Body composition, energy expenditure and caloric intake among breast cancer patients at a teaching hospital in Nigeria—a cross sectional study
Ogochukwu O Izuegbuna1, Toyin Sodiq2, Hannah O Olawumi1, Samuel A Olatoke3 and Olayide Agodirin3
1Department of Haematology and Blood Transfusion, University of Ilorin Teaching Hospital, Ilorin 241102, Nigeria
2Dietetics Unit, University of Ilorin Teaching Hospital, Ilorin 241102, Nigeria
3Department of Surgery, University of Ilorin Teaching Hospital, Ilorin 241102, Nigeria
Abstract
Objective: This cross-sectional study was conducted on the associations between body composition, energy expenditure and caloric intake among 45 Nigerian breast cancer patients.
Methods: Forty-five Nigerian breast cancer patients were measured and analysed for their body composition, energy expenditure and caloric intake. Statistical analyses included a chi-square test, Student’s t-test, paired t-test, Spearman correlation and linear regression using Statistical Package for the Social Sciences 23.0.
Results: The body fat indices (body mass index (BMI), fat mass index (FMI), and body fats percentage) show that more than 50% of breast cancer patients were either overweight or obese. The Spearman correlation showed that fat-free mass (FFM) was the most strongly correlated with energy expenditure (r = 0.84). BMI and (FMI – fat mass in relation to height) were significantly correlated with the Harris–Benedict equation for energy expenditure (p < 0.001; p = 0.002), but they were not correlated significantly with the Karnofsky performance status. A paired t-test showed that caloric intake was significantly higher than total energy expenditure (p < 0.001). FFM was the best predictor of resting energy expenditure (REE).
Conclusion: In conclusion, FFM remains the best predictor of REE. High body mass and high caloric intake indicate the need for support from nutritional programmes.
Keywords: body composition, energy expenditure, breast cancer, fat-free mass, dietary recall
Correspondence to: Ogochukwu O Izuegbuna
Email: Ogoizu@gmail.com
Published: 04/09/2023
Received: 03/05/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Breast cancer is the most common female malignancy, and it accounted for about 30% of all cancer diagnoses in women in 2019 [1]. In Nigeria, it was estimated to be responsible for about 11,500 deaths in 2018 with an age-standardised rate of 18.8/100,000 [2]. The mortality rate of breast cancer has dipped over the past three decades in the developed world [1], but unfortunately, mortality rates are disproportionately high especially in sub-Saharan Africa [3, 4]; and with Nigeria, Africa’s most populous country having the highest mortality rate [5]. Genetic mutations are known to account for only a small percentage of breast cancer, while environmental factors play a major role in association with other factors. Due to its growing public health effects, the identification of these environmental factors as well as a screening of at-risk populations is important.
It is postulated that such environmental factors as diet and dietary intake, body composition e.g., body mass index (BMI), reduced metabolic rate and reduced physical activity are associated with breast cancer risk [6–9]. Women with higher BMI have been found to have a higher risk of mortality from breast cancer [10, 11], while animal models have provided evidence that calorie restriction can inhibit mammary tumour development [12]. An increase in dietary intake and body weight has been observed in female rats exposed to exercise training, showing an association between energy intake, expenditure and body size [13]. While BMI has long been regarded as the surrogate marker for body composition, it does not reflect true body ‘fatness’ and cannot discriminate between fat mass (FM) and lean mass [14]. This limits its usefulness especially concerning energy expenditure as fat-free mass (FFM) is known as the single best predictor of resting energy expenditure (REE) [15].
VanItallie et al [16] suggested the use of fat mass index (FMI) and fat-free mass index (FFMI) for a better assessment of anthropometric measurement, according to body compartments, by calculation that considers the amount of FM and FFM. The units are also expressed the same as for BMI. With their use, four types of scenarios can be identified: low FFMI and high FMI, which corresponds to obesity; low FFMI and low FMI, to leanness; high FFMI and low FMI, to muscle hypertrophy; and high FFMI and high FMI, which correspond to combined excess of FFMI and FMI. However, their reference range is not yet a consensus in scientific works of literature.
