Incidental venous thrombosis in oncology in a sub-Saharan tertiary hospital
Etienne Okobalemba Atenguena1, Joseph Francis Nwatsock2, Berthe Sabine Esson Mapoko3, Lionel Fossa Tabola1, Kenn Chi Ndi1, Jérôme Boombhi4 and Paul Ndom1
1Medical Oncology, General Hospital, PO Box 5408, Yaoundé, Cameroon
2Radiology, General Hospital, PO Box 5408, Yaoundé, Cameroon
3Haematology Oncology, Central Hospital, PO Box 87, Cameroon
4Cardiology, General Hospital, PO Box 5408, Yaoundé, Cameroon
Abstract
The relationship between cancer and thrombosis was initially highlighted in the 19th century. Vascular complications in oncology can be arterial or venous thrombosis, and incidental pulmonary embolism is a growing challenge. We aimed to describe the frequency and clinical characteristics of cancer patients with incidental venous thromboembolism (iVTE). We conducted a descriptive study at the Yaounde General Hospital. We included patients with a confirmed diagnosis of cancer, followed up on an outpatient basis, in whom an iVTE was identified on a computed tomography scan performed to evaluate tumour status over a 6-month period. Of the 359 patients, 19 had venous thromboses, representing a frequency of 5.3%. The mean age was 51.2 years. The sex ratio was 1.1 in favour of males. Comorbidities found were diabetes, hypertension and obesity. Colon cancer (5), ovarian cancer (3) and lung cancer (3) were the most frequent diagnoses. All patients had advanced disease with 14 (73.7%) being naive to anticancer treatment. Pulmonary arteries were the most affected vessel (63.1%). The frequency of iVTE in a sub-Saharan context was around 5%.
Keywords: thrombosis, incidental, oncology, Cameroon
Correspondence to: Lionel Fossa Tabola
Email: magnantr@yahoo.fr
Published: 07/11/2024
Received: 20/03/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
The relationship between cancer and thrombosis was first demonstrated in the 19th century, with several studies following, in a bid to better understand the different mechanisms involved [1]. Components of Virchow’s triad (blood stasis, hypercoagulability and endothelial damage) are generally found in patients with cancer, predisposing them to a greater risk of thrombosis. Vascular complications in oncology can be arterial or venous thrombosis [2]. Of these two types, venous thromboembolism (VTE) is the most common and includes deep vein thrombosis and pulmonary embolism (PE). It can occur at any time during the follow-up of cancer and can sometimes reveal the disease [3]. Patients with cancer have a 5 to 7 times greater risk of developing VTE [4]. In cancer patients, VTE is associated with a worse prognosis and increased medical costs [5, 6]. These varying vascular events can be identified on a contrast-enhanced computed tomography (CT) scan. People with cancer often undergo multiple routine staging and response to treatment
CT examinations [7]. Advances in medical imaging studies, including CT, have allowed for improved visualisation of the pulmonary arterial tree, which has led to an increase in the detection of PE as an incidental finding [8, 9]. In low- and middle-income countries, as is the case in most of sub-Saharan Africa, the availability and cost of CT scans are obstacles to their use. There is less than one CT scan per one million population for low- and middle-income countries, compared to 40 scans per one million population for high-income countries [10]. In the literature, few studies on thrombosis in patients with cancer have been published in sub-Saharan Africa. Virtually all of the epidemiological data on VTE come from high-income countries [11]. A study on the rates of VTE in high-income, upper middle-income and lower middle/low-income countries concluded that the rates of VTE are substantially higher in high-income than in low-income countries [12]. We sought to describe incidental venous thromboembolism (iVTE) found on CT in outpatients with cancer in a sub-Saharan African country to obtain data on the subject. These data, which will make it possible to assess the efficiency of iVTE detection, will remind clinicians to treat these patients effectively. This study aimed to describe the frequency and clinical characteristics of cancer patients with iVTE.
Methods
We carried out a single-centre descriptive cross-sectional study at the Yaounde General Hospital, the referral hospital for the management of cancer in the city of Yaounde, the capital of Cameroon, a country in Central Africa. This study was conducted from January to June 2022. The study population consisted of consenting outpatients with pathologically confirmed cancer in whom a staging contrast-enhanced thoraco-abdomino-pelvic CT scan incidentally found VTE. iVTE was defined as a filling defect on contrast-enhanced CT scans performed for reasons other than clinical suspicion of VTE. In this study, iVTE was classified into two categories depending on whether the thrombosis occurred in or outside the thorax: incidental pulmonary embolism (iPE) and unusual deep VTE. iPE was defined as a filling defect in one or more pulmonary arteries in a contrast-enhanced chest CT. We defined unusual deep site VTE as thrombosis that affects any vein located either in the abdomen or in the pelvis.
