Core needle biopsy of breast tumours: comparison of diagnostic performance between surgery and radiology services at a national cancer centre in Latin America
Gonzalo Javier Ziegler-Rodriguez1,2,3, Miguel Ángel Pinillos Portella1,2, Gabriel De la Cruz Ku4, Sheila Eunice Vílchez Santillan1,2, Jorge Dunstan Yataco1, José Antonio Galarreta Zegarra1, Gabriela Calderón Valencia1 and José Manuel Cotrina Concha1
1Department of Breast and Soft Tissue Tumor Surgery, National Cancer Institute of Peru (INEN), Lima 15038, Peru
2Senology Unit, Clinica Ziegler, Lima 15036, Peru
3School of Medicine, Universidad Peruana de Ciencias Aplicadas, Lima 15023, Peru
4Universidad Cientifica del Sur, Lima 15067, Peru
Abstract
Introduction: Breast pathology is a very common reason for medical attention. Tissue diagnosis is usually obtained with core needle biopsy which could be performed by breast surgeons or interventional radiologists. Our aim was to assess the comparison of diagnostic performance between the two services.
Methods: A retrospective, descriptive and cross-sectional study was carried out on patients who had breast pathology at Instituto Nacional de Enfermedades Neoplasicas in 2019. Descriptive analyses, sensitivity and specificity were calculated using the R program version 4.2.3.
Results: From 1,082 patients with breast tumours who underwent core needle biopsy (CNB) during 2019, 782 cases were included. Breast surgeons performed 462 CNBs and radiologists performed 320 CNBs. The 87.5% were palpable tumours and 525 breast carcinomas were identified in the final pathology. The diagnostic performance showed that the sensitivity and specificity were greater than 95% and 98%, respectively. The waiting time in both showed that >95% underwent a CNB before 2 months. The breast surgery service performed the majority of the biopsies in less than 1 week since the indication of the execution of the CNB compared to the radiology service (90% versus 36%).
Conclusion: Both hospital services, breast surgery and radiology, are efficient in determining an accurate diagnosis using CNB. However, the breast surgery service performs CNB in a shorter time interval. Breast surgical oncologists are encouraged to perform CNB if there are understaffed radiology services to expedite the diagnosis and treatment of breast cancer patients.
Keywords: core needle biopsy, ultrasound-guided biopsy of the breast, breast surgical oncologist, interventional radiologist, breast diagnosis
Correspondence to: Gonzalo Javier Ziegler-Rodriguez and Gabriel De la Cruz Ku
Email: gonzaloziegler@gmail.com and gabrieldelacruzku@gmail.com
Published: 13/09/2024
Received: 04/04/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast pathology is one of the most frequent reasons for medical attention around the world. Breast cancer is the second most common pathology of the breast, behind benign breast conditions [1]. In developed countries, public prevention policies are established with the strategy of screening with mammography for women between 50 and 69 years old. This, in addition to the well-organised health services, ensures that asymptomatic patients reach a radiology service dedicated to breast cancer to diagnose malignant lesions at an earlier stage, which allows improvement of disease-free and overall survival [2–4]. These tissue diagnoses are usually obtained with core needle biopsy (CNB) which can be performed by breast surgical oncologists or radiologists; however, in developed countries, the radiology service is usually responsible for these procedures with high accuracy and precision [5].
In developing countries like Peru, the estimated age-adjusted incidence of breast cancer is 35.9 per 100,000 inhabitants, which is not too high if we compare it to a developed country like Spain with 77.5 cases per 100,000 inhabitants [6]. However, unfortunately, Peru does not have efficient public policies for cancer prevention and, despite breast cancer being the second cancer with the highest incidence, a public mammographic screening programme practically does not exist [7]. The public system is subdivided according to the entity in charge of the finances such as social security, the ministry of health and other entities [8]. This fact creates high heterogeneity in the service, infrastructure, processes, referrals and in the academic level of professionals who serve. Moreover, there are gaps in access to health services, where the government has not been able to intervene efficiently, public hospitals do not typically have a dedicated breast surgery service and radiology services do not typically have a distinct breast imaging area [9].
All these limitations have a high impact on the outcomes of our population when compared to other developed countries, highlighting the importance of the early diagnosis of breast cancer to continue multidisciplinary treatment [10–13]. Hence, our aim was to compare the results of CNB obtained by the breast surgery service and the breast imaging radiology service in a tertiary reference hospital.
