Mucinous intestinal adenocarcinoma arising from mature cystic teratoma of the ovary treated with FOLFOX-6 - a case report
Enrique Bedoya-Ismodes1,2,a, Karem Portugal1,2,b, Sandy Carmona-Lozano1,c, Mirna Dominguez-Gonzales1,d, Jaime Torres1 and Nestor Juarez-Herrera1,e
1Hospital Santa Rosa, Lima 15084, Perú
2Facultad de Medicina Humana, Universidad de San Martín de Porres, Lima, Perú
ahttps://orcid.org/0000-0002-6792-2371
bhttps://orcid.org/0000-0002-4851-8664
chttps://orcid.org/0000-0002-8954-3727
dhttps://orcid.org/0000-0002-4895-6824
ehttps://orcid.org/0000-0002-7414-5785
Abstract
The malignant transformation of mature cystic teratomas during pregnancy is a rare occurrence in medical literature. In this case report, we present a remarkable instance of mucinous intestinal adenocarcinoma arising from a mature ovarian cystic teratoma diagnosed during pregnancy. This is among the few reports detailing the effective use of the FOLFOX 6 chemotherapy regimen for treating this type of intestinal cancer. Furthermore, we emphasize the immunohistochemical results that confirm the colorectal histological origin.
Keywords: mature cystic teratoma, malignant transformation, mucinous intestinal adenocarcinoma, FOLFOX 6, colorectal origin
Correspondence to: Enrique Oswaldo Bedoya Ismodes
Email: e.bed10@outlook.com
Published: 04/06/2024
Received: 06/11/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Dermoid cysts, also known as mature cystic teratomas, are among the most diagnosed ovarian neoplasms and primarily affect women during their reproductive years. These cysts have the potential to originate from any one of the three primary germ layers [1].
While most teratomas are benign tumours, they pose a notable risk of malignancy. Malignant transformation within mature ovarian cystic teratomas is rare, occurring in fewer than 2% of cases [2]. Squamous cell carcinoma represents the most common form of malignancy, accounting for about 75% of such transformations, while the progression into mucinous intestinal adenocarcinoma is particularly rare, with a reported incidence of less than 6.8% [3, 4].
This transformation may manifest with clinical symptoms, yet it might also be an incidental finding in an asymptomatic patient during pregnancy or, as described in this case, during a cesarean section [1]. This report details a rare instance of malignant conversion to mucinous intestinal adenocarcinoma within a mature ovarian cystic teratoma that remained asymptomatic. The case was diagnosed and managed by the oncology department at Hospital Santa Rosa in Lima, Peru.
Case report
The patient, a 30-year-old woman from Venezuela, underwent an emergency cesarean section due to preeclampsia. Her medical history includes untreated hypertension, mild bronchial asthma and marijuana use. Her father was diagnosed with bladder cancer at the age of 50.
During the first-trimester ultrasound, a right ovary measuring 36 × 37 mm was identified, showing a follicle and a 26 mm mass, while the left ovary was obscured by an 80 × 50 mm complex cyst, predominantly solid (80%), indicative of a mature teratoma. Additionally, elevated levels of tumour markers were detected (Figure 1).
She underwent an emergency segmental cesarean section at 35 weeks of gestation due to severe preeclampsia. Intraoperatively, the uterus exhibited friable tissue and pinpoint erythematous lesions on the posterior surface, with ligaments adhering to the posterior surface, along with the involvement of the omentum and intestinal loops. Pinpoint lesions were also present on the ovaries. Additionally, there was 2,000 cc of citrine-yellow gelatinous fluid, alongside a tumour originating from the ovary, and a live single fetus.
Following the cesarean section, evaluation by the gynecologic oncology service recommended an exploratory laparotomy, with the possibility of cytoreduction.
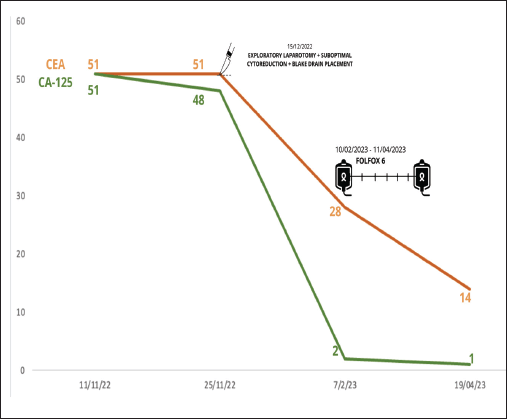
Figure 1. Tumour markers changes through surgery and chemotherapy with FOLFOX 6.
