Young Filipino breast cancer patients have worse survival outcomes
Ralph Victor Yap1,2,a, Deanne Lou Marquez1,b and Frances Marion De La Serna1,c
1Department of Surgery, Cebu Doctors’ University Hospital, Osmeña Blvd, Cebu City, 6000, Philippines
2Unified Minimally Invasive Surgery Training, Quezon City, Manila, 1112, Philippines
ahttps://orcid.org/0000-0003-0083-696X
bhttps://orcid.org/0009-0003-4203-8461
chttps://orcid.org/0000-0001-9632-5544
Abstract
Background: In the 2020 GLOBOCAN report, breast cancer is the 3rd most common cause of cancer-related mortality in the Philippines. The incidence of breast cancer in the young (≤40 years) was reported to be higher in the Philippines compared to other Asian countries. Several studies have consistently demonstrated poor survival outcomes in this age group due to its aggressiveness and unique tumour biology. However, data on survival outcomes of young Filipino breast cancer patients remains unknown in the Philippines.
Methods: A retrospective study was performed involving patients with stage I–III breast cancer who underwent definitive surgery from January 2010 to December 2015 at a single-tertiary institution. Patients were grouped according to age (≤40 and >40 years old). Their clinicopathological characteristics, treatment profile and 5-year survival outcomes were analyzed.
Results: A total of 524 Filipino patients (15.1% aged ≤40 years) were included. Younger patients were diagnosed at a higher stage and pathologic grade. A negative hormone receptor, high Ki67 status, and triple negative breast cancer (TNBC) subtypes were also more common among younger patients. The overall breast-conserving surgery rate was low at 8.9%. The use of adjuvant chemoradiotherapy was more common and both 5-year overall survival (OS) and disease-free survival (DFS) were lower (61.1% versus 77.1% and 31.1% versus 66.8%, respectively) in the ≤40-year-old group. In the multivariate analysis, age group, tumour size, and nodal status were significant predictors for DFS. However, only tumour size was significant for OS.
Conclusion: Young Filipino breast cancer patients have demonstrated unique pathologic characteristics with associated lower survival outcomes similar to the published literature. Increasing awareness of cancer screening practices among young women, provision of equitable access to healthcare, and prompt management of breast cancer in the young are crucial.
Keywords: breast cancer, young, survival, outcomes, Philippines
Correspondence to: Ralph Victor Yap
Email: rvyapmd@gmail.com
Published: 23/11/2023
Received: 09/07/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
According to the GLOBOCAN 2020 report, female breast cancer has become the most commonly diagnosed cancer worldwide with an estimated 2.3 million new cases [1]. In the Philippines, it is the 3rd most common cause of cancer-related mortality [2]. Additionally, it was reported that 1 out of 13 Filipino women is expected to develop breast cancer in their lifetime [3]. The incidence of breast cancer continues to increase with age while the median age at the time of breast cancer diagnosis is 61 years [4].
Interestingly, the incidence of breast cancer in young women has been on the rise, with breast cancer as the second leading cause of cancer-related mortality in women aged <39 years. Although the age cut-off for breast cancer in the young is inconsistently defined in previous literature (<45, <40, <35 years), the European School of Oncology – European Society for Medical Oncology 3rd international consensus guidelines in 2017 defined ‘young women’ as less than 40 years of age at the time of pathologic diagnosis [5].
Data analysed from the California Cancer Registry (1998–2004) have shown that migrant Filipino women were being diagnosed at a statistically younger age compared to Caucasians and other Asian women [6]. In Asia, up to 13% of women diagnosed with breast cancer are aged <40 years. The incidence of breast cancer among this age group is particularly higher in the Philippines (22.1 per 100,000 women) compared with Osaka (11.8), Hanoi (13.8), 14.7 (Shanghai), Bangkok (15.2) and Hong Kong (19.3) [7, 8].
Despite worldwide trends in improvements in early detection and treatment access of breast cancer, the recurrence and mortality rates among young women with breast cancer remain poor. The survival disparity in young breast cancer patients could be attributed to its aggressiveness and unique biological features compared to older women. Breast cancer in the young tends to present at an advanced stage, with higher rates of triple-negative or human epidermal growth factor 2 (HER2)-overexpression profiles, and with lower efficacy to hormonal therapy [6, 9].
Moreover, breast cancer with breast cancer gene (BRCA) mutations was more common among Filipino patients aged <45 years and associated with worse overall survival (OS) [10, 11]. Optimizing the care and outcomes for this population would require a multidisciplinary team while tailoring therapies that would also address specific issues on fertility preservation, pregnancy, survivorship and psychosocial support [12].