Materials and methods
A retrospective, observational, descriptive and cross-sectional study was conducted. The research protocol was approved by the institutional review committee (22-0250/INEN). The data collected was from 12 months from 1st January 2019, to 31st December 2019, from the medical records of patients who underwent CNB in a breast surgery service (10 surgeons) and the radiology service (6 radiologists).
Our inclusion criteria were a) Patients with a breast tumour, b) undergoing CNB at the institute, c) performed by breast surgeons in the clinic or by radiologists from the breast imaging area and d) patients who had breast surgery with an operating piece following the CNB. Our exclusion criteria were a) CNB performed in the inpatient setting or emergency department and b) by other specialists inside or outside the institution.
Variables
We included demographic, clinical and imaging characteristics, type of biopsies, number of attempts, waiting times and concordance with the final surgical specimen. We STARD guidelines were applied to all the biopsies as per protocol [14]. Patients were followed for at least 1 year since the indication of the CNB.
Statistical analysis
We performed a descriptive analysis of the demographic and clinicopathological information, frequencies and percentages were used for qualitative variables, while mean with standard deviation or median with range for quantitative variables. Bivariate analyses were performed with chi-square for qualitative variables according to the service which performed by the CNB. Sensitivity and specificity were assessed for both services. A p-value <0.05 was considered statistically significant for all the analyses performed. The R-4.3.3 program was for data processing.
Results
Demographic characteristics
Of 1,082 cases, 782 patients were included in the present study. The 99.6% of cases were women with a median age of 51 years (range 14–92 years). Moreover, two thirds of the population (63.2%) resided in Lima with better accessibility to the hospital and 93.1% of patients did not have a personal history of breast cancer Table 1.
Clinical presentation
Most of the cases presented with a palpable breast lesion (87.5%), and after a first breast surgery clinic appointment, 67.8% of patients had suspected breast cancer and were referred to different additional studies. Then, of these patients, 95.1% had a diagnostic ultrasound and 87.2% had a mammogram Table 1.
Table 1. Sociodemographic and clinical characteristics of patients who underwent CNB.
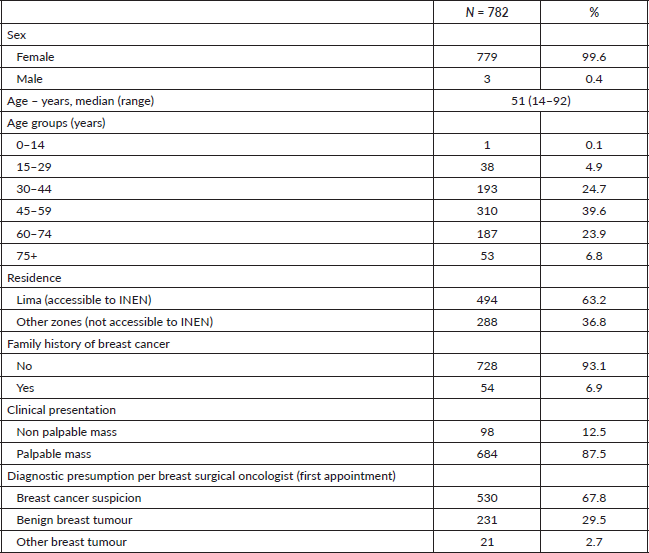
CNB according to service and equipment used to guide the sample collection
The CNB was performed according to medical criteria, availability of staff and material in the pharmacy (14-gauge needle and compatible biopsy gun). Of the 782 patients who underwent CNB, 462 (59.07%) were performed by breast surgeons and 320 (40.92%) by breast radiology. Regarding the use of equipment to guide the sample collection, 452 (57.82%) clinical records did not specify the equipment used such as the ultrasound machine, most of them were in the breast surgery service. In 314 (40.2%) cases, ultrasound guidance was used to assist the CNB, while the rest (2.0%) was performed by radiology using stereotactic guidance Table 2.
Need to repeat the biopsy and pathology results
Repeating the biopsy was not required in 714 (91.3%) cases; of them, 92.9% was performed by surgery and 89.1% by radiologist (p < 0.05). A total of 68 (8.7%) cases required a repeated biopsy. One group underwent surgery (surgical biopsy) (4.7%), and another group underwent re-BAG (4.0%). From the group with insufficient tissue who underwent CNB with the radiology department (35 patients), surgical consultation was obtained in 26 patients Table 2.