Thoraco-abdomino-pelvic CT scans were all requested and performed in the same hospital, which has three radiologists who each have more than 5 years of experience. The CT scan used realises slices of 1 mm thickness. Our sampling was consecutive and non-probabilistic. All new and old patients who visited the oncology outpatient unit during the study period were approached by the investigator to check for study eligibility, and signed informed consent forms were obtained.
A pre-established questionnaire was used to collect the data. We collected the different variables on a handwritten questionnaire before entering them into the analysis software.
The variables collected from patient files included patient demographics, past history, cancer characteristics, thrombosis diagnostic imaging data and initial VTE management. Demographic data included age and sex, and past history included the existence of comorbidities, the nature of these comorbidities and a history of COVID-19 infection. Cancer characteristics included primary site, histologic type and American Joint Committee on Cancer stage.
Thrombosis diagnostic imaging data included the reason for imaging and the site of thrombosis. The reason for imaging was one of the following: initial evaluation if the patient had not yet received any anticancer treatment, midterm evaluation if the patient had received some systemic anticancer treatment and surveillance if the patient had completed anticancer treatment. Radiology reports were reviewed to determine the location of the VTE.
The incidence rate was calculated by dividing the number of iVTE cases by the number of thoraco-abdomino-pelvic CT scans performed during a 6-months period.
Statistical analysis was performed using the Epi Info version 5 software. For continuous data, the number of subjects, average, standard deviation (SD), median, minimum value and maximum value are presented. For categorical data, the frequency and percentage are displayed, and the number of cases are shown for categories collected multiple times. The descriptive statistics (average, SD, median, minimum value, maximum value and percentage) are rounded up and presented to one decimal point.
We obtained a research authorization from the administration of the General Hospital of Yaounde. Free and informed consent was obtained from each patient prior to participation in the study.
Results
During our study period, we recruited 19 patients with incidental venous thrombosis among the 359 who underwent routine staging or treatment assessment CT scans. This represents a frequency of 5.3%.
Patient characteristics
The average age was 51.2 years. The sex ratio was 1.1 in favour of males. Of our patients, six had a comorbidity. Hypertension was the most frequent (n = 3) comorbidity. Three patients were found to have had a previous COVID-19 infection, (Table 1).
Disease characteristics
The largest number of incidental VTE were seen in patients with colon cancer (n = 5), followed by ovarian cancer (n = 3) and lung cancer (n = 3). Adenocarcinoma was the most frequent histological type (n = 13), followed by squamous cell carcinoma (n = 4). All 19 patients had advanced cancer: 5 had stage III cancer and the other 14 had stage IV cancer (Table 2).
Imaging indication
Fourteen patients underwent imaging for an initial evaluation of their cancer, four were at the mid-term evaluation. Following the diagnosis of VTE, 13 individuals (68.4%) were initially treated with an oral anticoagulant (rivaroxaban). The remaining six patients did not receive any anticoagulant treatment (Table 3).
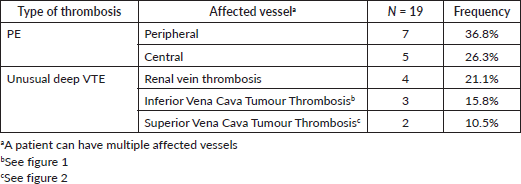
Anatomical location of thrombosis
Pulmonary arteries were the most affected vessel (63.1%). We had 47.4% of deep VTE occurring outside the thorax. Some patients had multiple affected vessels (Table 4).
Discussion
In our study, the frequency of iVTE was 5.3%. The rate of iVTE varies between studies, ranging from 1.4% to 9% [13, 14]. The wide variation in the incidence of iVTE, particularly iPEs can be explained by the high heterogeneity between studies. The slice thickness of the CT scans could also be a possible explanation. Dentali et al [14] reported a higher incidence of iPE when using CT scans with <5 mm slice thickness compared to >5 mm slice thickness (3.0% versus 2.0%). In our study, the thickness of the CT slices was less than 5 mm, which probably enabled us to obtain incidence rates closer to the high values found in the literature.
