Real-world data in patients with BRCA mutated breast cancer treated with poly (ADP-ribose) polymerase inhibitors
Evelyn Lilian Beas-Lozano, Haydeé Cristina Verduzco-Aguirre, Roberto Gonzalez-Salazar and Yanin Chavarri-Guerra
Department of Hematology and Oncology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14630, Mexico
Abstract
Breast cancer is the most common type of cancer globally. Hereditary breast cancer accounts for 10% of new cases and 4%–5% of cases are associated to pathogenic variants in BRCA1 or BRCA2 genes. In recent years, poly-adenosine-diphosphate-ribose polymerase inhibitors (PARPi) olaparib and talazoparib have been approved for patients with BRCA-associated, HER2 -negative breast cancer. These drugs have shown positive results in the early and advanced setting with a favourable toxicity profile based on the OlympiAD, OlympiA and EMBRACA phase 3 trials. However, patients included in these randomised trials are highly selected, making toxicity and efficacy in patients encountered in routine clinical care a concern. Since the approval of olaparib and talazoparib for advanced human epidermal growth factor receptor 2-negative (HER2-negative) breast cancer, several phase IIIb–IV trials, expanded access cohorts, and retrospective cohorts have provided information on the efficacy and tolerability of these treatments in patient subgroups underrepresented in the registration trials, such as older adults, patients with poor performance status, and heavily pretreated patients. The aim of this review is to present a critical review of the information regarding the use of PARPi in real-world breast cancer patients.
Keywords: breast cancer, BRCA1 gene, BRCA2 gene, poly(ADP-ribose) polymerase inhibitors
Correspondence to: Yanin Chávarri-Guerra
Email: yaninchg@gmail.com
Published: 21/11/2023
Received: 07/02/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Breast cancer is the most common malignant tumour globally, with an incidence of 47.8 cases per 100,000 habitants reported worldwide in 2020 [1]. Incidence and mortality vary among countries, in part due to access to optimal screening programs and novel therapies, as well as family history, environmental and ethnic factors [2, 3]. Although mortality rates have improved in the last 20 years, a contrast in survival persists between women living in high and low-middle income countries. As informed by the CONCORD cohort, 5-year survival in women diagnosed from 2005 to 2009 was greater than 80% in high-income countries, while survival was lower than 70% in low-middle income countries [4], highlighting the benefit of opportune diagnosis and access to better care. This was exemplified during 2010–2014, where 5-year survival for breast cancer in high-income countries such as Australia and the United States of America (USA) was 89.5% and 90.2%, respectively, in stark contrast to countries with a low income, with survival as low as 66.1% in India [5].
Hereditary breast cancer accounts for 10% of new breast cancer cases and 4%–5% are associated with pathogenic variants (PVs) in a high-penetrance gene, transmitted in an autosomal dominant fashion [3]. BRCA1 was first identified in 1990 on chromosome 17 by Hall et al [6] using analyses in families with suggestive pedigrees. Subsequently, BRCA2 was identified in chromosome 13 in 1994 [7]. Both genes express proteins that are crucial for deoxy nucleotide acid (DNA) double-strand repair. Although BRCA1 and BRCA2 PVs are inherited in an autosomal dominant fashion, a biallelic inactivation is required for its pathogenic phenotype [8]. This results in an elevated lifetime risk of breast cancer, with up to 80% for BRCA1 mutation carriers and 60% for BRCA2, compared to a lifetime risk of 12.9% in women in general population [9, 10]. Importantly, tumours associated with germline PVs in BRCA1 (gBRCA1m) tend to have higher histologic grades and are more likely to yield triple-negative tumours (58.1%) compared to tumours associated with germline PVs in BRCA2 (gBRCA2m) and noncarriers (34.4% and 7.5%, respectively) [9]. Nevertheless, it should be noted that this does not necessarily result in a worse prognosis. An analysis of 16 studies including 10,180 patients revealed that the presence of BRCA PVs did not lead to a worse overall survival (OS) rate (HR 1.06, 95% CI 0.84–1.34, p = 0.61). This conclusion was also supported when examining the impact of BRCA1 and BRCA2 PVs separately on OS (BRCA1: HR 1.20, 95% CI 0.89–1.61, p = 0.24; BRCA2: HR 1.01, 95% CI 0.80–1.27, p = 0.95) [11]. This might be a consequence of a higher sensitivity to drugs that cause DNA damage, such as platinum agents [12]. Hence, it was only after the advent of poly-adenosine-diphosphate-ribose polymerase inhibitors (PARPi) that this specific population had a chance for targeted treatment.
Based on the principle of synthetic lethality, PARPi hinders DNA double-strand repair (Figure 1). DNA damage response pathways target damage in either single or double-strand breaks. The three major DNA damage response pathways for single-strand breaks are base excision repair (BER), nucleotide excision repair and mismatch repair, while the two major pathways for double-strand breaks are homologous recombination repair (HRR) and non-homologous end joining (NHEJ) [13]. PARP enzymes play a key role in the BER pathway. They act by recognising the damaged site in DNA, binding to it, and catalysing the transfer of adenosine-diphosphate-ribose (ADP-ribose) units from NAD+ to itself, creating a polymer called poly(ADP-ribose) that can recruit and activate other repair enzymes to the site of the break. This process is known as PARylation [14]. PARP1 is a constitutively active enzyme that is always present in the cell and is involved in the initial detection and signalling of DNA damage. PARP2, on the other hand, is a more specialised enzyme that is activated only in response to specific types of DNA damage and plays a role in the later stages of repair [15]. Upon PARPi administration, single-strand breaks are not repaired, leading to the collapse of replication forks, leading to the generation of double-strand breaks [16]. In healthy cells, HRR would play a fundamental role in repairing the former. However, in BRCAm cells, HRR is compromised, and thus these double-strand breaks are not susceptible to repair. This results in an increase in genomic instability, which leads to eventual tumour cell death due to the activation of the remaining and imperfect NHEJ repair pathway [17].