Pathologic diagnosis
The pathology report obtained after the CNB identified 476 (60.9%) cases of infiltrating breast carcinomas and 29 (3.7%) cases of ductal carcinoma in situ, with a total of 505 of the 782 patients susceptible to undergo treatment for ‘breast cancer’ Table 3.
CNB operational performance
To assess the operational performance of CNB by both executors, validity measures were calculated according to the success in obtaining sufficient samples to reach a tissue diagnosis. Sensitivity and specificity of both were similar for both groups; sensitivity of 95.3% and 95.6%, and specificity of 98.3% and 98.5%, for the breast surgical oncologist and radiologist, respectively Table 4.
Evaluation of the waiting time to perform a CNB in both services
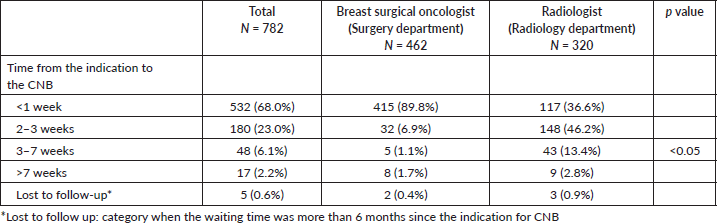
There was a difference between services regarding the waiting time, while breast surgeons performed 89.8% of cases during the first week after the procedure was indicated, radiologists were able to perform the CNB in 36.6% of their cases in this timeframe (p < 0.05). Specifically for patients who were diagnosed of ‘breast carcinomas’ (n = 525), no service took more than 2 months to perform a CNB, except for a minimal number of patients (<5%) Table 5.
Table 2. Characteristics of the CNB at the Instituto Nacional de Enfermedades Neoplasicas according to the service.
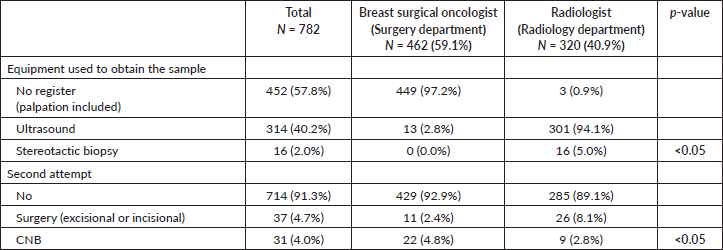
Table 3. Pathological characteristics of patients who underwent CNB.
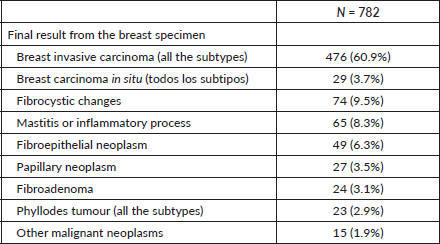
Table 4. Concordance and validity of the CNB and the pathology result of the surgical specimen according to the service who performed the biopsy.
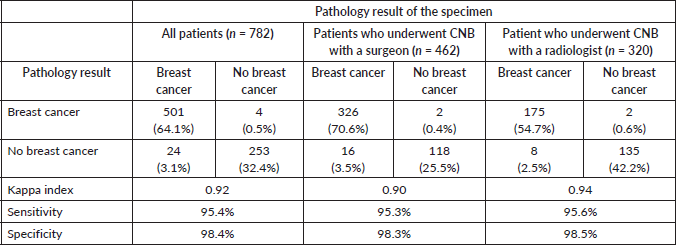
Table 5. Time from the indication to the CNB according to the service that performed the procedure.
Discussion
In the context of a deficient health system, the breast surgical oncologist has served to cover gaps from other professionals such as the shortage of radiologists dedicated to breast cancer, in the diagnosis through a CNB. One of the main roles of the breast surgical oncologist is to evaluate the operability of the case, as well as to be the treating doctor of the patient with breast pathology [15]. Our study showed that both services have high sensitivity and specificity regardless of the tool used to perform the CNB; however, breast surgical oncologists can do it in a shorter period of time since the indication to the biopsy compared to radiologists.