Of the 19 patients with iVTE, 10 were male. The role of gender in the occurrence of thrombosis varies between studies. Women are more likely to develop VTE, whereas men are more likely to develop arterial thrombosis [15, 16].
Several risk factors for iVTE have been described in the literature. We looked for different risk factors in patients in this study. Diabetes, hypertension and obesity were the comorbidities found in our patients. Among our patients, 2 (10.5%) were obese. Obesity is associated with a high risk of ischemic stroke, as well as VTE in patients of both sexes [17, 18]. In their study, Siegal et al [12] who worked on the effect of country income level on VTE risk, found that the effect of country income level on VTE risk was markedly stronger in people with a lower BMI compared to those with higher BMI (p < 0·001). In our sample, the majority of patients had a BMI of less than 30 (17/19). They would therefore be deemed to be at less risk of VTE.
Table 1. Patient characteristics.
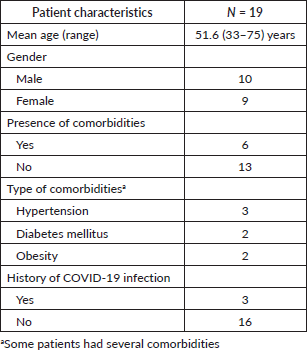
Table 2. Cancer characteristics.
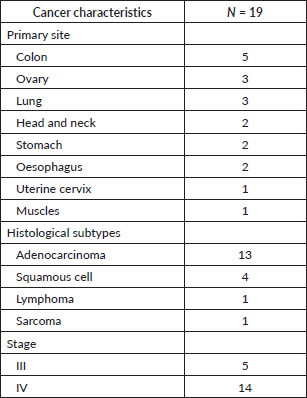
Table 3. Imaging indication.
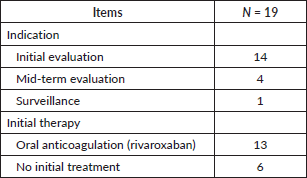
Table 4. Anatomical location.
Three of our patients had a COVID-19 infection in the past. After a respiratory or other infection, there is a two- to three-fold increased risk of VTE [19]. This risk decreases after infection resolution. However, this risk may persist for a year or more [19]. The risk of VTE after COVID-19 infection is mixed. A meta-analysis in patients with a previous severe infection in the first wave found a 13% incidence of VTE [20]. Another study including a control group did not find a higher risk in patients with a history of COVID-19 infection [21].
In our sample, the most common existing malignancy was colon cancer; followed by ovarian and lung cancers. The incidence of venous thrombosis differs according to the primary site of the cancer. It is higher in primary sites such as the liver, pancreas (about 4%) and ovary, compared to bladder or head and neck cancers (1%) [22, 23].
Adenocarcinoma was the most frequent histological type in our population. The histological type of cancer is related to the risk of VTE. The risk is higher for adenocarcinoma than for squamous cell carcinoma in patients with bronchial cancer [24]. Some studies suggest a higher frequency of VTE in patients with bronchial, pancreatic or gastrointestinal adenocarcinoma secreting mucin [25]. However, there was no significant difference between the different histological types of breast or colon cancers [26, 27].
All patients had advanced-stage cancer in our sample. Patients with advanced cancer would be at a higher risk of developing VTE [28]. Patients with metastatic disease had a 1.5-fold higher incidence of PE compared to patients without metastases [29].
Thrombosis was diagnosed in 15 patients (75%) at the time of initial evaluation. The period immediately following the diagnosis of cancer is a period in which the risk of developing VTE is high [30]. Of our patients, 4 (21.1%) had already started chemotherapy. Chemotherapy is an important risk factor for VTE in patients with cancer. Patients undergoing treatment have a 6- to 7-fold greater risk of developing VTE, and within 12 months of chemotherapy initiation, the rate of VTE is higher in patients with cancer [31]. Surgery, radiotherapy, hospitalisation, central venous catheters and other anti-cancer drugs [15, 32, 33] are likewise, risk factors for VTE. The mechanisms explaining treatment-related thrombosis are not fully understood. Drugs may activate and disrupt the integrity of the endothelium, decrease anticoagulants and increase pro-coagulants such as tissue factor leading to activation of coagulation or directly or indirectly activating platelets. It is difficult to differentiate between the pro-thrombotic actions of the tumour itself and those of the various anti-cancer treatments administered to patients.