Four PARPi are approved for the treatment of BRCA-associated cancers. However, only two PARPi have been approved for the treatment of breast cancer: talazoparib and olaparib. Talazoparib showed benefit in the metastatic setting based on the EMBRACA phase 3 trial, while olaparib has been approved in the adjuvant and metastatic setting based on the phase 3 OlympiA and OlympiAD trials, respectively [18–20]. Patients included in these trials were required to carry a deleterious gBRCA1/2m detected by BRACAnalysis by Myriad Genetics, Food Drug Administration (FDA)-approved test with high sensitivity and specificity [21]. However, PARPi have proven to be effective in a wider range of patients; those with somatic BRCA1/2m, homologous recombination deficiency (HRD) or mutations in HRR-related genes. This has been specially studied in the treatment of advanced ovarian cancer [22–25]. These alterations are not uncommon in breast cancer patients, especially in the triple negative subtype, with a reported incidence of HRD as high as 69% in a systematic review [26].
Almost 5 years have passed since the first approval of PARPi in breast cancer patients and real-world data has emerged regarding its use. This review offers an evaluation of the information available on this subject.
PARPi in the metastatic setting
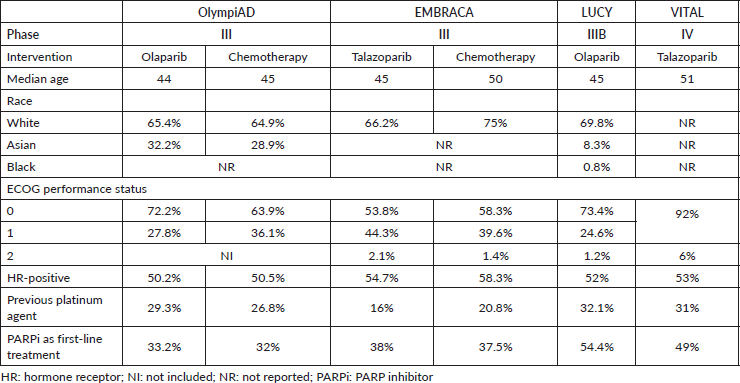
Olaparib and talazoparib currently are the only PARPi approved for patients with hormone receptor-positive/HER2-negative advanced breast cancer and a BRCA1 or BRCA2 mutation detected by germline sequencing. Table 1 includes demographic and clinical characteristics of the four trials in the metastatic setting that showed benefit with PARPi therapy.
In the OlympiAD trial, patients with metastatic breast cancer (MBC) who had received no more than two previous chemotherapy regimens were randomly assigned to receive olaparib or single-agent chemotherapy (capecitabine, eribulin or vinorelbine). Progression-free survival (PFS), the primary endpoint, was significantly longer for patients who received olaparib (7.0 months versus 4.2 months, HR 0.58); the response rate was also higher with olaparib (59.9% versus 28.8%). Grade 3+ toxicity was lower in patients who received olaparib (36.6% versus 50.5%) [20]. On longer follow-up, however, no statistically significant OS improvement was observed (19.3 months versus 17.1 months, HR. 0.90) [27], although the trial was not powered for this outcome.
Talazoparib was approved in the metastatic setting due to information from the EMBRACA trial, where patients with advanced breast cancer who have received no more than three previous cytotoxic regimens were randomly assigned to talazoparib or chemotherapy (capecitabine, eribulin, gemcitabine or vinorelbine). The primary endpoint of the trial was PFS, which was significantly longer in patients who received talazoparib (8.6 months versus 5.6 months, HR 0.54). Objective response rate (ORR) was higher with talazoparib (62.6% versus 27.2%). Hematologic grade 3–4 events were higher with talazoparib (55% versus 38%), while non-hematologic grade 3 events were lower with talazoparib than with chemotherapy (32% versus 38%) [28]. The final analysis of OS did not show a significant difference between the treatment arms (19.3 months versus 19.5 months, HR 0.84) [18].
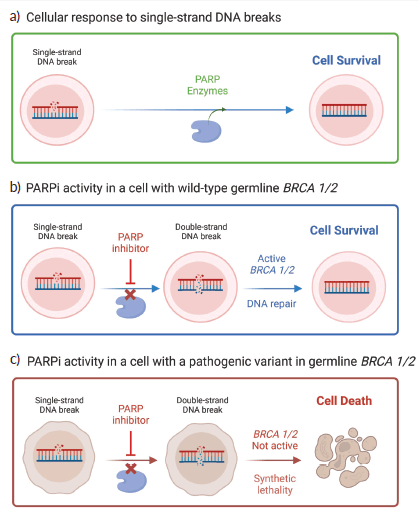
Figure 1. PARPi mechanism of action. (a): In healthy cells, the PARP enzymes play a key role in the repair of single-strand breaks because they recognise the damaged DNA site, bind to it and recruit other repair enzymes to the site of break. (b): By administering PARPi, the cell is unable to repair single-strand breaks due to a collapse of the replication fork which leads to double-strand breaks. In cells with wild-type germline BRCA (and other HRR -related genes), double-strand breaks are repaired via the HRR pathway. (c). In BRCA mutated cells, the HRR pathway is compromised and these double-strand breaks are not susceptible to repair which leads to eventual cell death. Adapted from ‘PARPi: Treatment for BRCA Mutant Breast Cancer’, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates.
Although, in the OlympiAD trial all subtypes of breast cancer (those with HR positive, HER2 negative disease and triple negative) had an improvement in PFS, triple negative breast cancer (TNBC) had the greatest benefit when compared to HR positive tumours: OR 0.56 (95% CI 0.34–0.98) and 0.65 (95% CI 0.47–0.91), respectively. Similarly, in the EMBRACA trial, both subgroups had an improvement in PFS with HR 0.60 (95% CI 0.41–0.87) and HR 0.47 (95% CI 0.32–0.71), respectively. Based on these subgroups analyses in both trials PARPi are recommended in the first-line for TNBC tumours and for endocrine refractory tumours according to the inclusion criteria.
Regarding OS and subgroup analyses, it is important to mention that the final OS in the OlympiAD trial showed a greater benefit among those patients who had not received prior chemotherapy for MBC: first-line treatment 22.6 versus 14.7 months (HR 0.51; 95% CI 0.29–0.90; p = 0.02) compared to those with prior chemotherapy 18.8 versus 17.2 months (HR 1.13; 95% CI 0.79–1.64; p = NS). One of the factors to take in consideration when interpreting these results is the higher sensitivity to platinum drugs in the BRCAm population [29, 30]. In fact, the population that received a previous platinum agent seems to have a greater benefit, HR 0.83 (CI 95% 0.49–1.45) versus HR 0.91 (CI 95% 0.64–1.33). Although it was not statistically significant which might be attributed to a higher proportion of patients receiving subsequent platinum agents in the control arm (42.3% versus 29.3%) [20]. On the other hand, in the EMBRACA trial benefit was not different among patients who received previous regimens of chemotherapy except for the patients who received previous platinum treatment with less benefit in PFS HR 0.76 (95% CI 0.40–1.45) versus 0.59 (95% CI 0.34–1.02). A limitation of both trials was to include platinum-based agents as an option in the standard therapy group to compare its effectivity against platinum therapy which opens the question of the best initial treatment option for patients with BRCAm.
