Challenges and opportunities in NMIBC management across Latin America: insights from healthcare providers and a patient advocacy group
Mark McCully1, Julia Lipkis1, Aryel Heller1 and Adrian Huñis2
1PharmaValue Partners, 603 Mattison Avenue, Suite 319, Asbury Park, NJ 07712, USA
2A. Hunis & Associates, Oncology Consultants, Hollywood, FL 33019, USA
Abstract
Non-muscle invasive bladder cancer (NMIBC) is characterised by high rates of recurrence and progression, requiring substantial healthcare resources. In Latin America, the incidence of NMIBC is set to increase due to an aging population and lifestyle changes. To better understand the current challenges for NMIBC treaters and patients, a mixed-methods approach was leveraged combining secondary research with qualitative interviews from healthcare providers in Brazil, Colombia, Mexico and Argentina. Our analysis found that significant challenges persist across the region, particularly due to Bacillus Calmette-Guérin shortages, inconsistent adherence to clinical guidelines and significant socioeconomic disparities for patients accessing healthcare services. Addressing these challenges requires improved patient advocacy, strategic use of clinical trials and better resource distribution to enhance NMIBC management across Latin America.
Keywords: NMIBC, patient advocacy, BCG, clinical trials, Latin America, urology, public health, Brazil, Colombia, Mexico, Argentina
Correspondence to: Mark McCully
Email: mmccully@pharmavaluepartners.com
Published: 07/06/2024
Received: 07/05/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Non-muscle invasive bladder cancer (NMIBC) is a treatable yet challenging form of bladder cancer, known for its high rates of recurrence and progression which require continuous monitoring and significant healthcare resources [1–4]. It has been reported that bladder cancer costs were associated with the highest per-patient cost of all cancers from diagnosis to death [1]. In the United States, a recent report by Williams et al [5], estimated that in 2020 treatment costs for bladder cancer approached nearly 6 billion USD, with median costs escalating from 29,459 USD in the first year to 55,267 USD in the second year, and reaching 117,361 USD by the fifth year following the start of Bacillus Calmette-Guérin (BCG) induction.
Latin America’s diverse healthcare systems pose significant and substantial challenges to effectively treat and manage NMIBC [6–17]. Moreover, the incidence of bladder cancer in the region is expected to increase over the coming years due to an aging population and changes in lifestyle [1].
The challenges of managing NMIBC in Latin America are multifaceted. One of the most pressing issues is the worldwide shortage of BCG, the primary treatment for high-risk NMIBC cases. Throughout Latin America, BCG is generally in low supply, which leads to its use being restricted to high-risk patients [18–20]. While chemotherapy can be used in the absence of intravesical BCG, its availability varies across countries. This shortage is compounded by varying standards of care, healthcare infrastructure constraints and significant disparities in access to healthcare services across urban and rural areas.
Additionally, lifestyle factors significantly influence the prevalence and management of NMIBC [21]. Smoking is closely linked with bladder cancer, and the prevalence of smoking in the region varies significantly by country, affecting disease incidence rates and complicating public health efforts to reduce cancer risks [1, 2]. Dietary factors also play a role, with changing diets potentially leading to higher rates of diseases previously less common in the region [2].
Finally, the quality of urology training programs in Latin America varies significantly, with a third of urology residents not receiving objective accreditation tests upon completion of their programs, and many lacking exposure to advanced surgical techniques and procedures [22]. Based on all these disparities, we selected four Latin American countries to explore the specific challenges and opportunities facing NMIBC patients and healthcare providers.
Methods
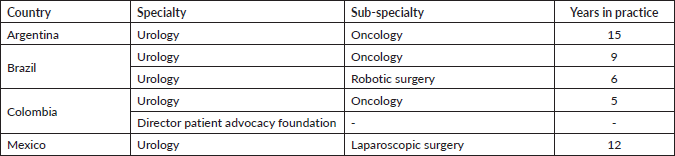
Utilising a mixed-methods approach, this paper integrates comprehensive secondary research with primary qualitative data gathered from interviews across four Latin American countries: Brazil, Colombia, Mexico and Argentina (Table 1). A review of existing literature and data was first performed to contextualise the current landscape of NMIBC care, as well as to inform primary interview questions and support subsequent findings. Primary interviews were conducted with healthcare providers experienced in the diagnosis and treatment of NMIBC patients, as well as with a representative from a major patient advocacy group who highlighted major challenges and barriers from a patient perspective navigating NMIBC diagnosis and care in Latin America. Each interview aimed to capture detailed insights into the current practices, challenges and perceptions regarding NMIBC management.