Of our patients, 26.3% had central iPE. The prevalence of central PE in incidental PE cases diagnosed varied from 23% to 63.6% in the literature [34, 35]. This infers that in many cancer patients, continuous prothrombotic states due to cancer contribute to the gradual expansion of pulmonary thrombosis resulting in hemodynamic adaptations in patients.
Some patients had unusual deep VTE, e.g., vena cava thrombosis. Generally, the signs and symptoms of superior vena cava obstruction are progressive. Isolated intravascular thrombosis is rare [36]. Cancers are the cause in 2/3 of cases. Occlusion is due to thrombosis in 1/3 of cases, reflecting the increased use of intravascular devices such as central venous catheters, catheter portals and pacemakers [37]. None of our patients had an intravascular device. Inferior vena cava thrombosis is rare [38]. Although inferior vena cava thrombosis is not frequently detected, it can be associated with complications such as post-thrombotic syndrome (90%), venous claudication (45%), PE (30%) and venous ulceration (15%) [39].
Of our patients, 13 (68%) received treatment for the iVTE. Those 13 patients all initially received rivaroxaban. Qdaisat et al [35] showed that cancer patients with iPE have a worse prognosis than those without PE. They argued that patients with IPE should be treated with proper management plans similar to their symptomatic counterparts [35].
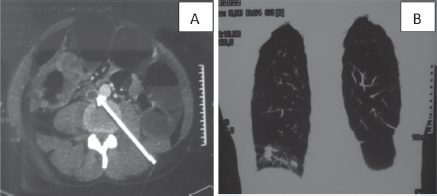
Figure 1. CT scan of a 37-year-old man: (a): Cross-section of inferior vena cava thrombosis (white arrow) and a circumferential mass of the right colon. (b): Frontal section of lung metastases.
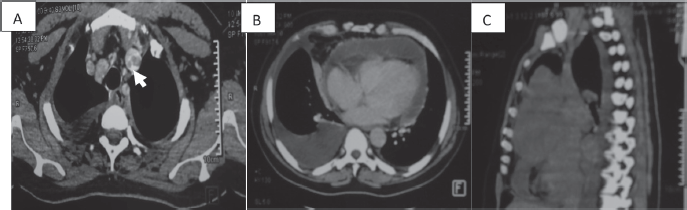
Figure 2. CT scan of a 58-year-old man (a): Cross-section of brachiocephalic trunk thrombosis (white arrow). (b): Cross-section of right pericardial and pleural effusion. (c): Sagittal section with a mediastinal mass.
This study on incidental venous thrombosis in oncology in a sub-Saharan tertiary hospital proves that this is a real clinical situation. Medical teams caring for cancer patients in sub-Saharan Africa must pay close attention to the CT scans performed in order to optimally manage any iVTE. This proper management will help to avoid potentially fatal acute complications.
Limitations
The present study has several limitations owing to its retrospective and single-centre design. The small sample size may limit the applicability of our findings. We did not collect respiratory symptoms data. Some patients may have had symptoms when questioned further, possibly increasing symptomatic VTE, and then decreasing the percentage of incidental VTE. Radiology reports were used without reviewing the CT images and iVTE may have been missed in initial reports.
Conclusions
We found that the incidence rate of incidental VTE in a sub-Saharan context is around 5%. iVTE was most frequently observed in patients with colon, ovarian and lung cancer. iPE was more frequent than unusual deep VTE. Policy makers in sub-Saharan Africa should take actions like: set up programmes to make CT scans accessible, and make available recommendations for the management of iVTE or nothing will change. They could consider oral anticoagulants, which solve a number of problems (few drug interactions, no need for biological monitoring).
Acknowledgments
The authors would like to thank the administration of the Yaounde General Hospital for permitting them to carry out this study. The authors are also grateful to the personnel of the medical oncology, cardiology and imaging units for their collaboration.
Conflicts of interest
All authors have no conflict of interest relevant to this study to declare.
Funding
This study was self-funded.