Furthermore, other trials have tried to answer the utility of combining platinum and PARPi, the BROCADE3 phase III trial, randomised patients to receive veliparib or placebo in combination with carboplatin and paclitaxel. Results showed a greater benefit in PFS in the veliparib group with 14.5 months versus 12.6 months (p = 0.0016), confirming its utility in this patient population [31].
Table 1. Demographic and clinicopathological characteristics among patients included in OlympiaD, EMBRACA, LUCY and VITAL.
Real world data on effectiveness of PARPi in metastatic breast cancer
The phase IIIb single-arm study LUCY assessed in a real-world setting, the clinical effectiveness of olaparib in a similar population to that of the OlympiAD trial: the trial enrolled 252 patients with HER2-negative MBC with a documented deleterious or suspected deleterious germline BRCA mutation (gBRCAm); in a later amendment, patients with somatic BRCA mutations were also included, but only 3 patients with this characteristic were enrolled in the study [32]. The median age of the cohort with a gBRCAm was 45 years; 52% had hormone receptor-positive disease, while 48% had TNBC. One hundred and thirty-seven patients (54.4%) received olaparib as first-line treatment (defined as no previous chemotherapy for advanced disease). Among patients with hormone receptor-positive disease, 19.1% had received therapy with CDK4/6 inhibitors.
In the final analysis of this trial [33], the median PFS was 8.2 months (95% CI 7.0–9.2) and the median OS was 24.9 months (95% CI 21.1–27.9). Median OS was longer in patients with hormone receptor-positive disease (27.4 months) than in those with hormone receptor-negative disease (21.1 months). OS was also longer in patients who received olaparib as a first-line treatment for MBC (27.4 months) compared to those who received it in the second or third line (22.7 months); no formal statistical comparisons were performed between subgroups, but mirrors what was already seen in OlympiAD.
The OS observed in the LUCY trial was longer than that previously reported in the OlympiAD trial; however, the rate of patients who received olaparib as a first-line treatment (defined as not having received chemotherapy in the advanced/metastatic setting) was higher in the former (54.4%) than in the latter (33.2%). Also, in the OlympiAD trial, patients were required to have previously received an anthracycline and a taxane; in the LUCY study, patients were able to enroll having received only one of these drugs – 86.7% of patients had previously received an anthracycline, while 88.6% had previously received a taxane.
The VITAL phase IV study aimed to ensure the effectiveness and safety of talazoparib in a real-world setting. This study consisted of two cohorts: the first included 86 patients treated through the French Early Access Program (93% with a germline BRCA1/2 mutation and 7% with a somatic mutation); the second enrolled 85 patients treated according to the European Marketing Approval. The primary endpoint of this study was the time to treatment discontinuation, which was 9.0 months in the first cohort. No difference was observed in time to treatment discontinuation according to hormone receptor status, type of mutation (BRCA1 versus BRCA2), prior treatment with platinum-based chemotherapy or number of previous lines of chemotherapy. In this first cohort, median OS was 25.6 months, 6.3 months longer than that reported in the EMBRACA trial. Interestingly, according to a poster presented by Delphine Loirat at the San Antonio Breast Cancer Symposium 2022 (SABCS 2022, Poster P4-01-20) [34], in the first cohort of the VITAL study, only 16% of patients had not received any previous chemotherapy in the metastatic setting, while 38% of patients in the EMBRACA trial had not received any cytotoxic regimens for advanced breast cancer. Results from the second cohort of VITAL were similar: a median time to treatment discontinuation of 9.1 months; this cohort is not yet mature for OS, as presented by Delphine Loirat at the SABCS 2022 (Poster P4-01-04) [35].
Outcomes for the few patients with somatic BRCA1/2 mutations included in the LUCY and VITAL studies have not been reported separately, but there are several reports on clinical benefit with olaparib in patients with somatic or combined germline/somatic BRCA1/2 alterations [36]. Remarkably, a 40-year-old woman with recurrent metastatic TNBC with lung and brain metastases underwent next-generation sequencing, which reported a large deletion of exon 2 in BRCA1, a novel (possibly pathogenic) somatic BRCA2-STARD13 rearrangement and copy number loss of RAD51. The patient was treated with olaparib after resection of the symptomatic cerebral metastatic disease, achieving >38 months of disease-free survival [37]. On the other hand, according to information recently presented by Nusrat Jahan at the SABCS 2022 about the Mayo Clinic’s Experience with PARPi, out of 65 patients with MBC and a BRCA1/2 mutation, 10 (15%) patients had a somatic mutation in homologous recombination-related genes, 7 of them in BRCA1/2. On multivariate analysis, somatic BRCA1/2 mutations were associated with a shorter time to treatment failure (4 months versus 7–8 months in patients with germline BRCA1/2 mutations).
While patients with active parenchymal central nervous system or leptomeningeal metastases were excluded from the registration trials of PARPi in breast cancer, olaparib has shown activity in the presence of leptomeningeal carcinomatosis, which usually carries a poor prognosis [38]. Thus, PARPi could represent a better-tolerated option compared to more invasive treatments with limited benefit in this situation, such as intrathecal chemotherapy or radiotherapy.
Real world data on toxicity and tolerability of PARPi in metastatic breast cancer
In the LUCY trial mentioned above, median total treatment duration was 8.0 months (range 0.2–43.3). Adverse events were observed in 85.1% of participants. Most adverse events were grade 1 or 2, with the most common being nausea, anemia, asthenia, fatigue, and vomiting. Grade 3+ adverse events occurred in 17.6% of participants, and almost exclusively consisted of anemia and neutropenia. The rate of discontinuation of olaparib due to adverse events was 4.3%. No toxic deaths were reported.