To the best of our knowledge, there have been no publications on the clinicopathologic and survival outcomes of young Filipino women with breast cancer. Thus, this study aims to compare the clinical and treatment profiles, OS, and disease-free survival (DFS) between the young (≤40 years) and older Filipino women with breast cancer treated at a private, tertiary hospital in the Philippines.
Methods
This was a retrospective study on all patients with pathologically confirmed primary stages I–III breast cancer aged 18 years old and above who underwent definitive surgery from January 01, 2010, to December 31, 2015, at Cebu Doctors’ University Hospital. This study was conducted in accordance with the Declaration of Helsinki, and the institution’s research ethics committee approved the study (protocol code 2-2019-034). The hospital’s medical records and outpatient clinic data were utilized.
The variables collected from the patient’s medical records included clinicopathologic characteristics – age (at the time of pathological diagnosis); histology, tumour size, nodal status, stage, tumour grade, lymphovascular invasion, hormonal and HER2/neu receptor status, Ki67 value and molecular subtype) from the records of the Department of Pathology. The operative records were reviewed to extract data on the surgical therapy to the breast (lumpectomy/breast-conserving surgery (BCS) or total mastectomy, and the method of axillary staging such as sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND). Neoadjuvant and adjuvant therapies (radiation therapy (RT) and systemic/chemotherapy) were reviewed using the patient’s medical charts, outpatient records, and/or records from the institution’s medical and radiation oncology departments. The hospital medical records and the prospectively maintained outpatient clinic records of the patients were reviewed to determine recurrence and survival status. DFS was defined as the time interval between the date of definitive surgery and the development of local/regional recurrence and/or development of distant metastasis. OS was defined as the time interval between the date of definitive surgery and mortality regardless of cause. The last follow-up date was January 01, 2021. Patients with a previous history of or synchronous malignancy other than the breast, whose records do not contain follow-up data, and male breast cancer were excluded from the study.
All collected data was encoded in Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Patients were grouped according to age (≤40 and >40 years old). Values were expressed as frequencies (percentages) and analysed using chi-square or Fisher’s exact test whenever appropriate. The primary and secondary endpoints of this study were to assess the 5-year OS and DFS between the 2 groups, respectively using the Log Rank (Mantel-Cox) test and Kaplan-Meier curve plot. Multivariate analyses for OS and DFS were done using Cox regression analysis. The following variables were included in the model: age groups (age <40 and age ≥40), pathologic T stage, N stage, final stage, grade, LVI, oestrogen receptor (ER), progesterone receptor (PR) and Ki67 status. We evaluated the hazard ratio and its 95% confidence interval for each variable. A p-value of <0.05 was considered significant in all analyses.