Results
Brazil
Standard of care
Despite a relatively well-structured healthcare system, adherence to NMIBC guidelines is somewhat inconsistent, primarily due to BCG supply issues [15]. Brazilian urologists typically follow the European Association of Urology (EAU) guidelines, but full adherence may vary, especially when treating patients with recurrent NMIBC. According to secondary sources, only about two-thirds of NMIBC treaters adhere to these guidelines post-BCG failure, due to persistent shortages [23]. Other challenges include limited access to chemotherapy agents as well as high costs, which can lead to deviations from recommended treatments [16, 21].
Table 1. Respondent information.
BCG use
Brazil faces significant BCG shortages, largely due to the recent closure of domestic manufacturing facilities over regulatory issues. As a result, hospitals rely on imported BCG, which is often in short supply [24].
Patient access
The patient journey from symptom onset to diagnosis is often a lengthy process, particularly in the public sector, where delays can compromise treatment outcomes [16]. Access to diagnostic and treatment facilities varies between 6 months and 2 years, with private patients generally receiving faster and more comprehensive care. Healthcare providers express frustration with cultural and systemic barriers, such as hesitancy to seek initial care and long waiting lists, that lead to delayed diagnosis.
Healthcare provider education
Brazilian urologists typically keep themselves updated on advances in the field through international guidelines, participation in congresses and ongoing professional education, indicating general engagement with both current practices and emerging research in their field. NMIBC treaters express interest in novel immunotherapies; however, high costs and limited clinical trials within the country slow the widespread adoption of newer therapies.
Colombia
Standard of care
Colombia exhibits significant variability in the management of NMIBC, largely influenced by geographic disparities in healthcare access. Adherence to international guidelines is inconsistent, especially in rural areas where healthcare resources are sparse. Urban centers like Bogotá have better infrastructure and are more likely to follow these guidelines closely [25]. Challenges include a severe shortage of BCG and a lack of trained urologists, which can delay or alter the standard treatment protocol [26, 27].
BCG use
Colombia faces acute shortages of BCG, more severe than in many other countries, affecting the standard care for NMIBC patients. The only BCG product with an approved health registration, ‘SII Onco BCG,’ is imported in limited quantities, and the supply does not meet the patient demand, leading to significant treatment delays and the use of alternative therapies where feasible [28].
Patient access
Patients in Colombia often experience lengthy delays from symptom onset to diagnosis and treatment, especially within the public health system. These delays are exacerbated by insufficient healthcare infrastructure and a centralised healthcare system that does not adequately serve rural and remote areas. Patient navigation is hindered by complex referral systems and limited access to specialised care.
Healthcare provider education
Healthcare professionals in Colombia face challenges in accessing the latest training and resources, particularly outside major urban centers. While there is interest in advancing NMIBC treatment through novel therapies and techniques, actual implementation is slow due to these educational gaps. Urologists and oncologists strive to stay informed through limited participation in international conferences and digital learning platforms.
Mexico
Standard of care
In Mexico, the approach to NMIBC care is relatively comprehensive in urban areas but is highly inconsistent across the country due to the uneven distribution of healthcare resources. Most major cities have adequate facilities to follow EAU guidelines, but rural areas often have insufficient access to specialised care, leading to significant deviations from these guidelines [17, 29].
BCG use
Mexico does not possess any local production centers for BCG and therefore depends upon imported strains of the virus. Shortages often occur which lead to distribution issues, forcing healthcare providers to ration BCG use or switch to alternative treatments.
Patient access
The disparity in healthcare access between urban and rural areas significantly affects the NMIBC treatment pathway in Mexico. Urban centers provide relatively quick access to diagnosis and treatment, while rural patients may face long travel times and delays in receiving care, impacting treatment outcomes.
Healthcare provider education
Mexican healthcare providers are generally well-engaged with the latest developments in NMIBC care, participating in national and international medical conferences and professional societies. However, the reach of this education may vary, with practitioners in remote areas having less access to ongoing education and resources [30].
Argentina
Standard of care
Argentina’s healthcare system provides relatively uniform access to NMIBC care, and most treaters are well-versed in international guidelines [11, 31, 32].