Post-surgery contrast-enhanced abdominal tomography revealed retroperitoneal paraaortic and mesenteric lymph nodes measuring less than 15 × 11 mm. Additionally, a multiseptated mass measuring 190 × 85 × 125 mm was identified, with a solid component measuring 62 × 44 mm. Pelvic magnetic resonance imaging with contrast displayed a complex cystic lesion characterised by thick and irregular septa, a hemorrhagic component, and solid areas. This made measurement of the left ovary challenging, with free fluid evident in the pelvic cavity.
The laparotomy yielded suboptimal cytoreduction, and a Blake drain was inserted. The surgical findings were as follows: no hepatic metastasis, 5,600 cc of mucinous citrine fluid was drained, and a 15 × 12 cm complex, solid, multilobulated mass was observed in the left ovary. This mass was flattened and adhered to the small intestine and rectum without infiltration. A mass was observed attached to the uterus at the cervical level, seemingly infiltrating it along its entire length but not involving the bladder. Additionally, on the cecum, a flattened mass originating from the cecal appendix was noted, extending into both the right and left paracolic areas. This mass displayed necrotic regions containing mucin, causing architectural distortion and apparent infiltration throughout its entirety.
The pathology report described the infiltration of the left ovary by a well-differentiated mucinous adenocarcinoma originating from a mature cystic teratoma. Immunohistochemistry revealed positive staining for CDX 2, CK20, SATB2 and focal PAX8, while CK7 was negative (Figure 2). The infiltration extended throughout the entire ovarian capsule, with evident capsule rupture, and also affected the external surface. However, vascular permeation was absent, and the uterine tube remained free. Deciduous changes were noted in the endometrium, and the myometrium harbored an intramural leiomyoma. Mucin lakes affected the uterine serosa, although surgical margins were clear of malignant neoplasm. Both the ovary and uterine tube were free of malignant neoplasm, as confirmed by serial sections. Subsequent cyto-reduction revealed fibroconnective tissue with acellular mucin lakes. Additionally, a scar was observed on a skin fragment, accompanied by chronic inflammatory infiltrate. Further immunohistochemistry studies (SATB2 and PAX 8) were requested to determine the primary origin. The appendicular region biopsy revealed vascularised fibroadipose tissue containing acellular mucin lakes, fibrin and necrotic tissue, with no evidence of appendicular tissue. Finally, the epiploon exhibited chronic inflammation, acellular mucin lakes and granulation tissue with foreign body giant multinucleated cells.
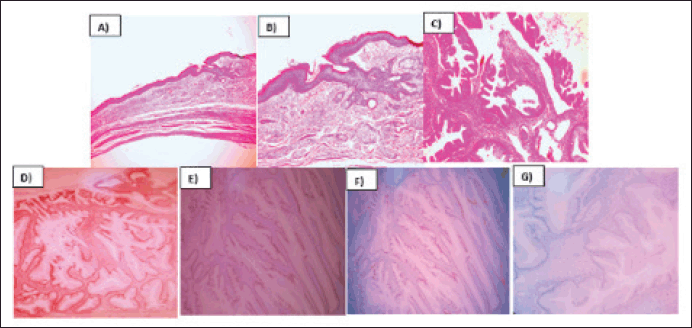
Figure 2. Mucinous intestinal adenocarcinoma arising from mature cystic teratoma of ovary. (a): H&E 10X, Mature cystic teratoma component of the ovary. (b): H&E 20X, Mature cystic teratoma component of the ovary. (c): H&E 40X, Malignant transformation of ovary´s cystic teratoma to well differentiated mucinous adenocarcinoma. (d): CK20, 40X, Membrane inmunoreactivity in mucinous adenocarcinoma. (e): SATB2, 40X, Nuclear inmunoreactivity in mucinous adenocarcinoma. (f): PAX8,40X, Nuclear inmunoreactivity in mucinous adenocarcinoma. (g): CK7, 40X, Lost inmunoreactivity in mucinous adenocarcioma.
The patient underwent a colonoscopy, during which no lesions or tumours were found.