All statistical analysis was performed using GraphPad Prism version 8.4.2 (GraphPad Software, San Diego, CA, USA) and IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA) for Windows.
Results
Clinicopathological characteristics
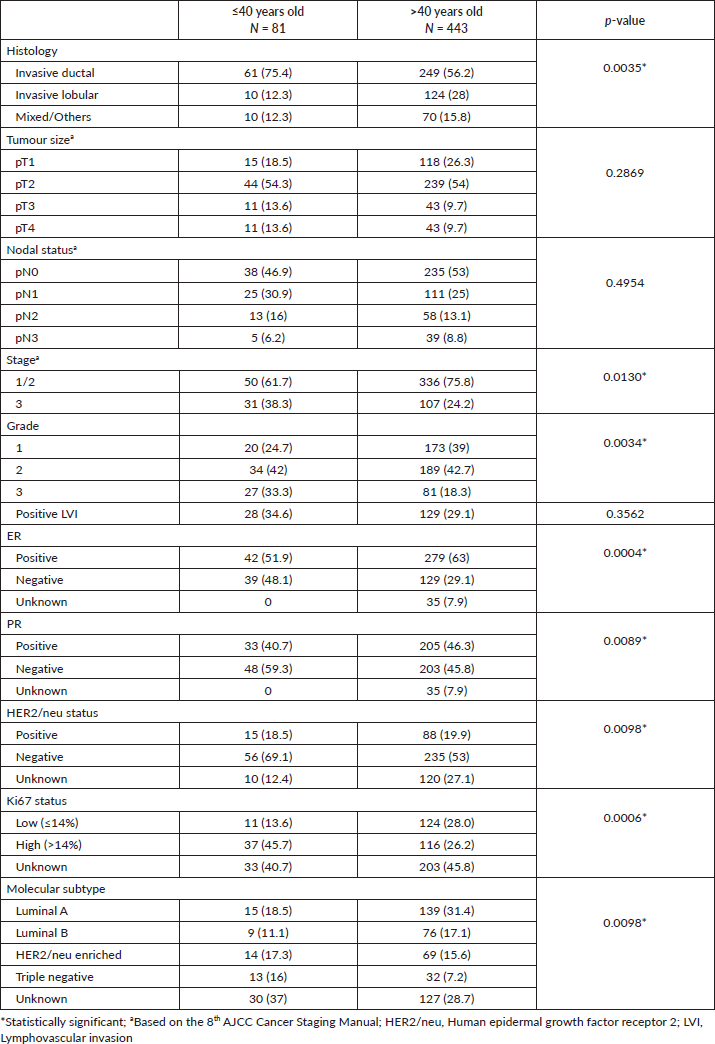
A total of 524 female Filipino breast cancer patients were included in the study. Of these, 81 (15.5%) patients were aged ≤40 years old (mean 34.9 ± 5.1) while 443 (84.5%) were aged >40 years old (mean 56.7 ± 10.6). Overall, invasive ductal carcinoma (IDC) was the most common histologic type (75.4% versus 56.2%, p = 0.0035). There were no significant differences between the 2 groups in terms of tumour size, nodal status and LVI. Patients aged ≤40 years old were diagnosed at a higher stage and pathologic grade, with higher negative rates for ER (48.1% versus 29.1%) and PR (59.3% versus 45.8%) receptor status, and Ki67 high status (45.7% versus 26.2%). A lower luminal A/B (29.6% versus 48.5%) and higher triple-negative (TNBC) (16% versus 7.2%) molecular subtypes were found among patients ≤40 years old (Table 1).
Table 1. Clinicopathological characteristics between the ≤40 and >40-year-old groups with breast cancer.
Treatment profile
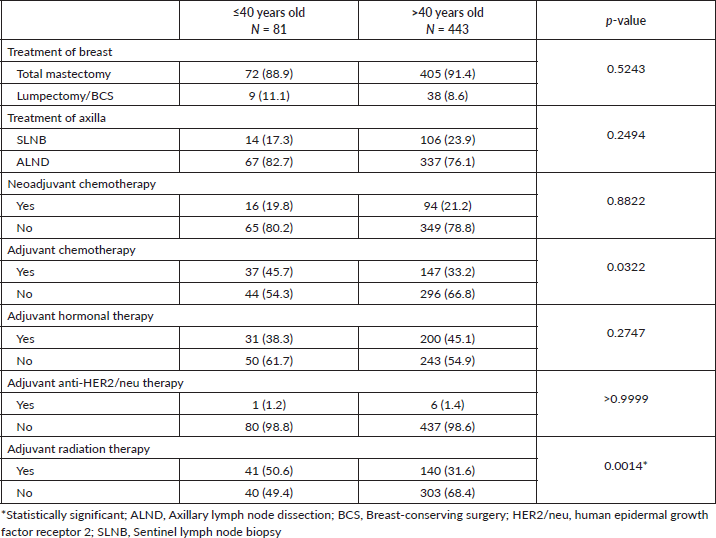
The total mastectomy rates between the ≤40 and >40-year-old groups were 88.9% and 91.4%, respectively. Overall, the BCS rate was only 8.9%. More patients in the ≤40-year-old group received adjuvant chemotherapy (45.7% versus 33.2%) and RT (50.6% versus 31.6%). There were no significant differences between the 2 groups in terms of the method of axillary staging, neoadjuvant chemotherapy and adjuvant hormonal and anti-HER2/neu therapies (Table 2).
Table 2. Treatment profile between the ≤40 and >40-year-old groups with breast cancer.
Survival outcomes
The Kaplan-Meier estimates indicated that the 5-year OS rate for the ≤40-year-old group was 61.1% and for the >40-year-old group was 77.1%. However, the Log Rank test indicated there was no statistically significant difference (p = 0.464) between the two survival rates (Figure 1). The mean OS time for the ≤40-year-old group was 74 months and for the >40-year-old group was 76.2 months. The 5-year DFS rate for the ≤40-year-old group was significantly lower (31.1% versus 66.8%, p = 0.000) than the >40-year-old group (Figure 2). The mean DFS time for the ≤40-year-old group was 50.5 months and for the >40-year-old group was 69 months. Using the Cox regression model, the multivariate analysis for DFS showed that younger age (≤40 years), higher tumour size, and nodal status were significant in the model controlling for all other variables. However, only tumour size was significant when analysing for OS. Patients in the ≤40-year-old group had 1.9 times the risk of their older counterparts in developing disease recurrence (95% CI (1.391–2.694), p = 0.000) (Table 3).