On the other hand, REE (which is the largest component of total energy expenditure (TEE)) is known to be variable in cancer, and it is influenced by several factors including body composition [17]. Furthermore, reduced energy expenditure plays an important role in the development of obesity by decreasing REE, energy activity, diet-induced thermogenesis or a combination of all of these components [14]. Thus, an increase in caloric intake with energy expenditure as well as body composition may be a risk factor for breast cancer. The objective of this study was to evaluate the body composition and REE of breast cancer patients as well as assess the association between dietary intake, as a function of energetic expenditure and energy requirements.
Materials and methods
This cross-sectional study was conducted at the University of Ilorin Teaching Hospital, Ilorin, from January 2018 to June 2018. Ethical approval was obtained from the Hospital’s Ethical Research Committee (ERC PAN/2015/12/1483). The study group comprised 45 histologically diagnosed female breast cancer patients who are >18 years old. Informed consent was obtained from each participant. The exclusion criteria included patients who were pregnant/breastfeeding, diagnosed with any other cancer, uncontrolled chronic comorbidities such as hypo/hyperthyroidism, on anti-hypertensive, on lipid-lowering drugs (HMG CoA reductase inhibitors (statins), bile acid sequestrants, nicotinic acid, fibric acids), on hormonal medications, with a Karnofsky performance status less than 50%, hospitalised or bedridden and patients with apparent nutritional impairment were excluded from this study. The patients were staged clinically according to the tumour, node and metastasis classification.
Height/weight
The participants’ weight was measured in kilograms (kg) using a bathroom scale with participants wearing minimal clothing and no shoes. Height was measured in meters using a stadiometer with the participants standing erect, bare-footed and looking straight ahead.
Body composition
The BMI was derived as weight divided by height squared expressed as kg/m2. A proprietary algorithm for calculating body fat percentage (BF%) was used. The equation of Deurenberg et al [18] was used to calculate the BF%. The FM was derived as a product of the BF% and body weight. FFM was determined through the formula: weight in kg × (1 − (BF%/100)). FMI and FFMI were determined by dividing the FM and the FFM by the square of the height as proposed by VanItallie et al [16]. The BMI was categorised as 18.5–24.9 kg/m2 as normal, 25–29.9 kg/m2 as overweight and >30 kg/m2 as obese,
while the BF% was classified as <23% underfat, 23%–33% healthy, 33%–39% overweight, 40% and above as obese. The categorisation of the FMI was <5 as underweight, 5–9 as normal, 9–13 as overweight and >13 as obese, while FFMI was <18 skinny, 18–20 average, 20–22 good and >22 excellent.
Energy expenditure
The REE predictive equation used in this study was the Harris–Benedict equation (HBE) [19]. The TEE was estimated by multiplying the REE calculated from HBE with the activity factor (AF) and the injury factor (IF).
TEE (from HBE) = REE (HBE) × AF × IF where AF = 1.3 and IF = 1.1
The energy requirement was further estimated using the 25–30 kcal/kg/day formula.
Dietary intake
Because dietary intake is a key component of energy balance, dietary intake was assessed using the 48 hours of dietary recall. The type and amount of food that the patient consumed in the last 48 hours were asked by a trained nutritionist. All recalls were analysed with the Nigerian Food Composition table version 1.0 [20].
The physical and functional ability were determined using the Karnofsky performance status scale.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences version 23.0. The descriptive data were given as mean ± SD. A correlation analysis was conducted to see the correlation between the parameters using the Spearman correlation analysis. The R-values were obtained to observe the strength and direction of the correlation. A paired T-test was performed to compare the mean difference between the 48 hours of dietary recall and TEE. Predictors of REE were evaluated using multiple linear regression analysis with REE as the dependent variable and body composition as the independent variables.