BCG use
The national INPB Mycobacteria Derivatives Service produces BCG domestically, which helps ensure better availability than in many surrounding countries. Despite this local production, shortages and distribution challenges still sometimes occur, leading to the rationing of BCG, especially during maintenance therapy [6, 33].
Patient access
While Argentina boasts a robust healthcare infrastructure, some disparities exist between urban and rural areas in terms of speed and quality of NMIBC care. Urban patients typically experience faster diagnosis and treatment initiation compared to those in rural areas, where healthcare resources are more limited.
Healthcare provider education
Argentinian healthcare professionals are active in both national and international urological communities, which contributes to a high level of knowledge and competency in NMIBC care. There is significant interest in integrating novel therapies into practice, although practical implementation can be hindered by regulatory and cost-related challenges.
Cross-country comparison and discussion
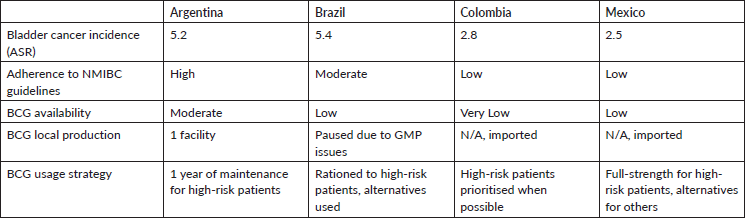
Table 2 provides a comparative analysis of the incidence, resources and healthcare practices related to NMIBC management in Argentina, Brazil, Colombia and Mexico.
Table 2. NMIBC SOC cross-country comparison [3].
Discussion
The management of NMIBC in Latin America is fraught with challenges, including widespread BCG shortages, inconsistent adherence to clinical guidelines and significant geographic and socioeconomic disparities. These issues, while prevalent across Argentina, Brazil, Colombia and Mexico, impact each country differently, influencing both patient outcomes and the overall effectiveness of NMIBC management.
A primary concern in the region is the scarcity of intravesical BCG, the fundamental treatment for high-risk NMIBC [18, 19, 21]. Brazil, once a producer of the Moreau BCG strain, has encountered severe supply disruptions. Production ceased due to compliance issues with good manufacturing practices (GMPs) and delays in establishing a new facility. Consequently, Brazil has had to import BCG at significantly higher costs, forcing hospitals to independently manage their BCG supplies. This has led to inconsistent treatment availability and compromised patient care [24].
In Colombia, the availability of BCG is critically low, with only one approved product [28]. High import costs and logistical challenges compound this issue, severely limiting access to essential treatments and necessitating that healthcare providers prioritise BCG for the most at-risk patients, often to the detriment of comprehensive care.
Argentina and Mexico also face significant BCG supply issues, though their challenges differ. Argentina struggles with production inconsistencies despite having manufacturing facilities, resulting in only moderate availability. In Mexico, vast geographic and economic inequalities exacerbate the shortage, particularly affecting rural areas where adherence to treatment guidelines is low, and patient outcomes vary significantly [29].
Healthcare disparities, particularly in rural and remote areas, limit access to well-trained medical personnel and infrastructure, thereby impacting NMIBC care. Investment in healthcare infrastructure, training programs and targeted initiatives can improve care in underserved areas.
These disparities in guideline adherence reflect broader issues of resource availability and the need to resort to alternative treatments, which are often less effective [21]. The role of patient advocacy is emerging but remains limited across the region. Advocacy groups, often focused on more common cancers, may overlook NMIBC given its lower incidence in the region. However, these groups could potentially help NMIBC survivors to establish their own NMIBC-specific advocacy initiatives, leveraging their unique experiences for support, public engagement and policy influence.
By addressing the unique challenges of each country with targeted policies and leveraging regional strengths, Latin America can improve NMIBC care outcomes and reduce the disease’s burden. Enhanced awareness, improved resource distribution and strategic use of clinical trials could become crucial for advancing NMIBC management in this diverse and dynamic region.
Based on our secondary and primary research we propose the following regional strategies to foster better NMIBC care:
1. Enhanced patient advocacy: Throughout the LATAM region, patient advocacy groups tend to be pan-tumor with more frequently occurring cancers prioritised. The establishment of NMIBC-specific advocacy groups across Latin America could lead to greater awareness of this increasingly prevalent cancer, resulting in the development of resources to help patients navigate a complex treatment journey, while also influencing country-specific health policies. As an example, patient advocacy groups might be able to lobby governments to subsidise BCG costs and expedite new treatment approvals.