Olaparib-related adverse events have also been studied through data mining of the USA FDA -Adverse Event Reporting System [39]. Reports in this system are consistent with the evidence from clinical trials and other cohorts: the most common adverse events reported were anemia, thrombocytopenia, nausea, and decreased appetite. Unexpected significant adverse events reported through this system were interstitial lung disease and renal impairment. The median onset time of reported adverse events was 61 days; most events occurred in the first 3 months since olaparib initiation. Other novel adverse events have been described in case reports of patients with MBC with a BRCA1/2 mutation, including recurrent episodes of erythema nodosum, which may be managed through dose reductions [40].
A retrospective study aimed to identify patient-related risk factors for the development of anemia during treatment with olaparib. This study included 113 patients with advanced breast or ovarian cancer, of which 7.1% of the cohort corresponded to patients with breast cancer. The incidence of grade 3+ anemia was 32.7%, while 61.1% of patients developed grade 1+ anemia. On multivariable logistic regression analysis, low baseline red blood cell (RBC) count, low baseline hematocrit, low baseline hemoglobin, and BRCA1/2 mutation were significantly associated with the development of grade 3+ anemia, while high baseline RBC, high baseline hematocrit, high baseline hemoglobin level and BRCA1/2 mutation were significantly associated with grade 1+ anemia [41].
According to the previously mentioned information presented by D. Loirat et al at the SABCS 2022, Cohort 1 of the VITAL study reported at least one adverse event in 83% of patients who received talazoparib: the most common adverse events were anemia (33%), thrombocytopenia (17%), neutropenia (12%), alopecia (9%), asthenia (9%) and nausea (8%). Serious adverse events occurred in 16% of patients, mainly anemia and thrombocytopenia. Dose modifications were required in 27% of patients, temporary interruptions in 42%, and in 6% talazoparib had to be permanently discontinued due to adverse events.
In another retrospective analysis of a disease-specific program in USA, European Union and Israel, patterns of treatment were described for 543 patients with advanced HER2-negative breast cancer and a germline BRCA1/2 mutation [42]. Seventy percent of patients had hormone receptor+/HER2- disease, while 30% had TNBC. Eighty-five percent of patients had a BRCA1 mutation, and 57% a BRCA2 mutation. In this cohort, 79 patients (14.5%) had received a PARPi; the proportion of patients who were treated with PARPi varied between patients with hormone receptor+/HER2- breast cancer (5% in first line, 11% in second line, and 12% in third line) and those with TNBC (18% in first line, 44% in second line, and 36% in third line). This study did not mention the specific PARPi that patients received, and survival was not reported in this analysis. Regarding tolerability, the median of days on treatment with a PARPi was 96 days, lower than for other reported therapies such as non-platinum-based chemotherapy (163 days) or endocrine therapy (493 days). Nausea was the most common adverse event, occurring in 32% of patients receiving a PARPi overall, 44% of those who received it in the first line and 31% of those who received it in the second line. Anemia was the second most common adverse event (24% overall; 12% in the first line, and 29% in second- or third-line therapy). Neutropenia was observed in 16% of patients.
Ongoing trials
Most of the ongoing trials with PARPi in patients with MBC with a BRCA1/2 mutation are focusing on combinations with other drugs, such as combining olaparib with immune checkpoint inhibitors such as pembrolizumab (NCT03025035), with trastuzumab for patients with HER2-positive disease (NCT03931551), or with sapacitabine, a DNA damage response inhibitor (NCT03641755). Talazoparib is currently being studied in combination with gedatolisib, a dual PI3K/mTOR inhibitor (NCT03911973). Phase IV studies with olaparib are also ongoing in India (NCT04330040).
PARPi in early breast cancer
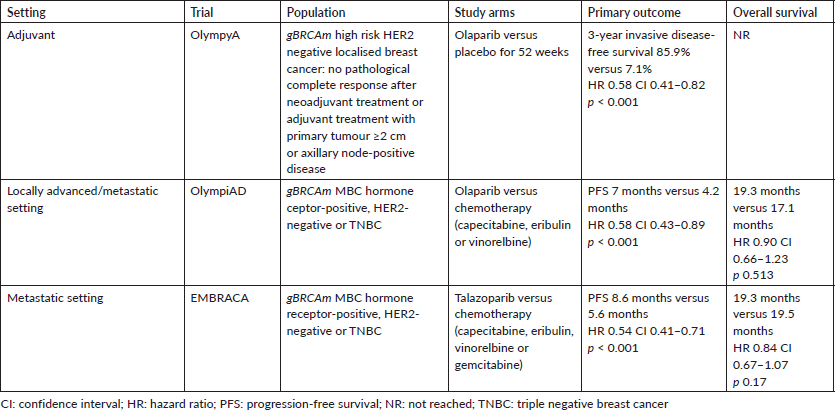
In early breast cancer, OlympiA was a large phase 3 randomised clinical trial testing effectivity of olaparib in patients with gBRCA1 and BRCA2 PVs HER2 negative high-risk breast cancer. Patients received neoadjuvant or adjuvant chemotherapy and were randomised to olaparib for a year or placebo. Patients with residual disease after neoadjuvant chemotherapy and TNBC or if hormone receptor positive disease should have a high tumour burden. For those who received adjuvant chemotherapy with TNBC (tumour size >2 cm or positive axillary disease) or hormone receptor-positive disease (and at least 4 positive axillary lymph nodes). At a median follow-up of 2.5 years interim analysis showed and improved invasive disease-free survival in the olaparib group (85.9% versus 77.1%; HR 0.58; p < 0.001) as well as in distant disease-free survival (87.5% versus 80.4%; HR 0.57; p < 0.0001) compared to the placebo group [19]. In the follow-up analysis OS was also improved with olaparib in comparison to placebo (HR 0.68; p 0.009) with 89.8% and 86.4% total deaths at 4 years, respectively. The benefit was consistent in all subgroups of patients (TNBC and hormone receptor-positive disease) [43]. At this time, this is the only trial that has shown benefit in the adjuvant setting.
Although PARPi has not been approved in the neoadjuvant setting, studies have showed activity with a high proportion of pathologic complete responses (pCR) in patients with gBRCAm. Neoadjuvant PARPi has shown pCR with monotherapy (53%–57%) or in combination with chemotherapy (48%–55%).