Discussion
Our study demonstrated that young Filipino women (≤40-year-old) with breast cancer were diagnosed with a higher pathologic grade and stage, had more IDC pathology, hormonal receptor negativity, high Ki67 status and TNBC molecular subtypes, and had more adjuvant chemo- and radiotherapy when compared with older patients. Moreover, young women with breast cancer had lower 5-year OS and DFS.
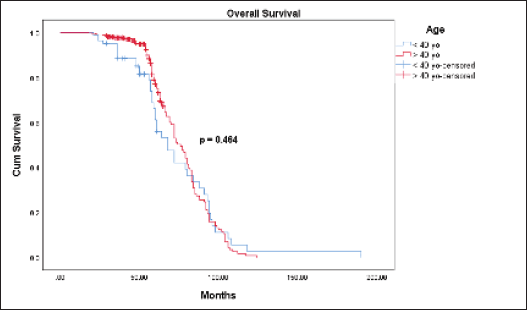
Figure 1. OS between the ≤40 and >40-year-old groups with breast cancer.
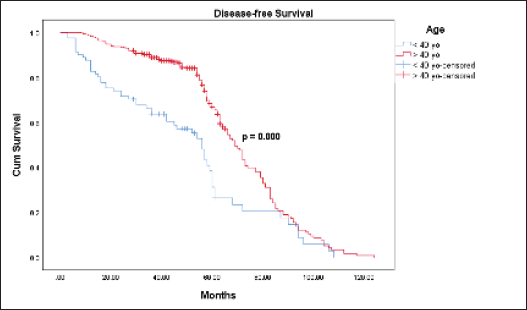
Figure 2. DFS between the ≤40 and >40-year-old groups with breast cancer.
We observed in our study that young breast cancer patients had significantly more IDC and less invasive lobular carcinoma (ILC) pathologies compared to the older cohort. A similar pathologic finding was also noted in a Jordanian cohort (970 patients). However, the young breast cancer patients in their study had significantly better OS and comparable DFS [13]. Previous reports from India (507 patients) and China (1,968 patients) did not find significant differences in histologic types between young (≤40-year-old) and non-young breast cancer patients [14, 15]. In a large cohort of 17,481 breast cancer patients in Sweden, ILC was found to be significantly associated with older age, ER positivity and well-differentiated tumours. Additionally, ILC was noted with improved survival during the first 5 years [16].
Table 3. Multivariate cox regression analysis for DFS and OS in the population.
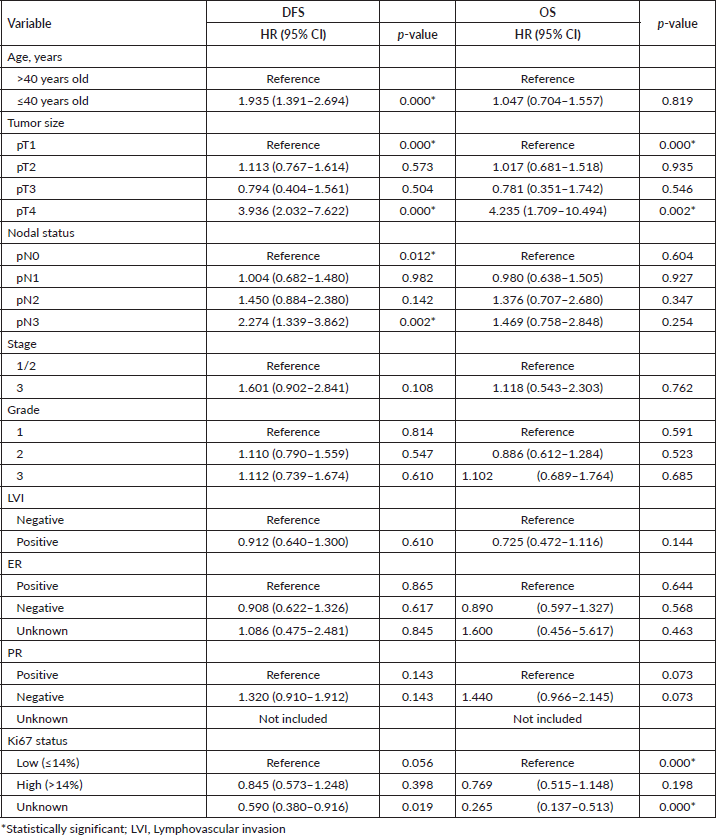
Young women with breast cancer have unique pathologic characteristics with an aggressive phenotype compared to older patients. Although our study did not demonstrate statistically significant differences in terms of pathologic tumour size, nodal status, and LVI, previous reports have shown that young breast cancer patients tend to present with T3/T4 tumour size (28.2% – 63.1% versus 13.8% – 15.2%), nodal positivity (73.2% – 86.7% versus 55.6% – 75.5%) and positive LVI (39.3% – 48.6% versus 27.7% – 39.4%) [13, 14, 17, 18]. Whether this is secondary to a small sample size and/or racial/geographical difference remains to be investigated. Other findings in our study (more pathologic grade 3, stage 3, higher Ki67 status and more hormonal receptor (ER/PR) negative, and TNBC subtypes) were consistent with previously published data from Jordan, India, Argentina and Croatia [13, 14, 18, 19]. All these variables are considered prognostic factors that negatively impact OS and DFS.
