The programme is endorsed by the Royal College of Paediatrics and Child Health (UK), which has strengthened the programme’s rigour and quality.
Conclusion
Children with cancer and their families experience tremendous suffering, much of which can be relieved through the PPC. Providing PPC for paediatric cancer patients is, therefore, an ethical imperative. To address current gaps and unmet needs, we should look to successful clinical models of integration, continue to advance research and better understand the priorities of patients and families and continue to expand educational opportunities in this area. Together we can make a world of difference in the global integration of PPC into paediatric cancer care.
Conflicts of interest
The author(s) declare that they have no conflict of interest.
Funding
This work was not financed.
References
1. Connor SR, Downing J, and Marston J (2017) Estimating the global need for palliative care for children: a cross-sectional analysis J Pain Symptom Manage 53(2) 171–177 https://doi.org/10.1016/j.jpainsymman.2016.08.020
2. World Health Organization EMRO International Childhood Cancer Day 2022-2023 [https://www.emro.who.int/fr/noncommunicable-diseases/campaigns/international-childhood-cancer-day-2022.html] Date accessed: 16/10/23
3. Steliarova-Foucher E, Colombet M, and Ries LAG, et al (2017) International incidence of childhood cancer, 2001-10: a population-based registry study Lancet Oncol 18(6) 719–731 https://doi.org/10.1016/S1470-2045(17)30186-9 PMID: 28410997 PMCID: 5461370
4. World Health Organization (2021) Childhood Cancer (Geneva: World Health Organization) [https://www.who.int/news-room/fact-sheets/detail/cancer-in-children] Date accessed: 16/10/23
5. Bhakta N, Force LM, and Allemani C, et al (2019) Childhood cancer burden: a review of global estimates Lancet Oncol 20(1) e42–e53 https://doi.org/10.1016/S1470-2045(18)30761-7 PMID: 30614477
6. Lam CG, Howard SC, and Bouffet E, et al (2019) Science and health for all children with cancer Science 363(6432) 1182–1186 https://doi.org/10.1126/science.aaw4892 PMID: 30872518
7. World Health Organization (2021) CureAll Framework: WHO Global Initiative for Childhood Cancer: Increasing Access, Advancing Quality, Saving Lives (Geneva: World Health Organization)
8. World Health Organization (2020) Global Health Estimates 2019: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019 (Geneva: World Health Organization)
9. Downing J, Boucher S, and Nkosi B, et al (2017) Palliative care for children in low and middle-income countries Cancer Control 2017: Cancer Care in Emerging Health Systems UK ed I MacGrath (INCTR and Global Health Dynamics) pp 71–76
10. Open Society Foundation (2015) Children’s Palliative Care and Human Rrights (New York: Open Society Foundation) [https://www. opensocietyfoundations.org/sites/default/files/childrens-palliative-care-human- rights- 20151008.pdf] Date accessed: 09/09/23
11. World Health Organization (2006) Cancer Control: Who Guide for Effective Programmes (Geneva: World Health Organization) [https://www.who.int/publications/i/item/9241546999] Date accessed: 09/09/23
12. World Health Assembly (2017) WHA70.12 Cancer Prevention and Control in the Context of an Integrated Approach (Geneva: World Health Assembly) [https://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_R12-en.pdf] Date accessed: 09/09/23
13. World Health Organization (2023) Universal Health Coverage (UHC) (Geneva: World Health Organization) [https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc)] Date accessed: 09/09/23