In combination with chemotherapy in the neoadjuvant setting, veliparib was tested in combination with carboplatin-paclitaxel followed by doxorubicin-cyclophosphamide, in both the ISP-Y 2 trial (randomised phase II study) and in BrighTNess (randomised phase 3) [44, 45] In both trials a higher pCR was reported in patients with TNBC in comparison with placebo. However, in none of these two trials a clear impact on the gBRCA status was found. On the other hand, in the GeparOLA study, olaparib in combination with paclitaxel followed by epirubicin-cyclophosphamide in HDR deficient tumours (gBRCAm, somatic BRCA mutation or with high HRD score) didn’t show a higher pCR compared to the control arm [46]. As monotherapy, 6 months of talazoparib before standard neoadjuvant chemotherapy showed 53% of pCR [47]. Olaparib as monotherapy also showed to be active in patients with TNBC in the PETREMAC trial with objective response in 56.3% of patients [48]. With this data the question of less intensive chemotherapy regimens for patients who achieved pCR needs further research [47–49].
The choice between olaparib versus other adjuvant strategies
Adjuvant capecitabine in patients with TNBC with residual disease after neoadjuvant chemotherapy is recommended by international guidelines since it improves OS [50]. However, the question about what therapy should be recommended in carriers of gBRCAm arises with the results of the OlympiA trial, where adjuvant capecitabine was not allowed. Taking in account the information in the metastatic setting, where single PARPi have been superior to single-agent chemotherapy and that in the OlympiAD trial olaparib in the first line of MBC was beneficial, suggests that using PARPi earlier might be beneficial [27, 28, 51]. Although there has not been a formal comparison between capecitabine and PARPi in this scenario, the information in the metastatic setting suggests that PARPi might be a better choice in patients with TNBC and gBRCAm.
Patients with TNBC might also be a candidate to receive immunotherapy according to the KEYNOTE-522 and GeparNuevo, where patients with stage II–III received immune check point inhibitors plus chemotherapy showed improved outcomes when compared to chemotherapy alone [52, 53]. However, there is no information comparing both strategies or the addition of a PARPi to immunotherapy in the adjuvant setting. In the metastatic setting combining both drugs have been reported to be an active combination, but it is unknown if it is a better choice than a PARPi alone. Therefore, until that information is available the combination of both drugs has been recommended to be individualised based on residual disease, clinical stage and tolerance to therapy [54].
On the other hand, patients with hormone receptor-positive tumours and high-risk characteristics are also candidates for adjuvant CDK4/6 inhibitor with abemaciclib due to improvement in invasive disease-free survival showed by the monarchE trial, where some patients with gBRCAm were included [55]. However, other CDK4/6 inhibitors in the adjuvant setting failed to show benefit and combination with PARPi and CDK4/6 inhibitors safety information is not available [56, 57].
Ongoing trials in early breast cancer
Further studies are investigating the benefit of PARPi in early breast cancer. The ZEST trial (NCT04915755), started in 2021, is a randomised phase III study that has been enrolling patients with localised breast cancer that is either triple negative or HR +/HER2- with a known BRCA tissue mutation (irrespective of BRCA status). All patients must have detectable circulating tumour DNA after surgery to be randomised to therapy with niraparib or placebo for up to 3 years. Within these patients, two cohorts (patients with wild-type tissue BRCA and mutated tissue BRCA) will be analysed. The SUBITO trial (NCT02810743), is a phase III study enrolling patients with stage III breast cancer with gBRCA1/2m or BRCA1-like copy number profile evaluated on tumour tissue. Patients in the control arm receive 1 year of adjuvant olaparib after completing dose dense neo- or adjuvant chemotherapy, while in the experimental patients receive two cycles of intensified alkylating chemotherapy with autologous peripheral stem cell transplantation. The PARTNER trial also tested olaparib in combination with carboplatin in TNBC or in patients with gBRCAm which has reported up-to-date tolerability and safety results, while efficacy results are pending. Due to preliminary results with high pCR, other trials in the neoadjuvant settings are also testing the efficacy of PARPi as monotherapy (NCT03329937) and (NCT03499353).
Special populations: older women
Based on genetic testing criteria, only a few older women with breast cancer will go through genetic risk assessment as compared to younger women [58]. However, a recent study of 1,035 women over the age of 65 in the USA and Latin America with breast cancer found that a significant percentage (10.4%) carried associated germline mutations. Among them, 37% were positive for BRCA1 and 42% were positive for BRCA2 [59]. This raises concern for underrepresentation of these women in pivotal phase 3 trials that gave approval to olaparib and talazoparib (only 7.3% in the OlympiAD trial and 3.1% in the OlympiA trial) [18–20].
One of the remaining queries is tolerability in this specific group of patients. A pooled analysis of 398 patients with ovarian cancer (included in phase 1–2 trials evaluating the use of olaparib) showed no differences in tolerability and toxicity between women younger than 65 years and their older counterparts [60]. While there is no data available regarding differences in adherence between the two approved formulations for olaparib, tablets might be preferred. Four tablets a day can be given instead of 16 capsules to achieve the same therapeutic dose. This might be advantageous in patients with underlying cognitive impairment or limited social support [61].
Patient eligibility criteria for the use of PARPi
FDA approval of the use of PARPi for the treatment of breast cancer is based on data from three randomised clinical trials that took place during different stages of the disease (Table 2). All three trials have in common that they defined eligible patients by the presence of pathogenic or likely PVs in germline BRCA1 and/or BRCA2.
Although FDA approval in all disease settings is currently limited to patients with gBRCAm, some studies have further included patients with somatic BRCA mutant (sBRCAm) tumours and tumours with HRD. For instance, in an effort to evaluate the clinical effectiveness and safety of olaparib in patients with HER2-negative MBC in a real-world setting, the phase IIIB study, LUCY, enrolled patients with sBRCAm tumours in addition to patients with PV in gBRCA [62]. Moreover, Talazoparib Beyond BRCA, a phase II single-arm trial, evaluated the use of talazoparib in patients with either germline or somatic likely pathogenic or PVs in HR-associated genes excluding BRCA1/2. Of 20 patients included, 13 had previously treated advanced HER-2 negative breast cancer, whereas seven patients had other types of cancer. Authors reported an ORR of 31% (CI 9%–61%) and a clinical benefit rate of 54% (CI 21%–81%) in breast cancer patients [63]. Likewise, in the early disease setting the GeparOLA trial included patients with HER2-negative disease with HRD as defined by a high HRD score and/or germline or somatic BRCA1/2 mutations to be randomised to receive neoadjuvant paclitaxel plus olaparib or paclitaxel plus carboplatin, both followed by epirubicin and cyclophosphamide [49]. Finally, the phase II study TBCRC 048 aimed to assess olaparib response in patients with wild-type germline BRCA (gBRCAwt) but with sBRCA mutations or germline or somatic mutations in the homologous recombination-related genes other than BRCA1/2. This study showed that patients with germline mutations in PALB2 and somatic mutations in BRCA1/2 had high responses to olaparib (82% for gPALB2m and 50% for sBRCA1/2m) [64].