Results
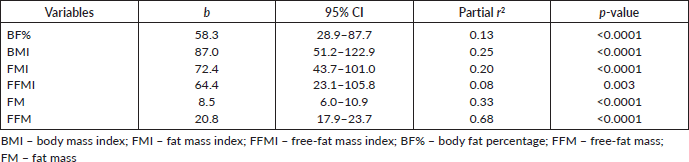
A total of 45 female breast cancer patients were included in this study. The mean patient age was 49.3 ± 14.8 years. The anthropometric characteristics and predicted energy requirement of the participants are presented in Table 1. The predicted REE which was the HBE positively correlated significantly with all components of body composition. Additionally, the ratios of HBE/FFM and HBE/BW were negatively correlated significantly as shown in Table 2. While BMI and FMI were significantly correlated with HBE, they were not correlated significantly with the Karnofsky performance status as shown in Figures 1 and 2.
Table 3 shows the energy requirements of the study with their daily caloric intake as well as the expected intake using a fixed amount of 25–30 kcal/kg of BW. The 48 hours recall showed a difference of 263 kcal compared to the TEE derived from the HB equation multiplied by the activity and the IF. Using the fixed amount of 25–30 kcal/kg of BW (i.e., 29 kcal/kg) a minor difference of 61 kcal was underestimated. Furthermore, the fixed amount of 25–30 kcal/kg of BW was more consistent with the TEE as there was no significant difference between both values.
The multiple linear regression analysis of REE was used to relate REE to the body composition variables. FFM was the single best predictor of REE among female Nigerian breast cancer patients in this study as shown in Table 4.
Table 1. Socio-demographic and anthropometric characteristics of study group.

Table 2. Spearman correlation of energy expenditures and body composition in breast cancer patients.
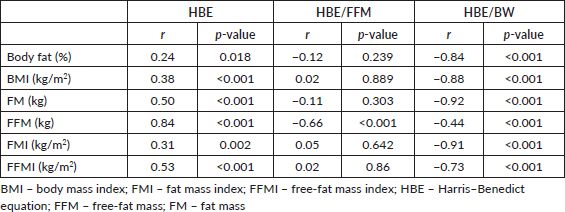
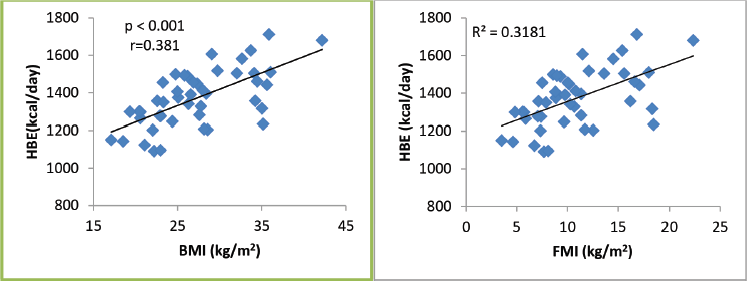
Figure 1. REE as related to BMI and FMI in 45 breast cancer patients. All correlations are significant.
Figure 2. Karnofsky performance status as related to BMI, FMI and FFMI in 45 breast cancer patients. Only the FFMI correlation is significant.
Table 3. TEE in women with breast cancer (n = 45).
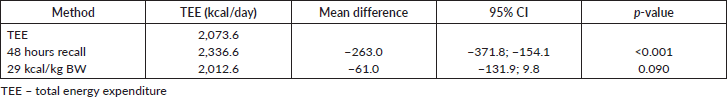
Table 4. Multiple linear regression analysis with the REE as a dependent variable.
Discussion
This study was embarked on to observe the body composition as well as the energy expenditure and its predictors among Nigerian breast cancer patients which have not been reported before now. The mean age (49.3 ± 14.8 years) of the study sample indicates breast cancer continues to be a disease of the middle-aged and younger age group (<40 years) in this part of the world [21].