2. Harmonised clinical trial regulations: As mentioned previously, the high level of regulatory diversity in the LATAM region impacts the running of clinical trials in the region as well as the approval of new medicines. More standardised clinical trial regulations could facilitate multi-country studies, speed up the approval process for new treatments and address BCG shortages. Using Colombia as an example, the government facilitated highly favorable clinical trials and R&D tax incentives to bring Colombia’s clinical research environment up to international standards and enhance its relative attractiveness. As reported, experts agree that Colombia could see over 100 new clinical trials every year and close to $500 million in economic gains per year [34]. By creating a harmonised regulatory environment for clinical trials, patients within these regions could get access to cutting-edge treatments years earlier.
3. Incentives for trial sponsor: Leveraging incentives such as tax breaks and fast-track approval processes for pharmaceutical companies conducting trials or investing in research within the region could accelerate access to novel NMIBC drugs and improve patient outcomes through increased resources and clinical education within the region [34].
4. Education and training programs: Initiate comprehensive education campaigns targeted at both healthcare providers and patients to enhance understanding of NMIBC, improve treatment compliance and promote guideline adherence. Enhance patient awareness programs to improve understanding of NMIBC treatment pathways and the importance of guideline adherence.
5. Infrastructure investment and localised patient recruitment: Promote clinical trials in rural and underserved regions to increase access to innovative treatments and enhance patient compliance by providing care closer to home. Develop mobile health clinics and telehealth services to extend reach in underserved areas [13].
Conclusion
Latin America faces significant challenges managing NMIBC due to complex and heterogeneous healthcare provisions. These challenges are further exacerbated by routine BCG supply shortages, disparities in healthcare access, lack of patient awareness about NMIBC and variability in guideline compliance. Addressing these issues requires a multifaceted approach that engages healthcare providers, governments, pharmaceutical companies and patient advocacy groups.
Key Challenges to Address
1. Healthcare disparities and resource distribution: Tackling disparities in healthcare access, especially in rural and remote areas, necessitates investments in infrastructure, professional training programs and initiatives that improve care delivery in underserved regions. Efficient distribution of resources – such as healthcare facilities, medical supplies and skilled personnel – is crucial to ensure equitable NMIBC care. Strategic planning and stakeholder collaboration will optimise resource allocation and help bridge the gap in healthcare access across different regions.
2. BCG supply shortages: BCG therapy, the standard treatment for NMIBC, is often in short supply globally, but shortages are uniquely pronounced in Latin America. This issue can be mitigated through diversification of suppliers, strategic stockpiling and international cooperation to ensure a consistent BCG supply.
3. Lack of patient awareness: Many patients in Latin America remain unaware of NMIBC symptoms, risk factors and treatment options. Public awareness campaigns, educational programs in healthcare facilities and partnerships with patient advocacy groups can promote early detection and timely treatment.
4. Variability in guideline compliance: Adherence to NMIBC diagnosis and treatment guidelines varies across healthcare providers and regions. Standardising protocols, additional training for healthcare professionals and implementing quality assurance measures can improve compliance and ensure high-quality care.
5. Tailored policies and strategies: Each country in Latin America has unique healthcare challenges and resources. Policies and strategies should be tailored to address specific needs while leveraging regional strengths, such as established research institutions and healthcare networks, to optimise NMIBC management.
6. Strategic use of clinical trials: Clinical research and trial participation can advance NMIBC management by evaluating new treatment methods, refining existing therapies and generating guidelines tailored to regional needs. Collaboration with academic institutions, pharmaceutical companies and regulatory agencies can facilitate trial conduct.
By implementing targeted policies and strategies tailored to each country’s unique challenges while harnessing regional strengths, Latin America can significantly enhance NMIBC care outcomes and reduce the disease’s impact across the region. Promoting greater awareness, optimising resource distribution and strategically utilising clinical trials will be vital to advancing NMIBC treatment throughout this diverse and dynamic area.
Conflicts of interest
The authors report no conflicts of interest.
Funding
Support for the research was provided by a Pfizer Global Medical Grant (GMG).
