Esophageal squamous cell carcinoma in a patient with BRCA1 mutation: a rare association
Mohammad Saad Salim Naviwala1, Mirza Rameez Samar1, Daania Shoaib1, Fizza Akbar2, Romana Idrees3 and Yasmin Abdul Rashid1
1Department of Medical Oncology, Aga Khan University Hospital, Karachi 74800, Pakistan
2Department of Women and Child Health, Aga Khan University Hospital, Karachi 74800, Pakistan
3Department of Pathology, Aga Khan University Hospital, Karachi 74800, Pakistan
Abstract
Background: Esophageal neoplasms rank as the 7th most common cancers in the world. Squamous cell carcinomas of esophagus (SCCE) are the predominant subset, linked to a number of genetic alterations. Gene-driven tumour pathways are being increasingly identified with the emerging role of next-generation sequencing.
Case presentation: We report a case of an 82-year-old male patient who was diagnosed with SCCE involving the cervical region. He received definitive concurrent chemoradiotherapy with Carboplatin and Paclitaxel. To trace the family history of malignancy, a genetic test was carried out which turned out to be a pathogenic BRCA1 variant.
Conclusion: SCCE arising in the context of known BRCA1 mutation has been rarely reported to date. Testing for these mutations should be considered in patients who present with esophageal cancer, especially in the backdrop of familial neoplasms.
Keywords: squamous cell carcinoma, next-generation sequencing, BRCA1, esophageal neoplasms
Correspondence to: Mohammad Saad Salim Naviwala
Email: saad.naviwala@aku.edu
Published: 16/07/2024
Received: 21/01/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Squamous cell carcinoma of esophagus (SCCE) is an aggressive neoplasm that is associated with a high relapse rate and mortality rate [1]. It has a complex carcinogenic mechanism that has been related to poor prognosis. Recent studies have demonstrated several critical genes and pathways crucial to tumorigenesis of esophageal cancer. The use of next-generation sequencing (NGS) platforms has played a pivotal role in providing comprehensive methods to detect somatic and germline genome alterations in cancers. Detection of disease-causing, pathogenic (P), or likely pathogenic (LP) variants (mutations) through the use of NGS is increasing which has improved our understanding of cancer biology. Researchers have exploited this technology to better understand the tumorigenesis of SCCE which is leading to finding several critical genes and pathways that provide drug targets to guide future precision medicine in oncology. In addition to the environmental risk factors associated with SCCE, there is growing evidence that supports the involvement of genetic and epigenetic risk factors in the tumorigenesis of ESCC.
Extensive work, enabled by NGS platforms; is being carried out; to understand the somatic genomic alterations in ESCC. Somatic variants attributing to tumorigenesis and having a possible disease prognostic value had been identified in genes including, TP53, EP300, NFE2L2, CSMD3, CCND1, CDKN2A, CREBBP, RB1, KMT2D, KMT2C, KDM6A, FAT1, FAT2, FAT3, FAT4, AJUBA, NOTCH1, NOTCH2, NOTCH3, FBXW7, PTCH1 and PIK3CA [2–4] Additionally, limited published work has however shown germline variants in TP53, BRCA1, BRCA2 and RECQL4 contributing to familial cases of ESCC [5, 6].
Of note, the most widely studied genes are two tumour suppressor genes, BRCA1 and BRCA2, both of which influence homologous recombination in DNA repair pathway [7]. Unquestionably, P and LP or disease-causing variants of these genes, increase the risk of malignancies, most notably breast and ovarian cancers. Apart from these, they are also associated with an increase in the risk of pancreatic cancers, prostatic cancers and male breast cancers [8]. With the development and exploration of more cost-effective gene sequencing methods, both somatic and germline and mutations in BRCA genes have been detected in several cancers. Testing for BRCA1/2 has exponentially increased as part of the patient’s clinical management and eligibility for targeted therapeutic agents with the introduction of poly-ADP ribose polymerase inhibitors in cancers with BRCA mutations [9]. In a family-based association review, the relative risk (RR) was found to be (RR 2.9, 95% CI 1.1–6) in esophageal and (RR 2.4, CI 1.2–4.3) in gastric cancer in families with known BRCA1 mutations [10]. Another study showed the cumulative lifetime risk of developing esophageal cancers in BRCA1/2 carriers by the age of 85 years at 5.2% (95% CI, 1.7%–8.5% [11].