All this becomes relevant because the population that could derive benefit from the use of PARPi for the treatment of breast cancer seems to extend beyond the carriers of a PV in gBRCA1/2, something similar to what has been found in ovarian cancer [65].
Table 2. Phase 3 trials for approved PARPi in breast cancer.
Conclusion
PARPi represent a novel targeted treatment for patients with BRCA associated cancers. Olaparib and talazoparib have been approved for a highly selected group of patients with an acceptable toxicity profile. These drugs have yielded promising results, especially for patients with advanced HER-2 negative breast cancer. Thus, we support routine genetic testing of these patients and the use of olaparib and talazoparib for both HR-positive and TNBC. We also favour its use earlier in the disease since patients without previous chemotherapy seem to benefit the most.
Real-world data studies have reproduced the effectiveness of these drugs and showed how they could be useful in different scenarios, especially those with HRD or HRR-related gene mutations. Encouraging results have been reported in the neoadjuvant setting either in monotherapy or in combination with cytotoxic agents, and its combination with immunotherapy is being explored. Real-world information is crucial to understand the effectiveness of these drugs in typically underrepresented patients in the pivotal trials that led to their approval and opened doors to further research for more extensive approval in breast cancer patients.
Conflicts of interest
Yanin Chávarri-Guerra: Roche research funding and travel expenses; Novartis travel expenses; INVITAE Speakers’ bureau; Astra Zeneca Advisory role.
Haydeé Cristina Verduzco-Aguirre: AstraZeneca travel expenses.
The rest of the authors declare no conflicts of interest.
Funding statement
The authors affirm that this paper has not been directly funded nor sponsored.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Ottini L, Rizzolo P, and Silvestri V, et al (2011) Inherited and acquired alterations in development of breast cancer Appl Clin Genet 4 145–158 https://doi.org/10.2147/TACG.S13226 PMID: 23776375 PMCID: 3681186
3. Collaborative Group on Hormonal Factors in Breast Cancer (2001) Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease Lancet 358(9291) 1389–1399 https://doi.org/10.1016/S0140-6736(01)06524-2 PMID: 11705483
4. Allemani C, Weir HK, and Carreira H, et al (2015) Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet 385(9972) 977–1010 https://doi.org/10.1016/S0140-6736(14)62038-9 PMCID: 4588097
5. Allemani C, Matsuda T, and Di Carlo V, et al (2018) Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries Lancet 391(10125) 1023–1075 https://doi.org/10.1016/S0140-6736(17)33326-3 PMID: 29395269 PMCID: 5879496
6. Hall JM, Lee MK, and Newman B, et al (1990) Linkage of early-onset familial breast cancer to chromosome 17q21 Science 250(4988) 1684–1689 2270482>https://doi.org/10.1126/science.2270482 PMID: 2270482
7. Wooster R, Neuhausen SL, and Mangion J, et al (1994) Localization of a breast cancer susceptibility gene, BRCA2 , to chromosome 13q12-13 Science 265(5181) 2088–2090 8091231>https://doi.org/10.1126/science.8091231 PMID: 8091231
8. Gudmundsdottir K and Ashworth A (2006) The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability Oncogene 25(43) 5864–5874 https://doi.org/10.1038/sj.onc.1209874 PMID: 16998501
9. Chen S and Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance J Clin Oncol 25(11) 1329–1333 https://doi.org/10.1200/JCO.2006.09.1066 PMID: 17416853 PMCID: 2267287
10. Giaquinto AN, Sung H, and Miller KD, et al (2022) Breast cancer statistics, 2022 CA Cancer J Clin 72(6) 524–541 https://doi.org/10.3322/caac.21754 PMID: 36190501
11. Templeton AJ, Gonzalez LD, and Vera-Badillo FE, et al (2016) Interaction between hormonal receptor status, age and survival in patients with BRCA1/2 germline mutations: a systematic review and meta-regression PLoS One 11(5) e0154789 https://doi.org/10.1371/journal.pone.0154789 PMID: 27149669 PMCID: 4858163
12. Mylavarapu S, Das A, and Roy M (2018) Role of BRCA mutations in the modulation of response to platinum therapy Front Oncol 8 16 https://doi.org/10.3389/fonc.2018.00016 PMID: 29459887 PMCID: 5807680
13. O’Connor MJ (2015) Targeting the DNA damage response in cancer Mol Cell 60(4) 547–560 https://doi.org/10.1016/j.molcel.2015.10.040
14. Helleday T (2011) The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings Mol Oncol 5(4) 387–393 https://doi.org/10.1016/j.molonc.2011.07.001 PMID: 21821475 PMCID: 5528309
15. Krishnakumar R and Kraus WL (2010) The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets Mol Cell 39(1) 8–24 https://doi.org/10.1016/j.molcel.2010.06.017 PMID: 20603072 PMCID: 2923840
16. Murai J, Huang SN, and Das BB, et al (2012) Trapping of PARP1 and PARP2 by clinical PARP inhibitors Cancer Res 72(21) 5588–5599 https://doi.org/10.1158/0008-5472.CAN-12-2753 PMID: 23118055 PMCID: 3528345
17. Ceccaldi R, Rondinelli B, and D’Andrea AD (2016) Repair pathway choices and consequences at the double-strand break Trends Cell Biol 26(1) 52–64 https://doi.org/10.1016/j.tcb.2015.07.009 PMCID: 4862604
18. Litton JK, Hurvitz SA, and Mina LA, et al (2020) Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial Ann Oncol 31(11) 1526–1535 https://doi.org/10.1016/j.annonc.2020.08.2098 PMID: 32828825
19. Tutt ANJ, Garber JE, and Kaufman B, et al (2021) Adjuvant olaparib for patients with BRCA1 - or BRCA2 -mutated breast cancer N Engl J Med 384(25) 2394–2405 https://doi.org/10.1056/NEJMoa2105215 PMID: 34081848 PMCID: 9126186
20. Robson M, Im SA, and Senkus E, et al (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation N Engl J Med 377(6) 523–533 https://doi.org/10.1056/NEJMoa1706450 PMID: 28578601