References
1. Khorana AA (2003) Malignancy, thrombosis and trousseau: the case for an eponym J Thromb Haemost 1 2463–2465 https://doi.org/10.1111/j.1538-7836.2003.00501.x PMID: 14675077
2. Eichinger S (2016) Cancer associated thrombosis: risk factors and outcomes Thromb Res 140 S12–S17 https://doi.org/10.1016/S0049-3848(16)30092-5 PMID: 27067965
3. Donnellan E and Khorana AA (2017) Cancer and venous thromboembolic disease: a review Oncologist 22 199–207 https://doi.org/10.1634/theoncologist.2016-0214 PMID: 28174293 PMCID: 5330704
4. Agnelli G and Verso M (2011) Management of venous thromboembolism in patients with cancer J Thromb Haemost 9 316–324 https://doi.org/10.1111/j.1538-7836.2011.04346.x PMID: 21781268
5. Okura Y, Ozaki K, and Tanaka H, et al (2019) The impending epidemic of cardiovascular diseases in patients with cancer in Japan Circ J 83 2191–2202 https://doi.org/10.1253/circj.CJ-19-0426 PMID: 31534064
6. Mahe I, Mayeur D, and Krakowski I (2016) Management of venous thromboembolism in cancer patients: the economic burden of hospitalizations Support Care Cancer 24 4105–4112 https://doi.org/10.1007/s00520-016-3224-0 PMID: 27146390
7. Carmona-Bayonas A, Jimenez-Fonseca P, and Font C, et al (2017) Predicting serious complications in patients with cancer and pulmonary embolism using decision tree modelling: the EPIPHANY index Br J Cancer 116(8) 994–1001 https://doi.org/10.1038/bjc.2017.48 PMID: 28267709 PMCID: 5396106
8. den Exter PL, van der Hulle T, and Hartmann IJ, et al (2015) Reliability of diagnosing incidental pulmonary embolism in cancer patients Thromb Res 136 531–534 https://doi.org/10.1016/j.thromres.2015.06.027 PMID: 26254194
9. Klok FA and Huisman MV (2017) Management of incidental pulmonary embolism Eur Respir J 49 1700275 https://doi.org/10.1183/13993003.00275-2017 PMID: 28663318
10. Hricak H, Abdel-Wahab M, and Atun R, et al (2021) Medical imaging and nuclear medicine: a lancet oncology commission Lancet Oncol 22(4) e136–e172 https://doi.org/10.1016/S1470-2045(20)30751-8 PMID: 33676609 PMCID: 8444235
11. Yusuf HR, Tsai J, and Atrash HK, et al (2012) Venous thromboembolism in adult hospitalizations – United States, 2007–2009 MMWR 61(22) 401–404
12. Siegal DM, Eikelboom JW, and Lee SF, et al (2021) Variations in incidence of venous thromboembolism in low-, middle-, and high-income countries Cardiovasc Res 117(2) 576–584 https://doi.org/10.1093/cvr/cvaa044
13. Callejas MF, Errazuriz JI, and Castillo F, et al (2014) Incidental venous thromboembolism detected by PET-CT in patients with cancer: prevalence and impact on survival rate Thromb Res 133(5) 750–755 https://doi.org/10.1016/j.thromres.2014.02.005 PMID: 24565275
14. Dentali F, Ageno W, and Becattini C, et al (2010) Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta-analysis Thromb Res 125 518–522 https://doi.org/10.1016/j.thromres.2010.03.016 PMID: 20451960
15. Khorana AA, Francis CW, and Culakova E, et al (2007) Frequency, risk factors, and trends for venous thromboembolism among hospitalised cancer patients Cancer 110 2339–2346 https://doi.org/10.1002/cncr.23062 PMID: 17918266
16. Khorana AA, Francis CW, and Culakova E, et al (2006) Thromboembolism in hospitalised neutropenic cancer patients J Clin Oncol 24 484 https://doi.org/10.1200/JCO.2005.03.8877 PMID: 16421425
17. Suk SH, Sacco RL, and Boden-Albala B, et al (2003) Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study Stroke 34 1586–1592 https://doi.org/10.1161/01.STR.0000075294.98582.2F PMID: 12775882
18. Stein PD, Beemath A, and Olson RE (2005) Obesity as a risk factor in venous thromboembolism Am J Med 118 978–980 https://doi.org/10.1016/j.amjmed.2005.03.012 PMID: 16164883
19. Clayton TC, Gaskin M, and Meade TW (2011) Recent respiratory infection and risk of venous thromboembolism: case-control study through a general practise database Int J Epidemiol 40 819–827 https://doi.org/10.1093/ije/dyr012 PMID: 21324940 PMCID: 3147071
