Survival trends in gastric cancer in Brazil: real-life data from a large cancer center
Angelo Borsarelli Carvalho Brito1, Tiago Cordeiro Felismino1, Diego Rodrigues Mendonca e Silva2, Maria Paula Curado2, Lais Corsino Durant3, Rodrigo Gomes Taboada1, Adriane Graicer Pelosof4, Alessandro Landskron Diniz3 and Felipe Jose Fernandez Coimbra3
1Department of Clinical Oncology, A. C. Camargo Cancer Center, Sao Paulo 01509-010, Brazil
2Hospital Cancer Registry, A.C. Camargo Cancer Center, São Paulo 01509-010, Brazil
3Department of Abdominal Surgery, A. C. Camargo Cancer Center, São Paulo 01509-010, Brazil
4Department of Endoscopy, A. C. Camargo Cancer Center, São Paulo 01509-010, Brazil
Abstract
Background: Gastric cancer (GC) is the fourth leading cause of cancer deaths globally. There is a paucity of real-life data on GC in Brazil. Our study aimed to evaluate survival trends in gastric adenocarcinoma (GA) in a large cancer center in Brazil during 2000–2017.
Methods: Based on our Hospital Cancer Registry Database, all individuals diagnosed with GA between 2000 and 2017, and treated at A.C. Camargo Cancer Center, were retrospectively included. The primary objectives were to describe the patient demographics, clinicopathological characteristics, treatment modalities and survival trends during four separate periods of diagnosis (2000–2004; 2005–2009; 2010–2014 and 2015–2017). χ2 test was performed between two specified periods (2000–2004 and 2015–2017) to compare categorical variables. Overall survival (OS) curves were stratified by four separate periods and compared with log-rank tests.
Results: This analysis included 1,406 individuals. Across all periods, most patients were men aged 50–69 and presented with Lauren’s intestinal subtype. The frequency of stage IV disease significantly decreased between 2000–2004 and 2015–2017 (43.6% to 32.8%, p < 0.001). In contrast, we observed a rise in stage II (9.4% to 24.8%, p < 0.001) in the same comparison. We noticed an increased utilization of a combined approach involving chemotherapy and surgery (12% in 2000–2004 and 36.3% in 2015–2017, p < 0.001). The predicted 5-year OS of patients with GA in 2000–2004 was 27.8%, which increased to 53.9% in 2015–2017 (p < 0.001).
Conclusion: Our retrospective cohort showed an upward trend in survival rates during the period. We observed that 5-year OS almost doubled among men and women during 2000–2017.
Mini Abstract: The present retrospective cohort showed an upward trend in survival rates during the period from 2000 to 2017, in which the OS almost doubled among men and women.
Keywords: gastric cancer, prognosis, epidemiology
Correspondence to: Angelo Borsarelli Carvalho Brito
Email: angelo.brito@accamargo.org.br
Published: 30/05/2024
Received: 16/12/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Gastric cancer (GC) is the fifth most common cancer in Brazil [1] and worldwide [2], ranking fourth in global cancer-related mortality [3]. There has been a decrease in the global incidence and mortality of GC over the past decades [4, 5]. Nevertheless, the incidence has increased among young adults below the age of 50 [6].
Gastric adenocarcinoma (GA) constitutes approximately 90% of the total cases of GC [7]. Significant progress has been achieved in the management of GA over the past two decades [8, 9]. Curative treatment often involves a multimodal approach comprising R0 resection, D2 lymphadenectomy, radiotherapy and chemotherapy [10]. For advanced-stage patients, palliative systemic therapy is the standard of care, though it remains associated with an unfavourable prognosis [11].
Improvements range from adequate clinical staging with modern imaging techniques [12, 13] and diagnostic laparoscopy [14], to perioperative chemotherapy (PCT) for locally advanced disease [15, 16], and minimally invasive surgery [17]. Furthermore, systemic chemotherapy [18] and targeted agents [19] have increased survival in the advanced setting.
There is still a paucity of real-life data on GA survival analysis from underrepresented countries. Herein, we aim to describe the survival trends in patients with GA, regardless of their clinical staging, treated at a large cancer center in Brazil during a period of 17 years (2000–2017).
Methods
This is a retrospective hospital-based cohort of patients diagnosed with GA between 2000 and 2017 who were treated at A.C. Camargo Cancer Center. All patients were identified from the Hospital Cancer Registry Database using ICD C16; data was extracted on 10 August 2022.