Subsequently, the patient received systemic chemotherapy with the FOLFOX 6 regimen (oxaliplatin, folinic acid and 5-fluorouracil every 2 weeks) based on the intestinal origin indicated by the immunohistochemistry results. She completed 12 cycles of FOLFOX 6 from 06/02/2023 to 27/07/2023.
Follow-up imaging studies revealed no abnormalities or signs of disease activity. The patient remains asymptomatic and free from malignant neoplasm. She will continue with periodic surveillance appointments.
Discussion
The discovery of adnexal masses during pregnancy is a rare occurrence, affecting fewer than 2% of patients. Among these masses, mature cystic teratomas are the most frequent. Most of these teratomas are totally asymptomatic and are identified incidentally during ultrasound examinations, occasionally complicated by the presence of the gravid uterus [1–5].
The malignant transformation of mature ovarian cystic teratomas is a rare occurrence, happening in less than 2% of cases. Squamous cell carcinoma represents the most commonly observed malignant transformation, accounting for 75% of cases. In contrast, the transformation into mucinous intestinal adenocarcinoma is exceptionally rare, with an incidence of less than 6.8% [6, 7].
This report outlines a remarkable case of malignant transformation into mucinous intestinal adenocarcinoma within a mature ovarian cystic teratoma during pregnancy. Notably, it is the sole documentation of employing systemic chemotherapy with the FOLFOX 6 regimen to address the intestinal neoplasm, yielding highly satisfactory outcomes.
Adenocarcinomas can arise from diverse tissues, such as sebaceous glands, mammary glands, salivary glands, thyroid and epithelia of the gastrointestinal and respiratory tracts. In this instance, immunohistochemistry results demonstrated positive staining for CDX2, CK20, SATB2 and focal PAX8, alongside negative staining for CK7, suggesting a colorectal origin histology [8].
Given the rarity of such cases, there is no established standard treatment approach, requiring individualised management and resulting in variable outcomes. For young patients, like the one depicted in this case, it is advisable to commence treatment with unilateral salpingo-oophorectomy, followed by surgical staging, assessment of pathological findings and the formulation of a tailored follow-up plan [9].
Huang et al [10] conducted a meta-analysis report elucidating the influence of multidisciplinary cancer conferences on overall survival. The study revealed a median survival time of 30.2 months in the multidisciplinary cancer conference group, contrasting with 19 months in the control group [10].
To date, the patient has remained asymptomatic for 12 months, with no observed changes or abnormalities in diagnostic imaging. Although existing clinical practice guidelines do not specifically address the use of tumour markers for monitoring in such cases, due to the initial elevation of these markers at diagnosis, it has been decided to conduct follow-up assessments every 3–6 months during the initial 2 years, followed by annual evaluations from the third year onwards, mirroring the approach outlined in clinical practice guidelines for ovarian cancer.
Conclusion
In summary, the malignant transformation into mucinous intestinal adenocarcinoma within a mature ovarian cystic teratoma during pregnancy is an exceedingly rare occurrence, with limited prior reports in the medical literature. Most instances of malignant transformation have been linked to the postmenopausal period. This case report stands out as one of the earliest to document the effective use of chemotherapy, specifically the FOLFOX 6 regimen, as part of the treatment protocol.
These findings underscore the importance of better understanding and awareness regarding the potential for malignant transformations in teratomas during pregnancy. These also emphasize the necessity of tailoring treatment in unique scenarios like the one depicted in this report.
Furthermore, this case underscores the significance of rigorous and prolonged follow-up for patients who have undergone this malignant transformation. The positive outcomes observed suggest that treatments such as chemotherapy with the FOLFOX 6 regimen could be a viable option in similar cases in the future. Gathering additional data and conducting research in this area is vital for enhancing our comprehension and management of this rare condition.
Conflicts of interest
Our authors have no competing interests, and the contents of this manuscript have not been published elsewhere. There are no conflicts of interest to disclose.
Funding
No external funding or funding by any author has been received for this study.
Ethical statement
Studies involving human participants underwent review and approval by the Ethics Committee of Hospital Santa Rosa. The patient provided written informed consent to participate in this study.
Authors’ contributions
The original manuscript was authored and approved by all contributing authors.
Enrique Bedoya-Ismodes: preparation of the document from its conception and design to the acquisition of the information, review of intellectual content, preparation of photos, participation in graphic designs and approval of the version sent to the editorial process.