The association between body fat and cancer risk has been noted in different studies [22, 23]. Traditionally, body fats are determined through the BMI which is known as a poor differentiator of lean mass and FM. On the other hand, FMI is a better marker of adiposity and obesity than BMI [24], although this was in Mexican Americans. However, BMI is strongly correlated with FMI and body fats according to a study carried out by Jeong et al [25], though such issues as body composition are linked to race and ethnicity [26]. Like in previous studies [27, 28], our study also showed that BMI is strongly correlated to FMI and body fats. However, while there is a similarity between BMI and FMI from our data, body fats estimated more people as being obese than BMI, the clinical significance is undetermined.
Body fat distribution is a well-known predictor of cardiovascular disease [29], some cancers [30, 31] and mortality [32, 33], thus accurate estimation of body fats is important in the management of obesity-related conditions. Equally, a meta-analysis of 221 datasets showed that the risk ratio for breast cancer incidence increased by 1.12 for every 5 kg/m2 increase in BMI for postmenopausal women [34]. In lieu of the aforementioned risks, our breast cancer patients in this study may need a weight management plan to prevent some morbidities.
The REE of women with breast cancer was determined using the HBE, which has been shown to give a higher value of REE compared to indirect calorimetry [35]. However, HBE and REE/BW were significantly correlated to the different body compositions showing that body compositions contribute to REE.
Individuals with cancer experience significant changes in body composition and REE with varying factors such as oral intake, physical activity, inflammatory processes, etc. REE is noted to be a result of metabolic activities within body tissues and organs especially organs like the liver and lungs. Elevated REE can potentially promote weight loss which can produce suboptimal clinical outcomes and increase morbidity and mortality risk. A relationship between REE, cachexia and survival were observed by Vazeille et al [36]. They also found that hypermetabolism, but not hypometabolism, was associated with reduced survival in metastatic cancer. Our study, however, did not evaluate survival with respect to levels of energy expenditure. It, however, showed that energy expenditure is significantly positively correlated with the various body composition indices with FFM having the strongest correlation as shown in Table 2. While tumour burden has been proposed to affect energy expenditure, our study did not show any significant correlation between REE and stage, tumour size and lymph node status. It, however, was significantly correlated with performance status.
The role of energy intake on breast cancer risk has been studied in both experimental and observational studies. According to the International Agency for Research on Cancer, there is sufficient evidence from experimental animal studies indicating that limiting body-weight gain by caloric restriction has a preventive effect on cancer of the mammary gland [30]. Energy intake, energy expenditure and body composition are three factors that are known to determine energy balance, and in turn, affect several biological pathways involved in cancer initiation. Caloric restriction is associated with decreased circulating levels of growth factors, anabolic hormones and cytokines which in turn lead to a reduction in growth factor signalling, vascular perturbations and inflammation. On the other hand, high energy intake has not been consistently associated with increased breast cancer risk in human studies [37]. Thus, a positive energy balance characterised by excess energy intake (nutritional intake) with little physical activity over a prolonged period, results in excess body fat and, consequently, contributes to increased postmenopausal breast cancer risk.
This present study also analysed the association between relative energy intake (dietary recall), and breast cancer with TEE and expected caloric estimate (25–30 kcal/kg BW). Our result shows that there is a positive energy balance with energy expenditure, and a caloric estimate of 29 kcal/kg BW is sufficient to meet daily requirements. This positive energy balance is also statistically significant thus the excess body fats in our subjects may be a result of nutritional intake. Lope et al [38] in the EPIGEICAM study, were able to show that high energy intake increases breast cancer risk, and caloric restriction could be protective.