21. Judkins T, Leclair B, and Bowles K, et al (2015) Development and analytical validation of a 25-gene next generation sequencing panel that includes the BRCA1 and BRCA2 genes to assess hereditary cancer risk BMC Cancer 15(1) 215 https://doi.org/10.1186/s12885-015-1224-y PMID: 25886519 PMCID: 4391687
22. Pujade-Lauraine E, Ledermann JA, and Selle F, et al (2017) Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial Lancet Oncol 18(9) 1274–1284 https://doi.org/10.1016/S1470-2045(17)30469-2 PMID: 28754483
23. Moore K, Colombo N, and Scambia G, et al (2018) Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer N Engl J Med 379(26) 2495–2505 https://doi.org/10.1056/NEJMoa1810858 PMID: 30345884
24. Monk BJ, Coleman RL, and Fujiwara K, et al (2021) ATHENA (GOG-3020/ENGOT-ov45): a randomized, phase III trial to evaluate rucaparib as monotherapy (ATHENA–MONO) and rucaparib in combination with nivolumab (ATHENA–COMBO) as maintenance treatment following frontline platinum-based chemotherapy in ovarian cancer Int J Gynecol Cancer 31(12) 1589–1594 https://doi.org/10.1136/ijgc-2021-002933 PMID: 34593565 PMCID: 8666815
25. González-Martín A, Pothuri B, and Vergote I, et al (2019) Niraparib in patients with newly diagnosed advanced ovarian cancer N Engl J Med 381(25) 2391–2402 https://doi.org/10.1056/NEJMoa1910962 PMID: 31562799
26. Chai Y, Chen Y, and Zhang D, et al (2022) Homologous recombination deficiency (HRD) ed Chai Y, Chen Y,and BRCA 1/2 gene mutation for predicting the effect of platinum-based neoadjuvant chemotherapy of early-stage triple-negative breast cancer (TNBC): a systematic review and meta-analysis J Pers Med 12(2) 323 https://doi.org/10.3390/jpm12020323 PMID: 35207810 PMCID: 8876589
27. Robson ME, Tung N, and Conte P, et al (2019) OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer Ann Oncol 30(4) 558–566 https://doi.org/10.1093/annonc/mdz012 PMID: 30689707 PMCID: 6503629
28. Litton JK, Rugo HS, and Ettl J, et al (2018) Talazoparib in patients with advanced breast cancer and a germline BRCA mutation N Engl J Med 379(8) 753–763 https://doi.org/10.1056/NEJMoa1802905 PMID: 30110579 PMCID: 10600918
29. Byrski T, Dent R, and Blecharz P, et al (2012) Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer Breast Cancer Res 14(4) R110 https://doi.org/10.1186/bcr3231 PMID: 22817698 PMCID: 3680929
30. Tutt A, Tovey H, and Cheang MCU, et al (2018) Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial Nat Med 24(5) 628–637 https://doi.org/10.1038/s41591-018-0009-7 PMID: 29713086 PMCID: 6372067
31. Diéras V, Han HS, and Kaufman B, et al (2020) Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial Lancet Oncol 21(10) 1269–1282 https://doi.org/10.1016/S1470-2045(20)30447-2 PMID: 32861273
32. Gelmon KA, Fasching PA, and Couch FJ, et al (2021) Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: phase IIIb LUCY interim analysis Eur J Cancer 152 68–77 https://doi.org/10.1016/j.ejca.2021.03.029 PMID: 34087573
33. Neven P, Fasching PA, and Chia S, et al (2022) Updated overall survival (OS) results from the first-line (1L) population in the phase III MONALEESA-3 trial of postmenopausal patients (pts) with HR+/HER2? advanced breast cancer (ABC) treated with ribociclib (RIB) + fulvestrant (FUL) Ann Oncol suppl_3 S194–S223 https://doi.org/10.1016/j.annonc.2022.03.280
34. Loirat D, Duboys de la barre M, Thery JC, et al (2022) Phase IV study evaluating talazoparib in patients with locally advanced or metastatic negative HER2 breast cancer and a somatic or germline BRCA1/2 mutation (ViTAL) – Analysis of cohort 1 according to hormonal receptor status. Abstracts from the 45th Annual San Antonio Breast Cancer Symposium 2022, Dec 6-10, 2022. Poster Session 4 [Internet] Available: https://www.sabcs.org/Portals/SABCS2016/2022%20SABCS/SABCS%202022%20Abstract%20Report.pdf?ver=2022-12-08-111637-860
35. Loirat D, Duboys de la barre M, Villanueva C, et al (2022) Phase IV multicenter study evaluating RWE and the safety of talazoparib in patients with locally advanced or metastatic negative HER2 breast cancer and a BRCA1/2 mutation (ViTAL) - Cohort 2: patients treated according to the EMA. Abstracts from the 45th Annual San Antonio Breast Cancer Symposium 2022, Dec 6-10, 2022. Poster Session 4 [Internet] Available: https://www.sabcs.org/Portals/SABCS2016/2022%20SABCS/SABCS%202022%20Abstract%20Report.pdf?ver=2022-12-08-111637-860
36. Schwartzberg LS and Kiedrowski LA (2021) Olaparib in hormone receptor-positive, HER2-negative metastatic breast cancer with a somatic BRCA2 mutation Ther Adv Med Oncol 13 175883592110069 https://doi.org/10.1177/17588359211006962
37. Wang X, Hu N, and Cui L, et al (2022) Durable disease-free survival in a patient with metastatic triple-negative breast cancer treated with olaparib monotherapy Curr Cancer Drug Targets 22(6) 530–536 https://doi.org/10.2174/1568009622666220214092207 PMID: 35156571 PMCID: 9906627
38. Exman P, Mallery RM, and Lin NU, et al (2019) Response to olaparib in a patient with germline BRCA2 mutation and breast cancer leptomeningeal carcinomatosis NPJ Breast Cancer 5 46 https://doi.org/10.1038/s41523-019-0139-1 PMID: 31815182 PMCID: 6884546
39. Shu Y, He X, and Liu Y, et al (2022) A real-world disproportionality analysis of olaparib: data mining of the public version of FDA adverse event reporting system Clin Epidemiol 14 789–802 https://doi.org/10.2147/CLEP.S365513 PMID: 35789689 PMCID: 9250344
40. Wheelden M, Cream L, and Sivik J, et al (2018) A novel adverse event associated with olaparib therapy in a patient with metastatic breast cancer Case Rep Oncol Med 2018 9529821 PMID: 30050710 PMCID: 6040243