Here, we report a patient with confirmed germline BRCA1 mutated SCCE.
Case report
An 82-year-old gentleman, with no known prior co-morbidities, and an Eastern Cooperative Oncology Group Performance Status score of 1, presented to our institution with complaints of difficulty swallowing for 3 months. He narrated that it started off with solids and gradually progressed to liquids as well. Along with this, he also reported an unintentional weight loss of around 4 kg in this interim. He did not report any history of substance abuse or past addictions.
However, further questioning highlighted a significant family history of malignancies. Three first-degree relatives (sister, brother and daughter) had been diagnosed with gastrointestinal malignancies and another daughter had been diagnosed with breast carcinoma, illustrated in Figure 1.
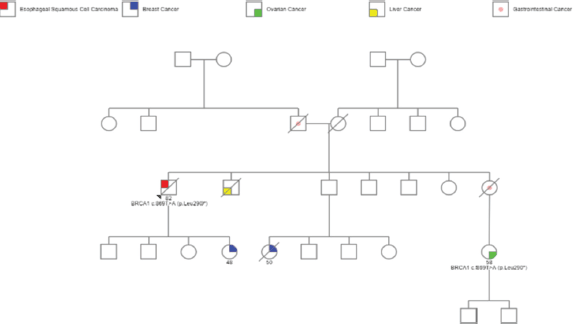
Figure 1. Flowchart showing patient’s pedigree information of malignancies, which includes patient’s diagnosis of SCCE, gastrointestinal and liver cancers in siblings, breast cancer in daughter and breast and ovarian cancers in nieces.
On general physical examination, he was of average build and all vital parameters were within reference ranges. Systemic examination was found to be unremarkable. Initial relevant blood investigations had been performed at a different healthcare facility which were all within the reference ranges. A Barium Swallow had revealed a short segment of irregularity and mucosal destruction with multiple filling defects at the lower cervical and upper dorsal esophagus (C7-T1 level). This was correspondent on a contrast-enhanced computed tomography scan of the neck and chest, which demonstrated an 6 cm upper esophageal thickening causing luminal narrowing.
He was advised and subsequently underwent an Esophagogastroduodenoscopy, where an upper esophageal mass was visualised just below the upper esophageal sphincter (Figure 2). Biopsy of this mass was performed and histopathology was consistent with moderately differentiated keratinizing squamous cell carcinoma (Figure 3).
A staging positron emission tomography with computed tomography (PET-CT) scan was performed which demonstrated a circumferential thickening of the cervical esophagus, 2.8 × 1.9 × 4 cm in size with a standardised uptake value (SUVmax) of 14 (Figure 4). This was seen abutting neighboring structures without any invasion. No regional or distant metastases were noted.
Definitive concurrent chemotherapy and radiation, i.e., combined modality treatment, were recommended for the patient. Before initiating therapy, he underwent percutaneous endoscopic gastrostomy tube placement. He received weekly Carboplatin (Area under the curve-2) and Paclitaxel (50 mg/m2) alongside 30 fractions of concurrent radiation therapy over the course of 5 weeks, which he tolerated without incident. However, tragically, the patient’s condition deteriorated rapidly, and he passed away before undergoing the end-of-treatment scans to assess his response to therapy. The cause of death was attributed to septic shock secondary to a severe pulmonary infection, highlighting the challenges in managing advanced-stage esophageal squamous cell carcinoma, particularly in elderly patients.
In view of his strong family history of multiple malignancies, he was referred to the Hereditary Cancer Clinic. After a detailed pre-test counselling session, a Multi-Cancer Genetic Panel (84 genes) at an outsourced commercial genetics laboratory (Invitae Genetics, US) was sent. He harbored a P variant in BRCA1 and additionally, two variants of uncertain significance (VUS) in ATM and CDH1, latter of which is unlikely to be contributing to disease, as they were present in the population database (gnomAD) and did not meet the criteria to be classified as P/LP variant. The variant classification is based on the American College of Medical Genetics and Genomics criteria. The P variant identified is BRCA1, Exon 10, c.869T>A (p. Leu290*). The variant creates a premature translational stop signal, leading to disruption or absence of protein product. The resultant loss-of-function variant in BRCA1 accelerates disease causation, this variant is absent from the population database (gnomAD) but have been found in the clinical database (ClinVar: 371981).