In 2017, a total of 52 mammography machines were reported in Peru, of which only 37 were operational. In a country of 33 million inhabitants, it is almost one mammogram per 1,000,000 inhabitants [7]. In addition, mammographic screening is not performed in several regions, despite being encouraged by several institutions and national health programmes such as ‘Plan Esperanza’ [16]. Unfortunately, this is a reality that is not repeated in other countries, where services are better distributed and access to quality health is more equitable [12, 17]. In this setting, the surgical oncology service has adapted to help close the gap that there are not enough radiologists or advanced practice radiographers available to be able to care for patients seeking help due to breast tumours [18, 19].
In our study, the majority of cases were from patients older than 45 years with a median age of 55.9 years, similar to the cases of breast cancer reported in the Lima population registry and other studies [20, 21]. Around 88% of patients went to the hospital for a symptomatic breast, which was usually a palpable breast tumour, and was evaluated by the breast surgeon, being the professional they first had in a clinic appointment. Moreover, two thirds of cases had suspected cancer, and one third came from other places other than Lima, which represents poor access to oncological care due to socioeconomic inequalities [22, 23]. As mentioned, there are several barriers that contribute to a late diagnosis and worse outcomes in developing countries, such as an inappropriate reassurance of a questionable benign mass, the need for education and awareness, access to health care, inappropriate referrals, established mammography screening programmes, long waiting times for clinic appointments, among others. There is an urgent need for new health policies, strategies and cancer programmes throughout the country to achieve improvement in our oncological indicators, especially in underserved communities [24–26].
Since around two decades ago, the CNB has been used for the diagnosis of breast pathology and has served to de-escalate costs and morbidity in breast diagnosis, while the place of surgery to establish the diagnosis of a breast tumour has been discontinued [27, 28]. Furthermore, technology has improved significantly with the combination of images to guide the CNB, becoming a key part of the diagnosis [29]. For instance, the association of CNB with ultrasound guidance is the ideal combination similar to stereotactic biopsies if the mass is unable to be palpated or seen in ultrasound but able to be identified in mammography or digital tomosynthesis [30, 31].
Several surgical specialties, including breast surgeons, have adapted throughout the years to minimally invasive diagnosis [28]. Also, radiologists have entered the interventional field with equipment and techniques that allow small tumours to be removed, due to the benefit to the patient in terms of morbidity and costs. To date, CNB with or without ultrasound guidance is considered one of the quality standards in the diagnosis of breast cancer [30]. This is why within the competencies of the breast surgical oncologist, ultrasound training is crucial for the triage of lesions and guiding procedures such as fine needle aspiration, vacuum aspiration biopsy (VAB) and placement of marking devices [32–35]. It has been demonstrated that surgical oncologists have outstanding outcomes with this tool in the outpatient setting and intraoperatively for excisional biopsies or lymph node excision, for instance, it has shown reduced need for wire-guided localisation of impalpable tumours [36–38]. Our study showed that the results of both services were similar in sensitivity and specificity, highlighting that there is no difference in the accuracy between specialties.
In developed countries where the population screening programme and the radiology service can be dedicated to the breast, biopsies are usually performed by radiologists [39]. However in developing countries where services are not entirely well organised or radiologists are not available, breast surgical oncologists have continued to perform non-surgical biopsies, mastering ultrasound as a tool to guide the collection of samples with CNB, FNA or VAB [40]. Currently, there are several opportunities to be trained in the use of ultrasound for procedures and surgeries such as specialised courses endorsed by important scientific societies [32, 33, 35, 41]. These capabilities are not intended to replace the work of a radiologist, much less fall into intrusiveness, but rather, as in other medical specialties, the use of the ultrasound in the hands of the surgeon complements the assessment and helps with early diagnosis.
The high operational performance rates of the BAG in terms of concordance, sensitivity and specificity with or without ultrasound guidance in the present study demonstrate that the professionals of both services in our hospital are optimal options for the diagnosis of breast cancer [21, 42, 43]. Furthermore, the Kappa indices 0.92 show an optimal concordance between the pathology of the CNB and the final surgical pathology results in both groups, which were similar or higher than previous studies [44–46].