Patients were considered eligible if they were 18 years old or above and had histological confirmation of GA independent of clinical staging. The date of diagnosis was determined as the date of histopathological analysis. We excluded individuals with esophageal squamous-cell carcinoma, gastrointestinal stromal tumours, gastric lymphomas and other rare histologies. The primary objectives were to describe the patient population demographics, clinicopathological characteristics, treatment modalities and survival trends during four distinguished periods of diagnosis (2000–2004; 2005–2009; 2010–2014 and 2015–2017).
Age group (<50, 50–69, 70+) and gender were analysed. We also collected the following information about the disease: histopathological type (according to Lauren’s classification), and clinical staging, according to the American Joint Committee on Cancer (AJCC) 7th Edition. Treatment modalities were grouped as follows: surgery alone, radiotherapy alone, chemotherapy alone or any combination of these approaches.
Descriptive statistics were used for demographics, clinicopathological characteristics and treatment modalities. χ2 tests were used to compare categorical variables between two specified periods (2000–2004 and 2015–2017).
Overall survival (OS) was calculated from diagnosis to date of death by any cause or last follow-up. Survival curves were stratified by four separate periods (2000–2004; 2005–2009; 2010–2014 and 2015–2017) and by sex. 5-year OS was estimated using Kaplan-Meier curves, and comparisons were performed with log-rank tests. All tests were considered statistically significant with a two-sided p-value of < 0.05.
Statistical analyses and 5-year survival probability curves were performed with IBM SPSS Statistics (version 23). The study protocol was approved by the institutional ethics committee (n. 2462/17) on 12 May 2017.
Results
The current analysis comprises a retrospective cohort of 1,406 patients (552 female and 854 male individuals). Demographics, clinicopathological characteristics and treatment modalities across the four periods of diagnosis are summarised in Table 1. The number of new cases per year was 46.8 during 2000–2004, and increased to 103.6 during 2015–2017. Across all periods, most patients were men aged 50–69 years and predominantly presented with Lauren’s intestinal subtype.
Table 1. Demographics, clinicopathological characteristics and treatment modalities during four separate periods of diagnosis.
There were significant differences between staging groups during the study. Specifically, between 2000 and 2004, stage IV varied from 43.6% to 32.8% in 2015–2017 (p < 0.001). The most substantial variation in nonmetastatic cases occurred in stage II, comprising 9.4% of cases from 2000 to 2004 and rising to 24.8% in 2015–2017 (p < 0.001).
Concerning treatment modalities, we observed an increased utilisation of a combined approach involving chemotherapy and surgery throughout the investigation. Specifically, from 2000 to 2004, only 12% of patients received a multimodal treatment regimen consisting of chemotherapy and surgery, whereas, between 2015 and 2017, this proportion increased to 36.3% (p < 0.001). Also, patients receiving surgery + radiotherapy + chemotherapy varied from 12.4% of patients in 2000–2004 to 4.5% in 2015–2017 (p < 0.001).

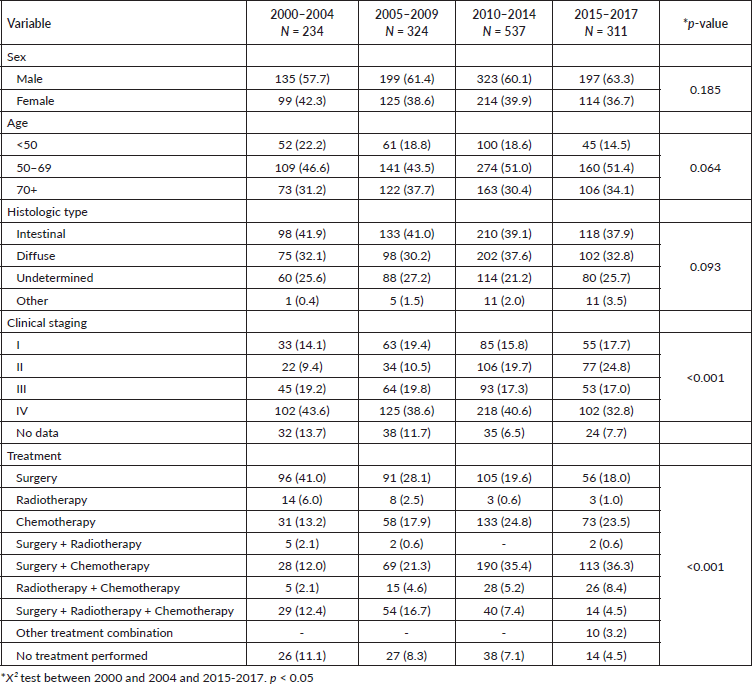
Figure 1. 5-year OS for GA stratified by diagnosis period.