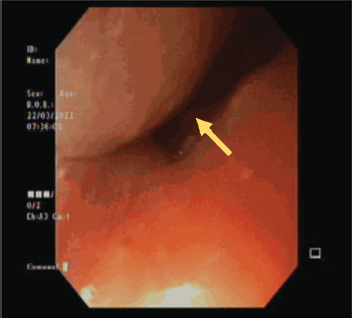
Figure 2. Endoscopic visualisation of the esophageal mass.
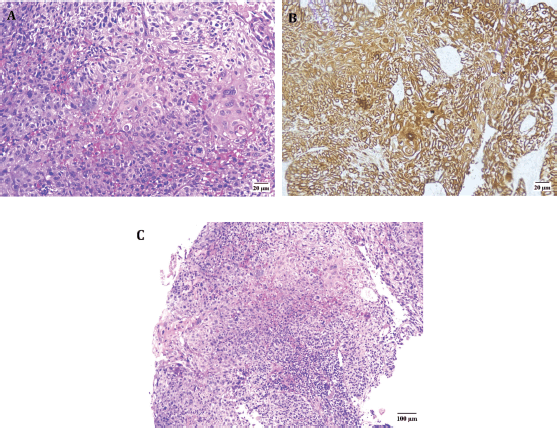
Figure 3. Microscopic findings of the upper esophageal mass. (a): High-power view (40x) displaying squamous cell carcinoma with areas of keratinization. (b): High-power view (40x) showing positive CK5/6 immunohistochemical stain in neoplastic cells. (c): Low-power image (10x) revealing squamous cell carcinoma.
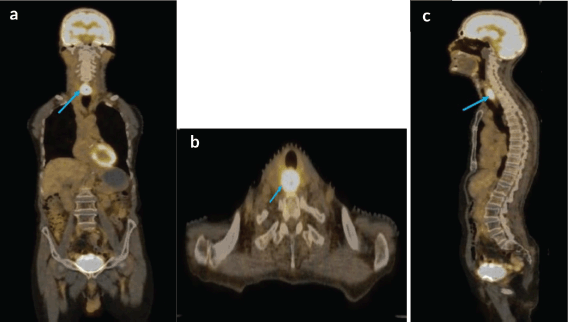
Figure 4. (a–c): Coronal axial and sagittal views respectively, of staging PET-CT scan showing FDG avid, cervical esophageal mass, with normal uptake seen over brain.
Discussion
Esophageal cancer is classified among the most common malignancies, as it ranked the 7th most frequent neoplasm in its occurrence and the 6th most significant cancer leading to mortality in 2020s [12]. Histologically, the two subgroups of esophageal cancer widely known are adenocarcinoma (AC) and squamous cell carcinoma. Between the two, squamous cell carcinoma is the leading cause, making up to more than 85% of the global burden among all esophageal cancer cases [13].
SCCE is prevalent in Northern Iran, Central Asia and North-Central China in contrast to AC, which is predominantly found in highly developed regions such as Europe and North America. Even in Pakistan, SCCE remains the dominant histology, with most cases found in Baluchistan [14]. These subtypes are highly influenced by a variety of risk factors. Smoking and alcohol use, diets rich in nitrosamines and polycyclic aromatic hydrocarbons, and human papillomavirus increase risk of SCCE whereas the major risk factors for AC include chronic gastro-esophageal reflux disease, Barrett’s esophagus, obesity, diets rich in saturated fat and red meat.
For patients who present with locally advanced esophageal cancer, the gold standard treatment is tri-modality i.e. the combination of neo-adjuvant chemoradiotherapy followed by surgery, based on the results of CROSS trial which showed median overall survival twice to that seen with surgery alone [15]. Kamarajah et al [16] reported superior survival rates in esophageal cancer with tri-modality than neo-adjuvant chemotherapy followed by surgery. Tri-modality not only improves the pathological complete response rate but also increases rate of R0 resection as reported by Yang et al [17].
The understanding of genomic impact on immune landscape in the modern era, have translated into emerging utilisation of NGS as a tool for multi-gene panel analysis. These genetic variants can lead to benign or malignant changes in the protein expression or function. Sawada et al [18] reported 15 distinct genes involved in the pathogenesis of SCCE with TP53 being the most common. Among the most widely studied are BRCA genes, which are involved in repair pathways of DNA duplex and are commonly associated with neoplasms involving breast, ovaries, prostate, pancreas, colo-rectum and stomach. Between the two commonly known BRCA genes, BRCA2 harbors a greater risk of gastrointestinal cancers, in particular colorectal cancers [19]. In addition to the BRCA1 mutation, the patient’s genetic testing revealed a VUS in CDH1. CDH1 or E-cadherin, is a tumour suppressor gene associated with various cancers. It regulates cell adhesion and proliferation, and its dysregulation can contribute to tumour progression and metastasis [20]. While the significance of the CDH1 variant in this case is unclear, its involvement underscores the importance of further investigation.