Additionally, younger patients with breast cancer were more likely to present with a positive HER2/neu receptor status, a marker for aggressive tumour phenotype, poor prognosis and targeted/chemotherapy [17, 18]. However, we could not confirm this in our study due to the disproportionate number of patients with unknown or unconfirmed HER2/neu status. In a 2015 local survey, more than 90% of medical oncologists and surgeons would request HER2/neu testing for their breast cancer patients. However, common barriers such as the unavailability of biomarker testing, patients’ refusal and limited finances remain a hindrance in pursuing this test in the Philippines [20, 21]. Secondly, only 1.3% of our patients had documented adjuvant anti-HER2/neu therapy. In a cost-utility analysis in the Philippines, adjuvant trastuzumab therapy (all cycles) for HER2/neu positive early breast cancer was not cost-effective (in addition to chemotherapy) and remains unaffordable at its 2017 government-negotiated price of PHP 619,667 (USD 11,161) [22].
No significant difference was found in terms of surgical therapy for the breast and method of axillary staging between the two age groups in our study. Only less than 12% of the young breast cancer patients had BCS, a rate similar to what we previously reported among early-stage breast cancer patients aged ≥18 years old in our institution. Multiple factors such as co-morbidities, education level, socio-economic and marital status, and mode of diagnosis may influence the decision to pursue BCS [23]. We also observed in our local practice that patients opt for total mastectomy because of the fear of having a second surgery for recurrence (for leaving breast tissue behind), and the additional financial burden of having to undergo adjuvant breast RT after lumpectomy. Similar to a recent survey among 383 Lebanese women with a median age of 32 years, ‘concern about residual cancer and/or cancer recurrence’, ‘cost of surgery and follow-up and the absence of health coverage’, and ‘side effects of radiotherapy’ were one of the main reasons for choosing mastectomy over BCS [24]. In a survival analysis of 15,611 breast cancer patients aged ≤40 years old utilizing the National Cancer Database (2006–2016), the BCS rate was 60.9% with an equivalent 5-year OS to mastectomy with or without RT [25].
In our study, the rates of adjuvant chemotherapy and radiation therapy were higher among women in the ≤40-year-old group. The unique pathologic characteristics as previously mentioned justify its increased use in this age group. While selecting chemotherapeutic regimens remains the same regardless of age, the comorbidities associated with older women may preclude the use or require dose modifications of systemic therapies [26]. Recommendations for adjuvant RT are similar to those in older women. However, the risk of local recurrence is higher among young women not only after BCS but also after mastectomy thus they are more likely to benefit from RT than their older counterparts [27].
Survival outcomes from neighbouring Southeast Asian countries
Our study demonstrated that the 5-year OS (61.1% versus 77.1%) and DFS (31.1% versus 66.8%) were lower in the ≤40-year-old group. We reviewed the literature on the survival outcomes of young breast cancer patients from other southeast Asian countries.
In Indonesia, a comparative study between young (≤40 years old) and elderly (≥60 years old) breast cancer patients showed that the former group had more positive lymph nodes, HER2/neu receptor positivity, adjuvant chemotherapy and tamoxifen use. Secondly, 5-year mortality, recurrence, and metastasis rates were also higher (30.4% versus 20%, 7.95 versus 5.7%, and 25% versus 22.8%, respectively) in the same group as well [28]. A more recent study from Indonesia analysing 115 young breast cancer patients showed a shorter progression-free survival in those with T4 tumours (16 versus 35 months), positive lymph nodes (24 versus 42 months), hormone receptor-positive (29 versus 24 months) and TNBC subtype (16 versus 38 months). Within 5 years of follow-up, 6.9% and 24.3% developed locoregional recurrence and distant metastasis, respectively [29]. In a cohort of stage III Indonesian breast cancer patients, those aged ≤40 years had a lower 3-year OS of 47% (versus 78%, p 0.010) [30].