Furthermore, our study showed that the surgery department performed most of the biopsies within a week since the indication, while only a third of the patients underwent this procedure by the radiology department and approximately 45% had the biopsy in 2–3 weeks. This might be due to the dynamics of the treating physician and the low availability of radiologists to perform procedures on the first visit. The surgeon can perform the CNB at the same time as the first visit, while in radiology procedures are usually scheduled according to available appointments after 2–3 weeks. Moreover, the number of breast surgeons is greater than the number of radiologists who work on breast imaging. These delays in our study emphasizes the long waiting time caused by the hospital system, a consequence of the national health system [47]. Moreover, the an urgent need for more radiology specialists in tertiary centres, and even more in community hospitals. The reference is 2 months to consider the diagnostic study in cancer cases, with the aim to start oncological treatment before 90 days to avoid impact on survival [3, 27]. In our study, around 3% and 2% of the patients had the CNB out of this timeframe for radiologists and breast surgical oncologists, respectively, which should be improved to offer the best treatment and outcome to the patients.
Breast cancer requires a multidisciplinary approach to have the best outcome for the patient [48]. Our results encourage the breast surgeons to continue to perform biopsies with ultrasound guidance when needed, and the purpose is to work together with the radiologist to achieve an early diagnosis of the patient and start the treatment as earliest as possible when resources are scarce due to staffing, schedules, shifts and referrals [49, 50]. To date, breast cancer incidence continues to increase and all the possible resources should be used in the most effective and efficient way, especially in countries with gaps in the health system [10, 51].
Our study has some limitations. With two different services, a comparison is difficult due to the heterogeneity of cases and the clinical context of both services; however, we presented and evaluated the data according to the performance of both CNB executors in a tertiary referral centre. The size of the breast tumours subjected to biopsy was not known, the only information available was the label ‘palpable tumour’ and ‘non-palpable tumour’. This is because we have focused on the performance of CNB by two different specialists, hence we did not consider the specific characteristics of the breast tumours that underwent CNB such as histology, biology or stage. There was no category of patient-dependent or physician-dependent delays in the context of diagnostic delays. Delays in starting cancer treatment after a pathology diagnosis were also not collected. Furthermore, we have not specified how many surgeons or radiologists took the biopsies. We did not find records of the use of ultrasound equipment in the surgery department, but based on our experience we estimate that the surgeons used ultrasound guidance in a much higher percentage than found in the clinical history reports, then we highly recommend the use of this tool by breast surgeons based on previous literature. Moreover, our results should be interpreted with caution when extrapolation is intended, as our study only included one institution, hence, we recommend future multicentric prospective studies that compare both services for the diagnosis of breast cancer. In addition, despite these potential biases, the cross-sectional and comparative design is ideal when the purpose of research is to establish the performance, precision or accuracy of a diagnostic test under evaluation, in terms of sensitivity and specificity.
Conclusion
To date, some developing countries still have patients with breast tumours that are self-diagnosed by palpation and not by mammography, a reality that should not be ignored. We demonstrate that CNB can be performed by either well-trained breast surgical oncologist or radiologist, both as effective resources to define pathology diagnosis of breast tumours, with the help of tools such as ultrasound to add precision and accuracy. Moreover, optimisation of time is crucial, and the surgery department is usually faster in terms of sampling the tissue in a shorter time since the indication. These results encourage the breast surgical oncologist to perform CNB when possible, to expedite diagnosis and treatment of the patients to achieve better oncological outcomes.
Acknowledgments
We thank Raul Mantilla from the Instituto Nacional de Enfermedades Neoplasicas for his contribution with the statistical analysis and support in methodology. We also thank The International Academy of Senology from University of Barcelona, where the initial idea of this paper was born. A special acknowledgment to Dr Sergi Ganau.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
This research has not received specific support from public sector agencies, the commercial sector or non-profit entities.
Ethical considerations
This manuscript has followed the work centre's protocols for the publication of patient data and was approved for publication by the institution’s research ethics committee. Privacy has been respected, keeping the patient’s identification data confidential. The use of informed consent was not required.
Author contributions
GJZR: Conceptualisation – Ideas; visualisation; writing – original draft; writing – review and editing
MAPP: Conceptualisation – Ideas; visualisation; writing – original draft; writing – review and editing
GDK: Conceptualisation – Ideas; visualisation; writing – original draft; writing – review and editing
SEVS: writing – review and editing
JDY: writing – review and editing
JAGZ: writing – review and editing
GCV: writing – review and editing
JMCC: visualisation; writing – original draft; writing – review and editing.
All authors participated in the conception, design of the work, writing and interpretation of the results, for the preparation of the manuscript.