20. Mansory EM, Srigunapalan S, and Lazo-Langner A (2021) Venous thromboembolism in hospitalised critical and noncritical COVID-19 patients: a systematic review and meta-analysis TH Open 5 e286–e294 https://doi.org/10.1055/s-0041-1730967
21. Mai V, Tan BK, and Mainbourg S, et al (2021) Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: a systematic review with meta-analysis Vasc Pharmacol 139 106882 https://doi.org/10.1016/j.vph.2021.106882
22. Blom JW, Doggen CJ, and Osanto S, et al (2005) Malignancies, prothrombotic mutations, and the risk of venous thrombosis J Am Med Assoc 293(6) 715–722 https://doi.org/10.1001/jama.293.6.715
23. Stein PD, Beemath A, and Meyers FA, et al (2006) Incidence of venous thromboembolism in patients hospitalised with cancer Am J Med 119(1) 60–68 https://doi.org/10.1016/j.amjmed.2005.06.058 PMID: 16431186
24. Chew HK, Wun T, and Harvey D, et al (2006) Incidence of venous thromboembolism and its effect on survival among patients with common cancers Arch Intern Med 166 458–464 https://doi.org/10.1001/archinte.166.4.458 PMID: 16505267
25. Haddad TC and Greeno EW (2006) Chemotherapy-induced thrombosis Thromb Res 118 555–568 https://doi.org/10.1016/j.thromres.2005.10.015 PMID: 16388837
26. Chew HK, Wun T, and Harvey DJ, et al (2006) Incidence of venous thromboembolism and the impact on survival in breast cancer patients J Clin Oncol 25 70–76 https://doi.org/10.1200/JCO.2006.07.4393 PMID: 17194906
27. Alcalay A, Wun T, and Khatri V, et al (2006) Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival J Clin Oncol 24 1112–1118 https://doi.org/10.1200/JCO.2005.04.2150 PMID: 16505431
28. Connolly G and Francis CW (2013) Cancer-associated thrombosis Haematol Am Soc Haematol Educ Program 2013 684–691 https://doi.org/10.1182/asheducation-2013.1.684
29. Bach AG, Schmoll HJ, and Beckel C, et al (2014) Pulmonary embolism in oncologic patients: frequency and embolus burden of symptomatic and unsuspected events Acta Radiol 55(1) 45–53 https://doi.org/10.1177/0284185113491569
30. Khorana AA and Connolly GC (2009) Assessing risk of venous thromboembolism in the patient with cancer J Clin Oncol 27 4839–4847 https://doi.org/10.1200/JCO.2009.22.3271 PMID: 19720906 PMCID: 2764392
31. Khorana AA, Dalal M, and Lin J, et al (2013) Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States Cancer 119 648–655 https://doi.org/10.1002/cncr.27772
32. Kuter DJ (2004) Thrombotic complications of central venous catheters in cancer patients Oncologist 9 207–216 https://doi.org/10.1634/theoncologist.9-2-207 PMID: 15047925
33. Oppelt P, Betbadal A, and Nayak L (2015) Approach to chemotherapy-associated thrombosis Vasc Med 20 153–161 https://doi.org/10.1177/1358863X14568705 PMID: 25832603 PMCID: 4655120
34. Banala SR, Yeung SJ, and Rice TW, et al (2017) Discharge or admit? Emergency department management of incidental pulmonary embolism in patients with cancer: a retrospective study Int J Emerg Med 10 19 https://doi.org/10.1186/s12245-017-0144-9 PMID: 28589462 PMCID: 5461224
35. Qdaisat A, Kamal M, and Al-Breiki A, et al (2020) Clinical characteristics, management, and outcome of incidental pulmonary embolism in cancer patients Blood Adv 4 1606–1614 https://doi.org/10.1182/bloodadvances.2020001501 PMID: 32311012 PMCID: 7189276
36. Wilson LD, Detterbeck FC, and Yahalom J (2007) Clinical practise. Superior vena cava syndrome with malignant causes N Engl J Med 356 1862–1869 https://doi.org/10.1056/NEJMcp067190 PMID: 17476012
37. Venturini E, Becuzzi L, and Magni L (2012) Catheter-induced thrombosis of the superior vena cava Case Rep Vasc Med 469619
38. McAree BJ, O’Donnell ME, and Fitzmaurice GJ, et al (2013) Inferior vena cava thrombosis: a review of current practise Vasc Med 18(1) 32–43 https://doi.org/10.1177/1358863X12471967 PMID: 23439778
39. Alkhouli M, Morad M, and Narins CR, et al (2016) Inferior vena cava thrombosis JACC Cardiovasc Interv 9(7) 629–643 https://doi.org/10.1016/j.jcin.2015.12.268 PMID: 26952909