A survival analysis of 868 breast cancer patients in Malaysia (comprised of 58% Malays, 25% Chinese and 17% Indians) showed a lower 5-year OS at 57.4% in the <40-year-old group [31]. A multivariable Cox regression analysis in 290 Thai women with breast cancer (9% were aged 35 years or younger) who underwent BCS showed that young age was a risk factor for locoregional (HR 4.1, p 0.01), and distant (HR 1.7, p 0.001) recurrences [32].
A 2005 study conducted in Singapore comparing 106 young breast cancer patients (higher tumour size, pathologic grade and more nodal positive) to 737 older counterparts showed a 5-year OS of 86.4% and 81.7%, respectively. However, this was not statistically significant [33]. In 2009, data from 10,287 Singaporean women with breast cancer with a median follow-up of 7.7 years was analysed. Those who were primarily diagnosed at the age of 35 to 54 years had a statistically significant 34% lower risk of mortality compared to patients aged <35 years [34]. A more recent (2018) Singaporean study comparing the survival outcomes between young (447) and old (2,045) breast cancer patients treated with BCS showed similar 5-year OS rates (94.1% versus 96.1%, respectively). However, the 5-year breast cancer-specific survival was significantly lower in the young group (96.7% versus 98.3%). Additionally, younger patients were more likely to have breast cancer recurrence (HR 1.92, p < 0.001) with 5-year local recurrence rates of 5.2% versus 3%, respectively [35].
The lower survival outcomes among young Filipino breast cancer patients were similar to other Southeast Asian countries. The lack of effective screening strategies for average-risk young women may result in the late stage of breast cancer presentation. A cross-sectional study involving 994 Filipino women (33% were aged 20–39 years) not diagnosed with breast cancer showed less reported knowledge about clinical breast exams (CBE) or mammograms. Adherence to CBE and monthly breast self-exams were only 15% and 25%, respectively. Only 8% of the participants had a prior mammogram which was generally done for diagnostic rather than screening purposes [3].
The high out-of-pocket healthcare costs, lack of organized national screening programs, centralization and/or unequal distribution of health resources/infrastructure and providers across the country and socioeconomic and cultural barriers preclude access to both breast cancer screening and timely surgical management [36, 37]. Lastly, specific challenges in the care of young breast cancer patients such as fertility preservation/future pregnancy, bone health maintenance, inherited breast cancer syndromes, and associated psychosocial issues should be included and discussed during multidisciplinary treatment planning [38, 39].
Our study is limited by its retrospective design, data incompleteness of hormonal receptor, HER2/neu, and Ki67 status, and a small study sample based solely on a single private tertiary institution, and outcomes may not be generalizable for the entire region/country. Secondly, a standardized and stringent research database/registry for breast cancer patients to optimize outcomes is still lacking in our setting.
Conclusion
We describe for the first time, survival outcomes of young (≤40 years) breast cancer patients in the Philippines. The unique pathologic characteristics and associated low OS and DFS in this group were consistent with published literature. Increasing awareness about breast cancer screening practices among young women, providing equitable/affordable access to healthcare, and prompt management of breast cancer in the young are imperative.
Disclosure
This paper was presented virtually as a poster presentation (by DLM) during the 11th Global Breast Cancer Conference held at Grand Walkerhill Hotel, Seoul last April 28–30, 2022.
Acknowledgments
None.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
The authors declare that they have not received any funding for the execution of this research/article.