References
1. Dixon J and Macaskill E (2015) Chapter 3. Management of benign breast disease Breast Disease Comprehensive Management eds AI Riker (New York: Springer) pp 51–77
2. World Health Organization (2017) Guide to Cancer Early Diagnosis [Internet] (Geneva: World Health Organization) Date accessed: 27/12/23 p 48 [https://apps.who.int/iris/handle/10665/254500]
3. Ginsburg O and Yip CH (2020) Breast cancer early detection: a phased approach to implementation Cancer 126(Suppl 10) 2379–2793 https://doi.org/10.1002/cncr.32887 PMID: 32348566 PMCID: 7237065
4. Houssami N, Ciatto S, and Martinelli F, et al (2009) Early detection of second breast cancers improves prognosis in breast cancer survivors Ann Oncol 20(9) 1505–1510 https://doi.org/10.1093/annonc/mdp037 PMID: 19297316
5. White RR, Halperin TJ, and Olson JA, Jr., et al (2001) Impact of core-needle breast biopsy on the surgical management of mammographic abnormalities Ann Surg 233(6) 769–777 https://doi.org/10.1097/00000658-200106000-00006 PMID: 11371735 PMCID: 1421319
6. IARC CT (2020) W. Estimated age-standardized incidence and mortality rates (world) in 2020, both sexes, all ages (excl. NMSC) Peru vs. Spain Population Comparison, ASR Indicator [Internet] Data accessed:27/11/23
7. Ministerio de Salud (2017) Dirección General de Intervenciones Estratégicas en Salud Pública. Dirección de Prevención y Control de Cáncer. Plan nacional para la prevención y control de cáncer de mama en el Perú 2017–2021 R.M. N°442-2017MINSA [Internet] Data accessed: 28/11/2023 [http://bvs.minsa.gob.pe/local/MINSA/4234.pdf]
8. Alcalde-Rabanal JE, Lazo-González O, and Nigenda G (2011) The health system of Peru Salud Publica de Mexico 53(Suppl 2) s243–s254
9. Carrillo-Larco RM, Guzman-Vilca WC, and Leon-Velarde F, et al (2022) Peru—progress in health and sciences in 200 years of independence Lancet Reg Health Americas 7 100148 https://doi.org/10.1016/j.lana.2021.100148
10. Francies FZ, Hull R, and Khanyile R, et al (2020) Breast cancer in low-middle income countries: abnormality in splicing and lack of targeted treatment options Am J Cancer Res 10(5) 1568–1591 PMID: 32509398 PMCID: 7269781
11. da Costa Vieira RA, Biller G, and Uemura G, et al (2017) Breast cancer screening in developing countries Clinics (Sao Paulo, Brazil) 72(4) 244–253 https://doi.org/10.6061/clinics/2017(04)09 PMID: 28492725 PMCID: 5401614
12. Manson EN and Achel DG (2023) Fighting breast cancer in low-and-middle-income countries—What must we do to get every woman screened on regular basis? Sci Afr 21 e01848
13. Breast Cancer in Developing Countries (2009) Lancet (London, England) 374(9701) 1567
14. Cohen JF, Korevaar DA, and Altman DG, et al (2016) STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration BMJ Open 6(11) e012799 https://doi.org/10.1136/bmjopen-2016-012799 PMCID: 5128957
15. DeVita V, Lawrence T, and Rosenberg S (2015) Chapter 79: malignant tumors of the breast DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology 10th edn (Amsterdam: Wolters Kluwer Health Adis)
16. Vidaurre T, Santos C, and Gómez H, et al (2017) The implementation of the plan Esperanza and response to the impact review Lancet Oncol 18(10) e595–e606 https://doi.org/10.1016/S1470-2045(17)30598-3 PMID: 28971826
17. Gutnik LA, Matanje-Mwagomba B, and Msosa V, et al (2016) Breast cancer screening in low- and middle-income countries: a perspective from Malawi J Global Oncol 2(1) 4–8 https://doi.org/10.1200/JGO.2015.000430
18. Ziegenhorn HV, Frie KG, and Ekanem IO, et al (2020) Breast cancer pathology services in sub-Saharan Africa: a survey within population-based cancer registries BMC Health Serv Res 20(1) 912 https://doi.org/10.1186/s12913-020-05752-y PMID: 33008380 PMCID: 7531092