Little has been known regarding the association of BRCA genes to esophageal cancers. Only a few studies have revealed the association of loss of function mutation in BRCA2 with familial esophageal squamous cell carcinomas [21]. Liang et al [6] also indicated the potential role of BRCA2 in familial esophageal squamous cell carcinomas. Recent literature has also shown the activity of platinum-based regimens in BRCA-mutated SCCE [22].
Our case had SCCE with an underlying BRCA1 mutation in the background of a significant family history for malignancies. While a comprehensive germline multi-cancer panel comprising of 84 genes, did not show any other P/LP variants in the patient, genomic alternations at the somatic level in the tumour were not investigated. As a result of comprehensive germline analysis, it seems that germline BRCA1 mutation is a driver mutation however further work is warranted to better elucidate this.
The presence of a BRCA1 mutation in this case suggests potential implications for treatment strategies. BRCA-mutated tumours, including SCCE, may exhibit heightened sensitivity to platinum-based chemotherapy regimens due to DNA repair defects. Consequently, incorporating platinum-based agents like carboplatin into the treatment regimen could enhance therapeutic outcomes. However, further research and clinical trials are necessary to validate the efficacy of platinum-based chemotherapy in BRCA1-mutated SCCE.
Overall, our findings suggest a possible association between BRCA1 and SCCE, hinting at the prospect of targeted therapies. Additionally, our study underscores the importance of tailored surveillance for associated malignancies and the identification of at-risk family members.
Conclusion
Esophageal cancers can be uncommonly driven by an underlying BRCA mutation. Patients who have a significant history of familial malignancies should be considered for genetic testing for timely identification of these P variants.
List of abbreviations
AC: Adenocarcinoma; LP: Likely pathogenic; NGS: Next generation sequencing; P: Pathogenic; SCCE: Squamous cell carcinoma of esophagus; VUS: Variant of uncertain significance.
Acknowledgments
The authors would like to acknowledge Dr Asra Ahmed, Resident II, Department of Radiology, Aga Khan University Hospital, for providing radiologic pictures and Ms Nida-e-Zehra, Research Associate Clinical Science, Department of Oncology, Aga Khan University Hospital, for helping in editing of the final manuscript.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
No specific funding has been used for manuscript writing or reporting.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
SN, MRS, DS gave a relevant contribution in writing the original manuscript. SN also contributed to the conception, formatting and revision of the final manuscript. FA was involved in reviewing and providing critically important content for the manuscript. YAR and RI reviewed the final version of the manuscript. All authors read and gave their approval for the final version to be published.
References
1. Starr J and Ramnaraign B (2020) Germline BRCA1 mutated esophageal squamous cell carcinoma Rare Tumors 12 2036361320972218 https://doi.org/10.1177/2036361320972218 PMID: 33224455 PMCID: 7649882
2. Deng J, Chen H, and Zhou D, et al (2017) Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations Nat Commun 8(1) 1533 https://doi.org/10.1038/s41467-017-01730-x PMID: 29142225 PMCID: 5688099
3. Gao YB, Chen ZL, and Li JG, et al (2014) Genetic landscape of esophageal squamous cell carcinoma Nat Genet 46(10) 1097–1102 https://doi.org/10.1038/ng.3076 PMID: 25151357
4. Munari FF, Dos Santos W, and Evangelista AF, et al (2021) Profile of esophageal squamous cell carcinoma mutations in Brazilian patients Sci Rep 11(1) 20596 https://doi.org/10.1038/s41598-021-00208-7 PMID: 34663841 PMCID: 8523676