Author contributions
RVY, DLM and FMD conceptualized the study design. DLM collected the data. RVY conducted the data analysis. RVY and DLM interpreted the results and wrote the manuscript. FMD gave critical comments and revisions to the manuscript. All authors approved the final version of the manuscript before submission.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA A Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660
2. International Agency for Research on Cancer (2020) Global Cancer Observatory Population Fact Sheet: Philippines [https://gco.iarc.fr/today/data/factsheets/populations/608-philippines-fact-sheets.pdf] Date accessed: 21/05/22
3. Wu TY and Lee J (2018) Promoting breast cancer awareness and screening practices for early detection in low-resource settings Eur J Breast Health 15(1) 18–25 https://doi.org/10.5152/ejbh.2018.4305
4. Alkabban FM and Ferguson T (2022) Breast Cancer (Treasure Island: StatPearls) [Internet] [http://www.ncbi.nlm.nih.gov/books/NBK482286] Date accessed: 21/05/22
5. Zhu JW, Charkhchi P, and Adekunte S, et al (2023) What is known about breast cancer in young women? Cancers (Basel) 15(6) 1917 https://doi.org/10.3390/cancers15061917 PMID: 36980802 PMCID: 10047861
6. Gomez SL, Quach T, and Horn-Ross PL, et al (2010) Hidden breast cancer disparities in Asian women: disaggregating incidence rates by ethnicity and migrant status Am J Public Health 100(Suppl 1) S125–S131 https://doi.org/10.2105/AJPH.2009.163931 PMID: 20147696 PMCID: 2837454
7. Anastasiadi Z, Lianos GD, and Ignatiadou E, et al (2017) Breast cancer in young women: an overview Updates Surg 69(3) 313–317 https://doi.org/10.1007/s13304-017-0424-1 PMID: 28260181
8. Gibson LJ, Héry C, and Mitton N, et al (2010) Risk factors for breast cancer among Filipino women in Manila Int J Cancer 126(2) 515–521 https://doi.org/10.1002/ijc.24769
9. Azim HA and Partridge AH (2014) Biology of breast cancer in young women Breast Cancer Res 16(4) 427 https://doi.org/10.1186/s13058-014-0427-5 PMID: 25436920 PMCID: 4303229
10. De Leon Matsuda ML, Liede A, and Kwan E, et al (2002) BRCA1 and BRCA2 mutations among breast cancer patients from the Philippines Int J Cancer 98(4) 596–603 https://doi.org/10.1002/ijc.10194 PMID: 11920621
11. Zhu Y, Wu J, and Zhang C, et al (2016) BRCA mutations and survival in breast cancer: an updated systematic review and meta-analysis Oncotarget 7(43) 70113–70127 https://doi.org/10.18632/oncotarget.12158 PMID: 27659521 PMCID: 5342539
12. Johnson RH, Anders CK, and Litton JK, et al (2018) Breast cancer in adolescents and young adults Pediatr Blood Cancer 65(12) e27397 https://doi.org/10.1002/pbc.27397 PMID: 30156052 PMCID: 6192832
13. Abdel-Razeq H, Iweir S, and Abdel-Razeq R, et al (2021) Differences in clinicopathological characteristics, treatment, and survival outcomes between older and younger breast cancer patients Sci Rep 11(1) 14340 https://doi.org/10.1038/s41598-021-93676-w PMID: 34253800 PMCID: 8275803
14. Thangjam S, Laishram RS, and Debnath K (2014) Breast carcinoma in young females below the age of 40 years: a histopathological perspective South Asian J Cancer 3(2) 97–100 https://doi.org/10.4103/2278-330X.130441 PMID: 24818103 PMCID: 4014656
15. Wang W, Tian B, and Xu X, et al (2022) Clinical features and prognostic factors of breast cancer in young women: a retrospective single-center study Arch Gynecol Obstet Online ahead of print Date accessed 08/12/22 https://doi.org/10.1007/s00404-022-06670-6
16. Chamalidou C, Fohlin H, and Albertsson P, et al (2021) Survival patterns of invasive lobular and invasive ductal breast cancer in a large population-based cohort with two decades of follow up Breast 59 294–300 https://doi.org/10.1016/j.breast.2021.07.011 PMID: 34388695 PMCID: 8361199
17. Sun H, Huang W, and Ji F, et al (2022) Comparisons of metastatic patterns, survival outcomes and tumor immune microenvironment between young and non-young breast cancer patients Front Cell Dev Biol 10 923371 https://doi.org/10.3389/fcell.2022.923371 PMID: 35912097 PMCID: 9329535
18. Fabiano V, Mandó P, and Rizzo M, et al (2020) Breast cancer in young women presents with more aggressive pathologic characteristics: retrospective analysis from an Argentine national database JCO Glob Oncol 6 639–646 https://doi.org/10.1200/JGO.19.00228 PMID: 32315233 PMCID: 7193768
19. Erić I, Petek Erić A, and Kristek J, et al (2018) Breast cancer in young women: pathologic and immunohistochemical features Acta Clin Croat 57(3) 497–502