14. World Health Organization and the UNICEF (2018) Declaration of Astana (Geneva: WHO)
15. UNDP 2023 Sustainable development goals. Goal 3. Good Health and Well Being [https://www.google.com/search?client=safari&rls=en&q=undp+sustainable+development+goals&ie=UTF-8&oe=UTF-8] Date accessed: 09/09/23
16. Lacerda A, Martinex MA, and Dumont B, et al (2023) Embracing paediatric palliative care in paediatric oncology from diagnosis onwards Pediatric Blood Cancer 70 e30561 https://doi.org/10.1002/pbc.30561 PMID: 37430425
17. Haines ER, Frost AC, and Kane HL, et al (2018) Barriers to accessing palliative care for pediatric patients with cancer: a review of the literature Cancer 124(11) 2278–2288 https://doi.org/10.1002/cncr.31265 PMID: 29451689
18. Grüneberg ES, Ramos-Guerrero J, and Pastrana T (2021) Challenges in the provision of pediatric palliative care in Mexico: a cross-sectional web-based survey J Palliat Care
19. Neha C and Vivek K (2016) Challenges to palliative care in pediatric patients J Palliat Care Med 6 256
20. Zahedi F, Kadivar M, and Khanali Mojen L, et al (2022) The ethical challenges of palliative care from the perspectives of pediatricians: qualitative study in Iran Front Pediatr 10 928476 https://doi.org/10.3389/fped.2022.928476
21. Rico-Mena P, Güeita-Rodríguez J, and Martino-Alba R, et al (2023) The emotional experience of caring for children in pediatric palliative care: a qualitative study among a home-based interdisciplinary care team Children 10(4) 700 https://doi.org/10.3390/children10040700 PMID: 37189949 PMCID: 10136408
22. Borhani F, Abbaszadeh A, and Mohsenpour M, et al (2013) Lived experiences of pediatric oncology nurses in Iran Iran J Nurs Midwifery Res 18(5) 349–354
23. World Health Organization (2021) Assessing the Development of Palliative Care Worldwide: A Set of Actionable Indicators (Geneva: World Health Organization)
24. Hawley P (2017) Barriers to access to palliative care Palliat Care Res Treat 10 1178224216688887 https://doi.org/10.1177/1178224216688887
25. Kagarmanova A, Mwesiga MD, and Sisk ML, et al (2022) Palliative care in Uganda: quantitative descriptive study of key palliative care indicators 2018-2020 BMC Palliat Care 21 55 https://doi.org/10.1186/s12904-022-00930-7
26. Downing J, Nakawesi J, and Kiwanuka R (2012) Paediatric palliative care in Uganda Pediatric Palliative Care: Global Perspectives ed C Knapp, V Madden, and S Fowler-Kerry (Dordrecht: Springer) pp 41–64
27. Downing J (Ed) (2021) Children’s Palliative Care: an International Case-Based Manual (Switzerland: Springer)
28. Ministry of Health (2000) Health Sector Strategic Plan 2000/01–2004/05 (Kampala: Government of Uganda)
29. Palliative Care Association of Uganda (2009) Audit Report of Palliative Care Services in Uganda (Kampala: PCAU)
30. Rassam RS, Abu-Saad Huijer H, and Noureddine S, et al (2023) Unfolding parental knowledge, attitudes, and beliefs toward palliative care for children with cancer Pediatr Blood Cancer e30484 https://doi.org/10.1002/pbc.30484 PMID: 37289143
31. Soueidan S, Osman H, and El-Jardali F (2018) K2P Policy Brief: Integrating Palliative Care into the Health System in Lebanon (Beirut: Knowledge to Policy (K2P) Center) [https://www.aub.edu.lb/k2p/Documents/K2P%20Policy%20Brief-%20Palliative%20Care-August%207%202018.pdf ] Date accessed: 04/01/2022