When the entire cohort was analysed, the predicted 5-year OS of patients with GA between 2000 and 2004 was 27.8%, which increased to 53.9% in the period from 2015 to 2017 (p < 0.001), as shown in Figure 1. Among females, 5-year OS increased from 31.3% between 2000 and 2004 to 58.5% between 2015 and 2017 (p = 0.001). Among males, 5-year OS increased from 25.2% to 51.0% in the same comparison (p < 0.001), as shown in Figure 2.
Discussion
This study represents a pioneering investigation of GA utilising a hospital-based cancer registry data in the Latin American context. Our findings describe the OS trends over 17 years in a tertiary cancer center. This milestone research contributes to understanding GA outcomes in our region, shedding light on critical aspects of patient prognosis and healthcare management.
The findings from our study reveal a consistent and notable increase in OS during the analysed period. This improvement may be attributed to several contributing factors, including a shift to earlier diagnoses, enhanced staging accuracy, more effective chemotherapy protocols, and improvements in surgical interventions. These factors collectively may elucidate the observed positive trends in survival outcomes.
Diagnosis of GA at earlier stages is related to higher OS [20, 21]. Our analysis revealed a significant trend towards more initial tumours, especially within AJCC 7th ed. clinical stage II. Although current guidelines do not recommend screening in the ‘average-risk’ population of Western countries [22], the more widespread availability of upper endoscopy [23] was an important factor leading to this transition of earlier staging GA cases.
Patients diagnosed with GA need a comprehensive staging evaluation to inform treatment decisions and enhance prognostication. In patients with locally advanced disease, the systematic use of staging laparoscopy detects occult peritoneal dissemination in 31% of patients [24]. A previous publication from our group highlighted that 57.7% of all GA patients underwent staging laparoscopy at our center [25].
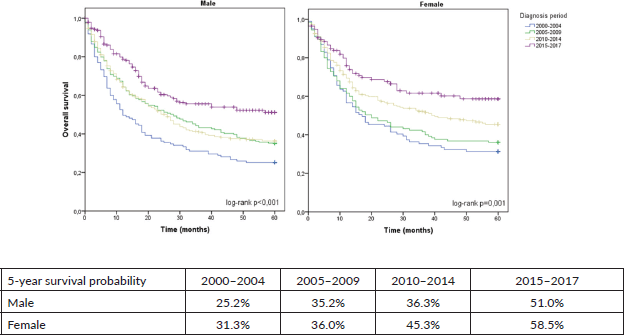
Figure 2. 5-year OS for GA stratified by sex and diagnosis period.
International guidelines in Western countries recommend, as a preference, performing PCT as a multimodal approach [10, 26]. The pivotal Magic trial [16], published in 2006, compared perioperative Epirrubicin, Cisplatin and 5-Fluorouracil (ECF) versus surgery alone in 503 locally advanced gastroesophageal cancer patients in the United Kingdom. PCT significantly improved OS (HR, 0.75; 95%CI, 0.60–0.93; p = 0.009) and progression-free survival (HR, 0.66; 95CI, 0.53–0.81; p < 0.001).
Another randomised trial from France [27] compared doublet PCT with Cisplatin and 5-Fluorouracil, versus surgery in 224 locally advanced gastroesophageal cancer patients. PCT also demonstrated improved OS (5-year rate 38% versus 24%; HR, 0.69; 95%CI, 0.50–0.95; p = 0.02). At our institution, patients with locally advanced disease (mainly >cT3 and cN+) have been systematically referred to PCT, since 2006. Our previously published data revealed that 65.5% of nonmetastatic patients underwent PCT [28].
More recently, perioperative 5-Fluorouracil, Oxaliplatin and Docetaxel (FLOT) were compared to ECF in a phase 2/3, randomised trial [29, 30] in Germany. Patients with locally advanced gastroesophageal cancer (>cT2 or cN+) were included. Perioperative FLOT was significantly superior to ECF in OS (HR, 0.77; 95%CI, 0.63–0.94 p = 0.012). The period of this publication aside, FLOT has become our standard protocol since 2017.
Surgery remains the cornerstone of curative-intended treatment. The main goals of surgery are achieving an R0 resection [31] and performing a D2 lymphadenectomy [32]. In recent years, minimally invasive techniques, such as laparoscopic and robotic procedures, have become available. Surgical teams’ expertise and the perioperative care protocols are also highly relevant. Previously published data from patients treated at our cancer center demonstrated a low surgical mortality rate of 4.4% [25]. Also, the frequency of adjuvant chemoradiation, evaluated in the Intergroup US 0116 Trial [33], decreased during the period. It is known that this adjuvant strategy is less common in the present day due to standard D2 lymphadenectomy, which was performed in less than 10% of pivotal trials. Previously published from our cancer center demonstrated that D2-lymphadenectomy was performed in 90.8% of the patients [34].