Karem Portugal: preparation of the document from its conception and design to the acquisition of the information, review of intellectual content, data collection, preparation of tables and approval of the version sent to the editorial process.
Sandy Carmona-Lozano: bibliographic review, intellectual content and approval of the version sent to the editorial process.
Mirna Dominguez-Gonzales: preparation of photos, bibliographic review, intellectual content and approval of the version sent to the editorial process.
Jaime Torres: intellectual content, approval of the version sent to the editorial process
Nestor Juarez-Herrera: intellectual content, approval of the version sent to the editorial process.
References
1. Cubas S, Vásquez V, and Velásquez O (2018) Mature cystic teratoma of the ovary, experience in a hospital in northern perú: case report Rev Cient Cienc Méd [Internet] 21(2) 67–72 [
2. Atwi D, Kamal M, and Quinton M, et al (2022) Malignant transformation of mature cystic teratoma of the ovary J Obstet Gynaecol Res 48(12) 3068–3076 https://doi.org/10.1111/jog.15409 PMID: 36053141
3. Ulker V, Gedikbasi A, and Numanoglu C, et al (2009) Mucinous adenocarcinoma arising in ovarian mature cystic teratoma in pregnancy Arch Gynecol Obstet 280(2) 287–291 https://doi.org/10.1007/s00404-008-0878-y
4. Schrag D, Shi Q, and Weiser MR, et al (2023) Preoperative treatment of locally advanced rectal cancer N Engl J Med 389(4) 322–334 https://doi.org/10.1056/NEJMoa2303269 PMID: 37272534 PMCID: 10775881
5. Hakoun AM, AbouAl-Shaar I, and Zaza KJ, et al (2017) Adnexal masses in pregnancy: an updated review Avicenna J Med 7(4) 153–157 https://doi.org/10.4103/ajm.AJM_22_17 PMID: 29119081 PMCID: 5655645
6. Chiang AJ, Chen MY, and Weng CS, et al (2017) Malignant transformation of ovarian mature cystic teratoma into squamous cell carcinoma: a Taiwanese Gynecologic Oncology Group (TGOG) study J Gynecol Oncol 28(5) e69 https://doi.org/10.3802/jgo.2017.28.e69 PMID: 28657230 PMCID: 5540728
7. Min KY, Jee BC, and Lee HS, et al (2006) Intestinal adenocarcinoma arising in a mature cystic teratoma of the ovary: a case report, pathology - research and practice Pathol Res Pract 202 (7) 531–535 https://doi.org/10.1016/j.prp.2006.03.005 PMID: 16713128
8. Aldaoud N, Erashdi M, and AlKhatib S, et al (2019) The utility of PAX8 and SATB2 immunohistochemical stains in distinguishing ovarian mucinous neoplasms from colonic and appendiceal mucinous neoplasm BMC Res Notes 12(1) 770 https://doi.org/10.1186/s13104-019-4816-9 PMID: 31771640 PMCID: 6880435
9. Mahdi H, Swensen RE, and Hanna R, et al (2011) Prognostic impact of lymphadenectomy in clinically early stage malignant germ cell tumour of the ovary Br J Cancer 105(4) 493–497 https://doi.org/10.1038/bjc.2011.267 PMID: 21772335 PMCID: 3170967
10. Huang RS, Mihalache A, and Nafees A, et al (2024) The impact of multidisciplinary cancer conferences on overall survival: a meta-analysis JNCI: J Nat Cancer Inst 116(3) 356–369 https://doi.org/10.1093/jnci/djad268
Appendix
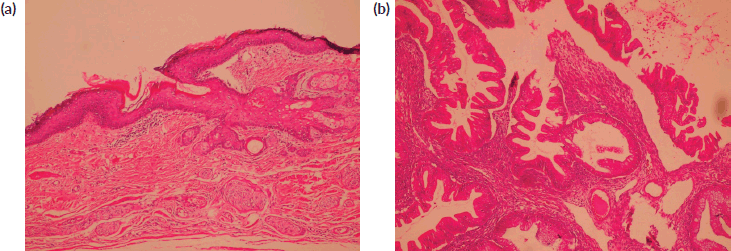
Figure 1. (a): Benign mature squamous epithelium (b): Malignant component displaying moderately diferentiated glandular structures (HE).