References
1. Grabe-Heyne K, Henne C, and Mariappan P, et al (2023) Intermediate and high-risk non-muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs Front Oncol 13 1170124 https://doi.org/10.3389/fonc.2023.1170124 PMID: 37333804 PMCID: 10272547
2. van Hoogstraten LMC, Vrieling A, and van der Heijden AG, et al (2023) Global trends in the epidemiology of bladder cancer: challenges for public health and clinical practice Nat Rev Clin Oncol 20(5) 287–304 https://doi.org/10.1038/s41571-023-00744-3 PMID: 36914746
3. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
4. He H, Xie H, and Chen Y, et al (2020) Global, regional, and national burdens of bladder cancer in 2017: estimates from the 2017 global burden of disease study BMC Public Health [Internet] 20(1) 1–9 [https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-020-09835-7] Date accessed: 03/05/24
5. Williams SB, Howard LE, and Foster ML, et al (2021) Estimated costs and long-term outcomes of patients with high-risk non–muscle-invasive bladder cancer treated with Bacillus Calmette-Guérin in the Veterans affairs health system JAMA Netw Open [Internet] 4(3) e213800 [https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2777975] Date accessed: 03/05/24 https://doi.org/10.1001/jamanetworkopen.2021.3800 PMID: 33787908 PMCID: 8013821
6. Argotti U, Leyens L, and Lisbona C, et al (2023) Comparison of the Latin America regulation landscape and international reference health authorities to hasten drug registration and clinical research applications Ther Innov Regul Sci 57(6) 1287–1297 https://doi.org/10.1007/s43441-023-00565-7 PMID: 37682461 PMCID: 10579156
7. Metzler I, Bayne D, and Chang H, et al (2020) Challenges facing the urologist in low- and middle-income countries World J Urol 38(11) 2987–2994 https://doi.org/10.1007/s00345-020-03101-6 PMID: 32034500 PMCID: 8186537
8. OECD/The World Bank (2020) Health at a Glance: Latin America and the Caribbean 2020 (Paris: OECD)
9. Mendoza-Valdes A (2009) Oncologic urology in Latin America Urol Oncol: Semin Ori Investig 27(2) 120–121 https://doi.org/10.1016/j.urolonc.2008.10.010
10. Piñeros M, Laversanne M, and Barrios E, et al (2022) An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean Lancet Reg Health Am 13 100294 PMID: 36189115 PMCID: 9483035
11. Videla EA, Arribillaga L, and Carrara S, et al (2021) Manejo del carcinoma de vejiga no músculo invasor en nuestro país Rev Argent Urol [Internet] 86(3) 125–134 [https://www.revistasau.org/index.php/revista/article/view/4432] Date accessed: 03/05/24
12. Carlos García-Ubaque J and Quintero-Matallana CS (2008) Barreras Geográficas y Económicas para el Acceso a los Servicios Oncológicos del Instituto Nacional de Cancerología en Bogotá Rev salud pública 10(4) 583–592 https://doi.org/10.1590/S0124-00642008000400008
13. Restrepo JG, Alarcón J, and Hernández A, et al (2023) Clinical outcomes in patients with solid tumors living in rural and urban areas followed via telemedicine: experience in a highly complex Latin American Hospital BMC Cancer 23(1) 253 https://doi.org/10.1186/s12885-023-10717-5 PMID: 36927771 PMCID: 10018944
14. Health in the Americas | Health in the Americas [Internet] [https://hia.paho.org/en] Date accessed: 03/05/24
15. Timoteo F, Korkes F, and Baccaglini W, et al (2020) Bladder cancer trends and mortality in the Brazilian public health system Int Braz J Urol 46(2) 224–233 https://doi.org/10.1590/s1677-5538.ibju.2019.0198 PMID: 32022511 PMCID: 7025845
16. Korkes F and Maluf F (20201) Increasing costs from bladder cancer in the Brazilian health system: the role of establishing public health policies Int Braz J Urol 47(2) 443–447 PMID: 33284548 PMCID: 7857771
17. Aggarwal A, Unger-Saldaña K, and Lewison G, et al (2015) The challenge of cancer in middle-income countries with an ageing population: Mexico as a case study Ecancermedicalscience 9 536 https://doi.org/10.3332/ecancer.2015.536 PMID: 26015805 PMCID: 4435755