5. Deng J, Weng X, and Ye J, et al (2019) Identification of the germline mutation profile in esophageal squamous cell carcinoma by whole exome sequencing Front Genet 10 47 https://doi.org/10.3389/fgene.2019.00047 PMID: 30833958 PMCID: 6387948
6. Liang Z, Hu W, and Li S, et al (2020) Germline BRCA2 truncating mutation in familial esophageal squamous cell carcinoma: a case controlled study in China Med Sci Monit 26 e923926 https://doi.org/10.12659/MSM.923926 PMID: 32579544 PMCID: 7331485
7. Powell SN and Kachnic LA (2003) Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation Oncogene 22(37) 5784–5791 https://doi.org/10.1038/sj.onc.1206678 PMID: 12947386
8. Akbar F, Siddiqui Z, and Waheed MT, et al (2022) Spectrum of germline pathogenic variants using a targeted next generation sequencing panel and genotype-phenotype correlations in patients with suspected hereditary breast cancer at an academic medical centre in Pakistan Hered Cancer Clin Pract 20(1) 24 https://doi.org/10.1186/s13053-022-00232-2 PMID: 35710434 PMCID: 9204946
9. Zimmer K, Kocher F, and Puccini A, et al (2021) Targeting BRCA and DNA damage repair genes in GI cancers: pathophysiology and clinical perspectives Front Oncol 11 662055 https://doi.org/10.3389/fonc.2021.662055 PMID: 34707985 PMCID: 8542868
10. Moran A, O’Hara C, and Khan S, et al (2012) Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations Fam Cancer 11(2) 235–242 https://doi.org/10.1007/s10689-011-9506-2
11. West HJ and Jin JO (2015) JAMA oncology patient page. Performance status in patients with cancer JAMA Oncol 1(7) 998 https://doi.org/10.1001/jamaoncol.2015.3113 PMID: 26335750
12. Wang ZX, Cui C, and Yao J, et al (2022) Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial Cancer Cell 40(3) 277–288.e3 https://doi.org/10.1016/j.ccell.2022.02.007 PMID: 35245446
13. Arnold M, Laversanne M, and Brown LM, et al (2017) Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030 Am J Gastroenterol 112(8) 1247–1255 https://doi.org/10.1038/ajg.2017.155 PMID: 28585555
14. Asghar MS, Khan NA, and Kazmi SJH, et al (2021) Clinical, epidemiological, and diagnostic characteristics of esophageal carcinoma in a Pakistani population Ann Saudi Med 41(2) 91–100 https://doi.org/10.5144/0256-4947.2021.91 PMID: 33818145 PMCID: 8020643
15. Shapiro J, van Lanschot JJB, and Hulshof M, et al (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial Lancet Oncol 16(9) 1090–1098 https://doi.org/10.1016/S1470-2045(15)00040-6 PMID: 26254683
16. Kamarajah SK, Phillips AW, and Ferri L, et al (2021) Neoadjuvant chemoradiotherapy or chemotherapy alone for oesophageal cancer: population-based cohort study Br J Surg 108(4) 403–411 https://doi.org/10.1093/bjs/znaa121 PMID: 33755097
17. Yang H, Liu H, and Chen Y, et al (2018) Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial J Clin Oncol 36(27) 2796–2803 https://doi.org/10.1200/JCO.2018.79.1483 PMID: 30089078 PMCID: 6145832
18. Sawada G, Niida A, and Uchi R, et al (2016) Genomic landscape of esophageal squamous cell carcinoma in a Japanese population Gastroenterology 150(5) 1171–1182 https://doi.org/10.1053/j.gastro.2016.01.035 PMID: 26873401
19. Oh M, McBride A, and Yun S, et al (2018) BRCA1 and BRCA2 gene mutations and colorectal cancer risk: systematic review and meta-analysis J Natl Cancer Inst 110(11) 1178–1189 https://doi.org/10.1093/jnci/djy148 PMID: 30380096
20. Shenoy S (2019) CDH1 (E-Cadherin) mutation and gastric cancer: genetics, molecular mechanisms and guidelines for management Cancer Manag Res 11 10477–10486 https://doi.org/10.2147/CMAR.S208818 PMID: 31853199 PMCID: 6916690
21. Ko JM, Ning L, and Zhao XK, et al (2020) BRCA2 loss-of-function germline mutations are associated with esophageal squamous cell carcinoma risk in Chinese Int J Cancer 146(4) 1042–1051 https://doi.org/10.1002/ijc.32619
22. Yan T, Cui H, and Zhou Y, et al (2019) Multi-region sequencing unveils novel actionable targets and spatial heterogeneity in esophageal squamous cell carcinoma Nat Commun 10(1) 1670 https://doi.org/10.1038/s41467-019-09255-1 PMID: 30975989 PMCID: 6459928