32. Republic of Lebanon Ministry of Public Health National Cancer Plan Official Version (Baabda: Republic of Lebanon Ministry of Public Health) [https://www.moph.gov.lb/userfiles/files/About%20MOPH/StrategicPlans/National%20Cancer%20Plan%202023-2028/NATIONAL%20CANCER%20PLAN%20OFFICIAL%20VERSION.pdf] Date accessed: 15/08/2023
33. Nath A, Mathur P, and Sudarshan KL, et al (2023) An assessment of childhood cancer care services in India – gaps, challenges and the way forward Lancet Reg Health Southeast Asia 16 100235 https://doi.org/10.1016/j.lansea.2023.100235
34. Ward E, DeSantis C, and Robbins A, et al (2014) Childhood and adolescent cancer statistics, 2014 CA Cancer J Clin 64(2) 83–103 https://doi.org/10.3322/caac.21219 PMID: 24488779
35. Weaver MS, Rosenberg AR, and Tager J, et al (2018) A summary of pediatric palliative care team structure and services as reported by centers caring for children with cancer J Palliat Med 21(4) 45262 https://doi.org/10.1089/jpm.2017.0405
36. Kaye EC, Weaver MS, and DeWitt LH, et al (2021) AAHPM Research Committee. The impact of specialty palliative care in pediatric oncology: a systematic review J Pain Symptom Manage 61(5) 1060–1079.e2 https://doi.org/10.1016/j.jpainsymman.2020.12.003
37. Levine DR, Baker JN, and Wolfe J, et al (2017) Strange bedfellows no more: how integrated stem-cell transplantation and palliative care programs can together improve end-of-life care J Oncol Pract 13(9) 569–577 https://doi.org/10.1200/JOP.2017.021451 PMID: 28898603 PMCID: 6366811
38. Selvaggi KJ, Vick JB, and Jessell SA, et al (2014) Bridging the gap: a palliative care consultation service in a hematological malignancy-bone marrow transplant unit J Community Support Oncol 12 50–55 https://doi.org/10.12788/jcso.0015 PMID: 24971405
39. Snaman JM, Kaye EC, and Levine DR, et al (2016) Pediatric palliative oncology: a new training model for an emerging field J Clin Oncol 34(3) 288–289 https://doi.org/10.1200/JCO.2015.63.0178
40. Lafond DA, Kelly KP, and Hinds PS, et al (2015) Establishing feasibility of early palliative care consultation in pediatric hematopoietic stem cell transplantation J Pediatr Oncol Nurs 32 265–277 https://doi.org/10.1177/1043454214563411 PMID: 25616372
41. Uber A, Ebelhar JS, and Lanzel AF, et al (2022) Palliative care in pediatric oncology and hematopoietic stem cell transplantation Curr Oncol Rep 24(2) 161–174 https://doi.org/10.1007/s11912-021-01174-z PMID: 35061198
42. Downing J (2016) Editorial: to research or not to research – an important question in paediatric palliative care Palliat Med 30(1) 902–903 https://doi.org/10.1177/0269216316675570 PMID: 27864501
43. Ethier JL, Paramsothy T, and You JJ, et al (2018) Perceived barriers to goals of care discussions with patients with advanced cancer and their families in the ambulatory setting: a multicenter survey of oncologists J Palliat Care 33 125–142 https://doi.org/10.1177/0825859718762287 PMID: 29607704
44. Hilden JM, Emanuel EJ, and Fairclough DL, et al (2001) Attitudes and practices among pediatric oncologists regarding end-of-life care: results of the 1998 American Society of Clinical Oncology survey J Clin Oncol 19 205–212 https://doi.org/10.1200/JCO.2001.19.1.205 PMID: 11134214
45. Davies B, Sehring SA, and Partridge JC, et al (2008) Barriers to palliative care for children: perceptions of pediatric health care providers Pediatrics 121 282–288 https://doi.org/10.1542/peds.2006-3153 PMID: 18245419
46. Szymczak JE, Schall T, and Hill DL, et al (2018) Pediatric oncology providers’ perceptions of a palliative care service: the influence of emotional esteem and emotional labor J Pain Symptom Manage 55 1260–1268 https://doi.org/10.1016/j.jpainsymman.2018.01.019 PMID: 29425881 PMCID: 5908218