18. Implications of the national BCG drug shortage (2023) [Internet] [https://info.enddrugshortages.com/External/WCPages/WCWebContent/webcontentpage.aspx?ContentID=68] Date accessed: 03/05/24
19. Alhogbani MM, Picard JA, and Fassi-Fehri MH, et al (2017) Prognostic impact of Bacillus Calmette-Guérin interruption at the time of induction and consolidation Urol Ann 9(4) 315–320 https://doi.org/10.4103/UA.UA_115_17 PMID: 29118530 PMCID: 5656953
20. Korkes F, Timóteo F, and Ferrari KL, et al (2021) Bacillus Calmette-Guérin (BCG) Brazilian backstage in bladder cancer Int Braz J Urol 47(2) 232–236 https://doi.org/10.1590/s1677-5538.ibju.2021.02.02
21. Wroclawski ML, Schutz FA, and Cha JD, et al (2019) Alternative therapies to Bacillus Calmette-Guérin shortage for nonmuscle invasive bladder cancer in Brazil and other underdeveloped countries: management considerations J Glob Oncol 5 1–9 PMID: 31454284 PMCID: 6776038
22. Angulo JC, Figueroa C, and Gómez R, et al (2018) Current status of urological training in Latin America Arch Esp Urol 71(1) 23–33 PMID: 29336329
23. Reis LO, Moro JC, and Ribeiro LFB, et al (2016) Are we following the guidelines on non-muscle invasive bladder cancer? Int Braz J Urol 42(1) 22–28 https://doi.org/10.1590/S1677-5538.IBJU.2015.0122 PMID: 27136464 PMCID: 4811222
24. Governo intervém em fábrica de vacinas após rombo milionário e “obra sem fim” - BBC News Brasil [Internet] [https://www.bbc.com/portuguese/articles/cv28ex2y41yo] Date accessed: 03/05/24
25. García-Perdomo HA (2017) Centros de excelencia del cáncer en Colombia: una manera fundamental de trabajar juntos Urol Colomb 26(3) 155–156 https://doi.org/10.1016/j.uroco.2017.08.001
26. Murillo R, Ojeda K, and Solano J, et al (2019) The Colombian medical oncologists workforce J Glob Oncol 5 1–4 PMID: 31657979 PMCID: 6825248
27. Murcia E, Aguilera J, and Wiesner C, et al (2018) Oncology services supply in Colombia Colomb Med 49(1) 89–96
28. Comentarios al proyecto de Circular de regulación de precios 2021 [Internet] [https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/MET/consolidado-circular-regulacion-precios-2021.pdf] Date accessed: 03/05/24
29. Bourlon MT, Remolina-Bonilla YA, and Velázquez HE, et al (2021) Current status of urologic oncology clinics in Mexico Gac Mex Oncol [Internet] 20(1) 12–19 [http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2565-005X2021000100012&lng=es&nrm=iso&tlng=en] Date accessed: 03/05/24
30. Linden-Castro E, Pelayo-Nieto M, and Ramírez-Galindo I, et al (2016) Urology training in Mexico: residents’ perspective Gac Med Mex 152(3) 339–344 PMID: 27335189
31. Angel MO, Pupareli C, and Soule T, et al (2021) Implementation of a molecular tumour board in LATAM: the impact on treatment decisions for patients evaluated at Instituto Alexander Fleming, Argentina Ecancermedicalscience 15 1312 https://doi.org/10.3332/ecancer.2021.1312
32. Straw C, Antelo VS, and Paolino M, et al (2022) Acceptability, appropriateness and feasibility of the Latin American and Caribbean code against cancer: perceptions of decision-makers and health professionals in Argentina Ecancermedicalscience [Internet] 16 1375 [/pmc/articles/PMC9116998/] Date accessed: 03/05/24 https://doi.org/10.3332/ecancer.2022.1375 PMID: 35702416 PMCID: 9116998
33. Producción de BCG intravesical para el tratamiento del cáncer superficial de vejiga y vacuna BCG | Argentina.gob.ar [Internet] [https://www.argentina.gob.ar/ciencia/dnpe/proyectos/produccion-de-bcg-intravesical-para-el-tratamiento-del-cancer-superficial-de] Date accessed: 03/05/24
34. Latin America a compelling region to conduct your clinical trials [Internet] [https://www.clinicalleader.com/doc/latin-america-a-compelling-region-to-conduct-your-clinical-trials-0001] Date accessed: 03/05/24