20. Orolfo-Real I, Tanael S, and Avila JM, et al (2015) Understanding current attitudes to HER2 testing for breast cancer at Tertiary Referral Hospitals of Metro Manila, Philippines Acta Med Philipp 49(2) Date accessed: 02/01/2023 https://doi.org/10.47895/amp.v49i2.970
21. Catedral LI, Tan HN, and Chua A, et al (2020) Patterns of biomarker use in cancer treatment among medical oncologists in the Philippines JCO Glob Oncol 6 1593–1608 https://doi.org/10.1200/GO.20.00265 PMID: 33079606 PMCID: 7605373
22. Genuino AJ, Chaikledkaew U, and Guerrero AM, et al (2019) Cost-utility analysis of adjuvant trastuzumab therapy for HER2-positive early-stage breast cancer in the Philippines BMC Health Serv Res 19(1) 874 https://doi.org/10.1186/s12913-019-4715-8 PMID: 31752849 PMCID: 6873585
23. Yap RV and De La Serna FM (2020) Outcomes of sentinel lymph node biopsy using blue dye method for early breast cancer – a single-institution experience in the Philippines Breast Cancer (Dove Med Press) 12 37–44
24. Naccour J and El-Helou E (2023) Factors influencing the decision-making process for breast surgery in women likely to face breast cancer: a cross-sectional study Ann Med Surg (Lond) 85(5) 1648–1654 PMID: 37229100 PMCID: 10205190
25. Orozco JIJ, Keller JK, and Chang SC, et al (2022) Impact of locoregional treatment on survival in young patients with early-stage breast cancer undergoing upfront surgery Ann Surg Oncol 29(10) 6299–6310 https://doi.org/10.1245/s10434-022-12190-z PMID: 35896922
26. Guay E, Cordeiro E, and Roberts A (2022) Young women with breast cancer: chemotherapy or surgery first? An evaluation of time to treatment for invasive breast cancer Ann Surg Oncol 29(4) 2254–2260 https://doi.org/10.1245/s10434-021-11102-x
27. Radecka B and Litwiniuk M (2016) Breast cancer in young women Ginekol Pol 87(9) 659–663 https://doi.org/10.5603/GP.2016.0062 PMID: 27723074
28. Aryandono T and Harijadi S (2006) Breast cancer in young women: prognostic factors and clinicopathological features Asian Pac J Cancer Prev 7(3) 451–454 PMID: 17059343
29. Anwar SL, Raharjo CA, and Herviastuti R, et al (2019) Pathological profiles and clinical management challenges of breast cancer emerging in young women in Indonesia: a hospital-based study BMC Women’s Health 19(1) 28 https://doi.org/10.1186/s12905-019-0724-3
30. Deanasa RS, Umar M, and Fitri AD (2022) Overall survival for stage III breast cancer patients at DR. Mohammad Hoesin General Hospital Palembang and the influencing factors Indones J Cancer 16(4) 231–237 https://doi.org/10.33371/ijoc.v16i4.905
31. Ibrahim NI, Dahlui M, and Aina EN, et al (2012) Who are the breast cancer survivors in Malaysia? Asian Pac J Cancer Prev 13(5) 2213–2218 https://doi.org/10.7314/APJCP.2012.13.5.2213 PMID: 22901196
32. Lertsithichai P, Sakulchairungreung B, and Chirappapha P, et al (2016) Effect of young age, positive margins, and triple negative status on disease recurrence after breast conserving therapy Gland Surg 5(1) 15–23 PMID: 26855904 PMCID: 4716862
33. Foo CS, Su D, and Chong CK, et al (2005) Breast cancer in young Asian women: study on survival ANZ J Surg 75(7) 566–572 https://doi.org/10.1111/j.1445-2197.2005.03431.x PMID: 15972049
34. Tan BKT, Lim GH, and Czene K, et al (2009) Do Asian breast cancer patients have poorer survival than their western counterparts? A comparison between Singapore and Stockholm Breast Cancer Res 11(1) R4 https://doi.org/10.1186/bcr2219 PMID: 19166618 PMCID: 2687707
35. Wong FY, Tham WY, and Nei WL, et al (2018) Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy Cancer Commun 38(1) 39 https://doi.org/10.1186/s40880-018-0310-3
36. Ho FDV, Arevalo MVPN, and de Claro PTS, et al (2022) Breast and cervical cancer screening in the Philippines: challenges and steps forward Prev Med Rep 29 101936 https://doi.org/10.1016/j.pmedr.2022.101936 PMID: 35959499 PMCID: 9361315
37. Co LMB, Dee EC, and Eala MAB, et al (2022) Access to surgical treatment for breast cancer in the Philippines Ann Surg Oncol 29(11) 6729–6730 https://doi.org/10.1245/s10434-022-12311-8 PMID: 35913672 PMCID: 9341402
38. Gabriel CA and Domchek SM (2010) Breast cancer in young women Breast Cancer Res 12(5) 212 https://doi.org/10.1186/bcr2647 PMID: 21067532 PMCID: 3096966
39. Tesch ME and Partridge AH (2022) Treatment of breast cancer in young adults Am Soc Clin Oncol Educ Book 42 1–12 PMID: 35580291