47. Dalberg T, Jacob-Files E, and Carney PA, et al (2013) Pediatric oncology providers’ perceptions of barriers and facilitators to early integration of pediatric palliative care Pediatr Blood Cancer 60 1875–1881 https://doi.org/10.1002/pbc.24673 PMID: 23840035 PMCID: 3966071
48. Ehrlich BS, Movsisyan N, and Batmunkh T, et al (2020) A multicountry assessment in Eurasia: Alignment of physician perspectives on palliative care integration in pediatric oncology with World Health Organization guidelines Cancer 126(16) 3777–3787 https://doi.org/10.1002/cncr.33001 PMID: 32530519 PMCID: 7385991
49. Ehrlich BS, Movsisyan N, and Batmunkh T, et al (2020) Assessing Doctors’ attitudes on palliative treatment (ADAPT) research group. Barriers to the early integration of palliative care in pediatric oncology in 11 Eurasian countries Cancer 126(22) 4984–4993 https://doi.org/10.1002/cncr.33151 PMID: 32813913 PMCID: 7981844
50. McNeil MJ, Ehrlich BS, and Wang H, et al (2022) Physician perceptions of pediatric palliative care for children with cancer in Latin America JAMA Network Open 5(3) e221245 https://doi.org/10.1001/jamanetworkopen.2022.1245
51. McNeil MJ, Godfrey A, and Loggetto P, et al (2023) Physician perceptions of and barriers to pediatric palliative care for children with cancer in Brazil JCO Glob Oncol 9 e2300057 https://doi.org/10.1200/GO.23.00057 PMID: 37535886 PMCID: 10581636
52. McNeil MJ, Ehrlich B, and Wang H, et al (2023) Ideal vs actual timing of palliative care integration for children with cancer in Latin America JAMA Netw Open 6(1) e2251496 https://doi.org/10.1001/jamanetworkopen.2022.51496 PMID: 36656580 PMCID: 9857245
53. Saad R, Abu-Saad Huijer H, and Noureddine S, et al (2022) Pediatric palliative care through the eyes of healthcare professionals, parents and communities: a narrative review Ann Palliat Med 11(10) 3292–3314 https://doi.org/10.21037/apm-22-525 PMID: 36267010
54. Rhee JY, Luyirika E, and Namisango E, et al (2017) APCA Atlas of Palliative Care in Africa (Houston: IAHPC Press)
55. Downing J, Adongo EA, and Kuma-Aboagye P, et al (2023) Report of the Needs Assessment and Situational Analysis for Children’s Palliative Care in Ghana (ICPCN and WCC)
56. Friedrichsdorf SJ, Remke S, and Hauser J, et al (2019) Development of a pediatric palliative care curriculum and dissemination model: education in palliative and end-of-life care (EPEC) pediatrics J Pain Symptom Manage 58(4) 707–720.e3 https://doi.org/10.1016/j.jpainsymman.2019.06.008 PMID: 31220594 PMCID: 6754756
57. Postier AC, Wolfe J, and Hauser J, et al (2022) Education in palliative and end-of-life care-pediatrics: curriculum use and dissemination J Pain Symptom Manage 63(3) 349–358 https://doi.org/10.1016/j.jpainsymman.2021.11.017
58. Widger K, Wolfe J, and Friedrichsdorf S, et al (2018) National impact of the EPEC-pediatrics enhanced train-the-trainer model for delivering education on pediatric palliative care J Palliat Med 21(9) 1249–1256 https://doi.org/10.1089/jpm.2017.0532 PMID: 29782212
59. McNeil MJ, Ehrlich B, and Yakimkova T, et al (2023) Regional adaptation of the education in palliative and end-of-life care pediatrics (EPEC-Pediatrics) curriculum in Eurasia Cancer Med 12(3) 3657–3669 https://doi.org/10.1002/cam4.5213 PMCID: 9939085
60. ICPCN (2023) International Children’s Palliative Care Network Impact Report March 2023 (ICPCN)