19. Shulman LN, Willett W, and Sievers A, et al (2010) Breast cancer in developing countries: opportunities for improved survival J Oncol 2010 595167 https://doi.org/10.1155/2010/595167
20. Payet Meza E Registro de Cáncer de Lima Metropolitana 2012–2015 [Internet] (Lima: Instituto Nacional de Enfermedades Neoplasicas (INEN)) [https://portal.inen.sld.pe/wp-content/uploads/2022/01/REGISTRO-DE-CANCER-DE-LIMA-METROPOLITANA-2013-2015.pdf]
21. Dahabreh IJ, Wieland LS, and Adam GP, et al (2014) AHRQ Comparative Effectiveness Reviews. Core Needle and Open Surgical Biopsy forDiagnosis of Breast Lesions: an Update to the 2009 Rreport (Rockville: Agency for Healthcare Research and Quality (US))
22. Díaz-Ruiz R, Vargas-Fernández R, and Rojas-Roque C, et al (2024) Socioeconomic inequalities in the use of medical consultation services in Peru, 2019 Int J Equity Health 23(1) 10 https://doi.org/10.1186/s12939-024-02099-2
23. Chávez Sosa JV, Guerra Pariona HN, and Huancahuire-Vega S (2022) Association between perceived access to healthcare and the perception of illness among peruvian adults with chronic diseases during COVID-19 pandemic INQUIRY J Health Care Org Prov Financ 59 469580221112832
24. Torres-Roman JS, Martinez-Herrera JF, and Carioli G, et al (2020) Breast cancer mortality trends in Peruvian women BMC Cancer 20(1) 1173 https://doi.org/10.1186/s12885-020-07671-x PMID: 33261561 PMCID: 7706041
25. Lameijer JRC, Voogd AC, and Pijnappel RM, et al (2020) Delayed breast cancer diagnosis after repeated recall at biennial screening mammography: an observational follow-up study from The Netherlands Br J Cancer 123(2) 325–332 https://doi.org/10.1038/s41416-020-0870-2 PMID: 32390006 PMCID: 7374543
26. Goodson WH, III and Moore DH, II (2002) Causes of physician delay in the diagnosis of breast cancer. Arch Int Med 162(12) 1343–1348 PMID: 12076232
27. Bleicher RJ (2018) Timing and delays in breast cancer evaluation and treatment Ann Surg Oncol 25(10) 2829–38
28. Balch CM (1995) The needle biopsy should replace open excisional biopsy … but will the surgeon’s role in coordinating breast cancer treatment be diminished? Ann Surg Oncol 2(3) 191–192 https://doi.org/10.1007/BF02307021 PMID: 7641012
29. Hoda SA, Brogi E, and Rosen PP, et al (2017) Rosen’s Diagnosis of Breast Pathology by Needle Core Biopsy [Internet] (Lima: Wolters Kluwer) [https://books.google.es/books?id=lEn3jwEACAAJ]
30. Commission on Cancer (CoC) Quality Measures [Internet] Date accessed: 20/11/23 [https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/quality-measures]
31. Ames V and Britton PD (2011) Stereotactically guided breast biopsy: a review Insights Imaging 2(2) 171–176 https://doi.org/10.1007/s13244-010-0064-1
32. American Medical Association (AMA) Privileging for Ultrasound Imaging H-230.960 [Internet] [https://policysearch.ama-assn.org/policyfinder/detail/Ultrasoundimaging?uri=%2FAMADoc%2FHOD.xml-0-1591.xml]
33. Snider H (2010) Chapter 31A. Basic breast ultrasound, certification, and accreditation for the surgeon ed Kuerer HM, Kuerer’s Breast Surgical Oncology [Internet] (New York: The McGraw-Hill Companies) Date accessed: 31/12/2023 [accesssurgery.mhmedical.com/content.aspx?aid=6411109]
34. The American Society of Breast Surgeons (ASBrS) (2018) The American Society of Breast Surgeons Performance and Practice Guidelines for Breast Ultrasound [Internet]. Date accessed: 19/02/2024 [https://www.breastsurgeons.org/docs/statements/Performance-and-Practice-Guidelines-for-Breast-Ultrasound.pdf]
35. Breast ultrasound: An introductory/refresher course—2022 (2022) Annual Meeting [Internet] Date accessed: 25/02/2024 [https://www.breastsurgeons.org/meeting/2022/pre/ultrasound]