To the best of our knowledge, this analysis represents the largest real-life GA database in our country. There might be some imprecision in collected information because of the extensive period covered in our research and changes made in the AJCC staging system over that period. It is also important to highlight that most of our patients were treated in the context of a private hospital. Hence, our results cannot be extrapolated to patients in the public health system. Moreover, interesting findings related to differences in OS, such as better survival in females, have not been fully clarified thus far and warrant further investigation.
Conclusion
Our hospital-based data shows an upward trend in survival rates across patients with GA. We observed that 5-year OS almost doubled among men and women during the analysed period. Recent improvements in staging methods and treatment have increased cure rates. Nevertheless, further investigation is essential to determine significant differences in OS between males and females.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
None.
References
1. INSTITUTO NACIONAL DE CÂNCER JOSƒ ALENCAR GOMES DA SILVA Estimativa 2023: incidência do Câncer no Brasil (Rio de Janeiro: INCA Dehwgb)
2. Morgan E, Arnold M, and Camargo MC, et al (2022) The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: a population-based modelling study EClinicalMedicine 47 101404 (In eng) https://doi.org/10.1016/j.eclinm.2022.101404
3. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 (In eng) https://doi.org/10.3322/caac.21660 PMID: 33538338
4. Bray F, Ferlay J, and Laversanne M, et al (2015) Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration Int J Cancer 137(9) 2060–2071 (In eng) https://doi.org/10.1002/ijc.29670 PMID: 26135522
5. Bertuccio P, Chatenoud L, and Levi F, et al (2009) Recent patterns in gastric cancer: a global overview Int J Cancer 125(3) 666–673 (In eng) https://doi.org/10.1002/ijc.24290 PMID: 19382179
6. Sung H, Siegel RL, and Rosenberg PS, et al (2019) Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry Lancet Public Health 4(3) e137–e147 (In eng) https://doi.org/10.1016/S2468-2667(18)30267-6 PMID: 30733056
7. Karimi P, Islami F, and Anandasabapathy S, et al (2014) Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention Cancer Epidemiol Biomarkers Prev 23(5) 700–713 (In eng) https://doi.org/10.1158/1055-9965.EPI-13-1057 PMID: 24618998 PMCID: 4019373
8. Joshi SS and Badgwell BD (2021) Current treatment and recent progress in gastric cancer CA Cancer J Clin 71(3) 264–279 (In eng) https://doi.org/10.3322/caac.21657 PMID: 33592120 PMCID: 9927927
9. Alsina M, Arrazubi V, and Diez M, et al (2023) Current developments in gastric cancer: from molecular profiling to treatment strategy Nat Rev Gastroenterol Hepatol 20(3) 155–170 https://doi.org/10.1038/s41575-022-00703-w
10. Lordick F, Carneiro F, and Cascinu S, et al (2022) Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up Ann Oncol 33(10) 1005–1020 (In eng) https://doi.org/10.1016/j.annonc.2022.07.004 PMID: 35914639
11. Hu HM, Tsai HJ, and Ku HY, et al (2021) Survival outcomes of management in metastatic gastric adenocarcinoma patients Sci Rep 11(1) 23142 https://doi.org/10.1038/s41598-021-02391-z PMID: 34848751 PMCID: 8633380
12. Abdalla EK and Pisters PW (2004) Staging and preoperative evaluation of upper gastrointestinal malignancies Semin Oncol 31(4) 513–529 (In eng) https://doi.org/10.1053/j.seminoncol.2004.04.014 PMID: 15297943
13. Mocellin S and Pasquali S (2015) Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer Cochrane Database Syst Rev 2015(2) Cd009944 (In eng) PMID: 25914908 PMCID: 6465120
14. Lowy AM, Mansfield PF, and Leach SD, et al (1996) Laparoscopic staging for gastric cancer Surgery 119(6) 611–614 (In eng) https://doi.org/10.1016/S0039-6060(96)80184-X PMID: 8650600
15. Xiong BH, Cheng Y, and Ma L, et al (2014) An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer Cancer Invest 32(6) 272–284 (In eng) https://doi.org/10.3109/07357907.2014.911877 PMID: 24800782
16. Cunningham D, Allum WH, and Stenning SP, et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer N Engl J Med 355(1) 11–20 (In eng) https://doi.org/10.1056/NEJMoa055531 PMID: 16822992