41. Tashiro R, Kawazoe H, and Mamishin K, et al (2022) Patient-associated risk factors for severe anemia in patients with advanced ovarian or breast cancer receiving olaparib monotherapy: a multicenter retrospective study Front Oncol 12 898150 https://doi.org/10.3389/fonc.2022.898150 PMID: 36267984 PMCID: 9577461
42. Mahtani R, Niyazov A, and Arondekar B, et al (2022) Real-world study of patients with germline BRCA1/2-mutated human epidermal growth factor receptor 2‒negative advanced breast cancer: patient demographics, treatment patterns, adverse events, and physician-reported satisfaction in the United States, Europe, and Israel Breast 66 236–244 https://doi.org/10.1016/j.breast.2022.10.009 PMID: 36368161 PMCID: 9650077
43. Geyer CE, Garber JE, and Gelber RD, et al (2022) Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer Ann Oncol 33(12) 1250–1268 https://doi.org/10.1016/j.annonc.2022.09.159 PMID: 36228963 PMCID: 10207856
44. Rugo HS, Olopade OI, and DeMichele A, et al (2016) Adaptive randomization of veliparib–carboplatin treatment in breast cancer N Engl J Med 375(1) 23–34 https://doi.org/10.1056/NEJMoa1513749 PMID: 27406347 PMCID: 5259561
45. Loibl S, O’Shaughnessy J, and Untch M, et al (2018) Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial Lancet Oncol 19(4) 497–509 https://doi.org/10.1016/S1470-2045(18)30111-6 PMID: 29501363
46. Fasching PA, Jackisch C, and Rhiem K, et al (2019) GeparOLA: a randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients (pts) with HER2-negative early breast cancer (BC) and homologous recombination deficiency (HRD) [abstract] J Clin Oncol 37 506–506 https://doi.org/10.1200/JCO.2019.37.15_suppl.506
47. Litton JK, Scoggins ME, and Hess KR, et al (2020) Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant J Clin Oncol 38(5) 388–394 https://doi.org/10.1200/JCO.19.01304 PMCID: 7351336
48. Eikesdal HP, Yndestad S, and Elzawahry A, et al (2021) Olaparib monotherapy as primary treatment in unselected triple negative breast cancer Ann Oncol 32(2) 240–249 https://doi.org/10.1016/j.annonc.2020.11.009
49. Fasching PA, Link T, and Hauke J, et al (2021) Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study) Ann Oncol 32(1) 49–57 https://doi.org/10.1016/j.annonc.2020.10.471
50. National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines. Breast Cancer. Version 4.2022 (Plymouth Meeting) [https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf] Date accessed: 30/01/23
51. Robson M, Goessl C, and Domchek S (2017) Olaparib for metastatic germline BRCA -mutated breast cancer N Engl J Med 377(18) 1792–1793 [10.1056/NEJMc1711644] https://doi.org/10.1056/NEJMoa1706450 PMID: 29091556
52. Loibl S, Schneeweiss A, and Huober J, et al (2022) Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response Ann Oncol 33(11) 1149–1158 https://doi.org/10.1016/j.annonc.2022.07.1940 PMID: 35961599
53. Schmid P, Cortes J, and Dent R, et al (2022) Event-free survival with pembrolizumab in early triple-negative breast cancer N Engl J Med 386(6) 556–567 https://doi.org/10.1056/NEJMoa2112651 PMID: 35139274
54. Tung N and Garber JE (2022) PARP inhibition in breast cancer: progress made and future hopes NPJ Breast Cancer 8 47 https://doi.org/10.1038/s41523-022-00411-3 PMID: 35396508 PMCID: 8993852
55. Johnston SRD, Harbeck N, and Hegg R, et al (2020) Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol 38(34) 3987–3998 https://doi.org/10.1200/JCO.20.02514 PMID: 32954927 PMCID: 7768339
56. Mayer EL, DuReck AC, and Martin M, et al (2021) Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study Lancet Oncol 22(2) 212–222 https://doi.org/10.1016/S1470-2045(20)30642-2 PMID: 33460574
57. Loibl S, Marmé F, and Martin M, et al (2021) Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer – the penelope-B trial J Clin Oncol 39(14) 1518–1530 https://doi.org/10.1200/JCO.20.03639 PMID: 33793299
58. National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High Risk Assessment: Breast, Ovarian and Pancreatic. Version 2.2023 (Plymouth Meeting) [https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf] Date accessed: 09/01/23
59. SIOG (2017) SIOG 2017 – abstract submission – oral presentations J Geriatr Oncol 8 S17–S37 https://doi.org/10.1016/S1879-4068(17)30224-2
60. Dockery LE, Tew WP, and Ding K, et al (2017) Tolerance and toxicity of the PARP inhibitor olaparib in older women with epithelial ovarian cancer Gynecol Oncol 147(3) 509–513 https://doi.org/10.1016/j.ygyno.2017.10.007 PMID: 29037805
61. Moore KN and Birrer MJ (2018) Administration of the tablet formulation of olaparib in patients with ovarian cancer: practical guidance and expectations Oncologist 23(6) 697–703 https://doi.org/10.1634/theoncologist.2017-0485 PMID: 29593098 PMCID: 6067940
62. Gelmon KA, Walker GP, and Fisher GV, et al (2018) LUCY: a phase IIIb, real-world study of olaparib in HER2-negative metastatic breast cancer patients with a BRCA mutation [abstract] Ann Oncol 29 viii120 https://doi.org/10.1093/annonc/mdy272.355
63. Gruber JJ, Afghahi A, and Timms K, et al (2022) A phase II study of talazoparib monotherapy in patients with wild-type BRCA1 and BRCA2 with a mutation in other homologous recombination genes Nat Cancer 3(10) 1181–1191 https://doi.org/10.1038/s43018-022-00439-1 PMID: 36253484 PMCID: 9586861
64. Tung NM, Robson ME, and Ventz S, et al (2020) TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes J Clin Oncol 38(36) 4274–4282 https://doi.org/10.1200/JCO.20.02151 PMID: 33119476
65. Ray-Coquard I, Pautier P, and Pignata S, et al (2019) Olaparib plus bevacizumab as first-line maintenance in ovarian cancer N Engl J Med 381(25) 2416–2428 https://doi.org/10.1056/NEJMoa1911361 PMID: 31851799