36. Patel B, Govindarajulu S, and Sahu A (2021) 534 use of ultrasound by breast surgeons reduces the need for wire-guided localisation of impalpable breast tumours by radiologists Br J Surg 108(Supplement_6) znab259.10 https://doi.org/10.1093/bjs/znab259.210
37. Whitehouse PA, Baber Y, and Brown G, et al (2001) The use of ultrasound by breast surgeons in outpatients: an accurate extension of clinical diagnosis Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 27(7) 611–616
38. Ahmed M, Abdullah N, and Cawthorn S, et al (2014) Why should breast surgeons use ultrasound? Breast Cancer Res Treat 145(1) 1–4 https://doi.org/10.1007/s10549-014-2926-6 PMID: 24706083
39. Winkler NS (2021) Ultrasound guided core breast biopsies Tech Vasc Interv Rad 24(3) 100776 PMID: 34861968
40. Jana S, Yadav SK, and Sharma D, et al (2021) A Low-cost model of breast biopsy for the training of surgical residents during COVID-19 pandemic Trop Doct 52(1) 107–119 https://doi.org/10.1177/00494755211050134 PMID: 34723752 PMCID: 8891898
41. Chakedis JM, Tang A, and Kuehner GE, et al (2021) Implementation of intraoperative ultrasound localization for breast-conserving surgery in a large, integrated health care system is feasible and effective Ann Surg Oncol 28(10) 5648–5656 https://doi.org/10.1245/s10434-021-10454-8 PMID: 34448055 PMCID: 8418593
42. Wang M, He X, and Chang Y, et al (2017) A sensitivity and specificity comparison of fine needle aspiration cytology and core needle biopsy in evaluation of suspicious breast lesions: a systematic review and meta-analysis Breast (Edinburgh, Scotland) 31 157–166 https://doi.org/10.1016/j.breast.2016.11.009
43. Cortés-Reyes E and Rubio-Romero J (2010) Statistical methods for evaluating diagnostic test agreement and reproducibility Rev Colomb Obstet Ginecol 61 247–255 https://doi.org/10.18597/rcog.271
44. Dekker TJ, Smit VT, and Hooijer GK, et al (2013) Reliability of core needle biopsy for determining ER and HER2 status in breast cancer Ann Oncol 24(4) 931–937 https://doi.org/10.1093/annonc/mds599
45. Fersis N, Smyczek-Gargya B, and Krainick U, et al (2001) Clinical experience with large-core needle biopsies of the breast and evaluation of histopathology Zentralbl Gynakol 123(3) 132–135 https://doi.org/10.1055/s-2001-12509 PMID: 11340952
46. Deshpande A, Garud T, and Holt SD (2005) Core biopsy as a tool in planning the management of invasive breast cancer World J Surg Oncol 3(1) 1 https://doi.org/10.1186/1477-7819-3-1 PMID: 15631625 PMCID: 544846
47. Kern KA (2009) Chapter 94—Delayed Diagnosis of Symptomatic Breast Cancer ed Bland KI and Copeland EM The Breast (Fourth Edition) [Internet] (Philadelphia: W.B. Saunders) pp 1483–1511 [https://www.sciencedirect.com/science/article/pii/B9781416052210000942]
48. Shao J, Rodrigues M, and Corter AL, et al (2019) Multidisciplinary care of breast cancer patients: a scoping review of multidisciplinary styles, processes, and outcomes Curr Oncol 26(3) e385–e397 https://doi.org/10.3747/co.26.4713 PMID: 31285683 PMCID: 6588064
49. Afaya A, Ramazanu S, and Bolarinwa OA, et al (2022) Health system barriers influencing timely breast cancer diagnosis and treatment among women in low and middle-income Asian countries: evidence from a mixed-methods systematic review BMC Health Serv Res 22(1) 1601 https://doi.org/10.1186/s12913-022-08927-x
50. Pinto JA, Pinillos L, and Villarreal-Garza C, et al (2019) Barriers in Latin America for the management of locally advanced breast cancer Ecancermedicalscience 13 897 PMID: 30792814 PMCID: 6372299
51. Xu S, Murtagh S, and Han Y, et al (2024) Breast cancer incidence among US women aged 20 to 49 years by race, stage, and hormone receptor status JAMA Netw Open 7(1) e2353331 https://doi.org/10.1001/jamanetworkopen.2023.53331 PMID: 38277147 PMCID: 10818222