17. Vi–uela EF, Gonen M, and Brennan MF, et al (2012) Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies Ann Surg 255(3) 446–456 (In eng) https://doi.org/10.1097/SLA.0b013e31824682f4 PMID: 22330034
18. Cunningham D, Starling N, and Rao S, et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer N Engl J Med 358(1) 36–46 (In eng) https://doi.org/10.1056/NEJMoa073149 PMID: 18172173
19. Bang YJ, Van Cutsem E, and Feyereislova A, et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial Lancet 376(9742) 687–697 (In eng) https://doi.org/10.1016/S0140-6736(10)61121-X PMID: 20728210
20. Onodera H, Tokunaga A, and Yoshiyuki T, et al (2004) Surgical outcome of 483 patients with early gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer Hepatogastroenterology 51(55) 82–85 (In eng) PMID: 15011835
21. Maharjan U and Kauppila JH (2022) Survival trends in gastric cancer patients between 1987 and 2016: a population-based cohort study in Finland Gastric Cancer 25(6) 989–1001 https://doi.org/10.1007/s10120-022-01326-5 PMID: 35933683 PMCID: 9587955
22. Lansdorp-Vogelaar I, Meester RGS, and Laszkowska M, et al (2021) Cost-effectiveness of prevention and early detection of gastric cancer in Western countries Best Pract Res Clin Gastroenterol 50-51 101735 (In eng) https://doi.org/10.1016/j.bpg.2021.101735
23. Januszewicz W and Kaminski MF (2020) Quality indicators in diagnostic upper gastrointestinal endoscopy Therap Adv Gastroenterol 13 1756284820916693 (In eng) https://doi.org/10.1177/1756284820916693 PMID: 32477426 PMCID: 7232050
24. Sarela AI, Lefkowitz R, and Brennan MF, et al (2006) Selection of patients with gastric adenocarcinoma for laparoscopic staging Am J Surg 191(1) 134–138 (In eng) https://doi.org/10.1016/j.amjsurg.2005.10.015 PMID: 16399124
25. Coimbra FJF, de Jesus VHF, and Ribeiro HSC, et al (2019) Impact of ypT, ypN, and adjuvant therapy on survival in gastric cancer patients treated with perioperative chemotherapy and radical surgery Ann Surg Oncol 26(11) 3618–3626 (In eng) https://doi.org/10.1245/s10434-019-07454-0 PMID: 31222685
26. Ajani JA, D’Amico TA, and Bentrem DJ, et al (2022) Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology J Natl Compr Canc Netw 20(2) 167–192 (In eng) https://doi.org/10.6004/jnccn.2022.0008 PMID: 35130500
27. Ychou M, Boige V, and Pignon JP, et al (2011) Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial J Clin Oncol 29(13) 1715–1721 (In eng) https://doi.org/10.1200/JCO.2010.33.0597 PMID: 21444866
28. Diniz TP, da Costa WL, and Gomes CC, et al (2022) Symptomatic recurrence and survival outcomes after curative treatment of gastric cancer: does intensive follow-up evaluation improve survival? Ann Surg Oncol 29(1) 274–284 https://doi.org/10.1245/s10434-021-10724-5
29. Al-Batran SE, Hofheinz RD, and Pauligk C, et al (2016) Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial Lancet Oncol 17(12) 1697–1708 (In eng) https://doi.org/10.1016/S1470-2045(16)30531-9 PMID: 27776843
30. Al-Batran SE, Homann N, and Pauligk C, et al (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial Lancet 393(10184) 1948–1957 (In eng) https://doi.org/10.1016/S0140-6736(18)32557-1 PMID: 30982686
31. Roder JD, Bšttcher K, and Siewert JR, et al (1993) Prognostic factors in gastric carcinoma. Results of the German gastric carcinoma study 1992 Cancer 72(7) 2089–2097 (In eng) https://doi.org/10.1002/1097-0142(19931001)72:7<2089::AID-CNCR2820720706>3.0.CO;2-H PMID: 8374867
32. Wu CW, Hsiung CA, and Lo SS, et al (2006) Nodal dissection for patients with gastric cancer: a randomised controlled trial Lancet Oncol 7(4) 309–315 (In eng) https://doi.org/10.1016/S1470-2045(06)70623-4 PMID: 16574546
33. MacDonald JS, Smalley SR, and Benedetti J, et al (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction N Engl J Med 345 725–730 https://doi.org/10.1056/NEJMoa010187 PMID: 11547741
34. Costa Jr WL, Coimbra FJ, and Fogaroli RC, et al (2012) Adjuvant chemoradiotherapy after d2-lymphadenectomy for gastric cancer: the role of n-ratio in patient selection. Results of a single cancer center Radiat Oncol 7 169 https://doi.org/10.1186/1748-717X-7-169 PMID: 23068190 PMCID: 3542168