While excess fats are a breast cancer risk, especially in postmenopausal women, it should be noted that FM contributes to REE. FFM is the single best predictor of REE [15] as also noted in our study; both FFM and FM were predictors of REE. Interestingly while FFM has the strongest positive correlation with REE in our study (r = 0.84; p < 0.01), it has the weakest negative correlation with HBE/kg of BW, while FM has the strongest negative correlation with HBE/kg of BW (r = −0.92; p < 0.01). In healthy adults, a decrease in FFM explains approximately 60% of the decrease in REE observed with age [39]. However, FFM is known to be a heterogeneous body composition compartment that comprises tissues with differing metabolic activity thus in metastatic cancer, it is unable to distinguish between changes in tissue, organs and tumours. A highly metabolic organ like the liver will be expected to have a greater REE impact in metastatic disease to the liver than on other less metabolic organs. Thus, two individuals with the same disease stage may not necessarily have the same REE. In our study, overall REE decreased in patients with stage III disease and increased in patients with stage IV disease (data not shown), which could be suggestive of extra tumour burden. This was also reported by Purcell et al [17] in colorectal cancer patients. In the correlation test, both BMI and FMI were positively correlated with REE (Figure 1). This may be because obese people tend to have increased FFM to probably support the FM. Nielsen et al [15] also reported the predictive significance of FFM and FM in REE, although their study did not include breast cancer patients.
In summary, our study shows that excess body fats among other variables in our breast cancer patients may explain their breast cancer status. The excess body fats may also be a result of increased caloric intake as derived from their 48 hours of dietary recall. This is the first study in Nigerian breast cancer patients that examine their body composition in association with energy expenditure as well as caloric intake. It thus brings to the fore the need for public health awareness of overweight and obesity and its association with the increasing numbers of breast cancer cases in Nigerian society. The increased consumption of fast foods in the country [40] also contributes to the obesity epidemic and high caloric intake. It is reported that what contributes to obesity and related diseases is not the number of calories in specific foods but rather the amount and type of carbohydrates these foods contain. Foods that are highly processed, and mostly comprised of rapidly absorbable sugars and starches, may be of greatest concern. Such carbohydrates may induce neurohormonal alterations that lead to ‘eating more’ and ‘moving less’ [38]. Individual and public health strategies should thus be implemented to help curb it. Caloric restriction may play a beneficial role in health and well-being. Finally, simple measures of body fat distribution and body composition have been found to be useful tools for identifying Black women with a higher risk of death after a breast cancer diagnosis. Bandera et al [41] very recently reported that higher adiposity was associated with higher all-cause and breast cancer-specific mortality among Black breast cancer survivors. Weight gain rather than weight loss is reported to occur in breast cancer patients especially during chemotherapy [42]. It is postulated that this weight gain is related to changes in caloric intake, reduced physical activity and metabolic rate and hormonal changes [6]. This weight gain increases the likelihood of complications, increases the risk of recurrence and results in a low survival rate [43].
Based on the results of this study, we propose that a nutrition planning/weight control programme and physical workout plan should be part of the supportive care of Nigerian breast cancer patients.
Limitations
Several limitations should be considered when interpreting our findings. First, it is a single-centre, cross-sectional study. A multi-centre prospective study would need to be carried out to further confirm our findings. Second, the small number of our study group may increase bias. A larger multi-centre study group may be needed to validate our results. Third, due to cost and limited expertise, most patients did not carry out immunohistochemistry tests such as oestrogen, progesterone receptors, HER-2 and Ki-67, so they could not be reported. While indirect calorimetry is the gold standard for measuring REE,
due to non-availability at our centre, logistics and its impractical nature for clinical use could not be assessed for this study. Indirect calorimetry is expensive, time-consuming and requires trained technicians, and to the best of our knowledge is not available in our country so the need to use a predictive equation. Body composition especially body fats could not be assessed by a bioelectrical impedance analyser or dual-energy X-ray absorptiometry due to non-availability at our centre, we, therefore, used anthropometric equations to derive our body composition. The self-reported dietary recall is subject to bias based on the extent of recall of diets taken during the period examined.
This study equally has its strength. First, this is the first study to examine the body composition among breast cancer patients in Nigeria, most importantly looking at other indices other than BMI e.g., FMI and FFMI. Second, to our knowledge, this is the first study on energy expenditure in Nigerian breast cancer patients and its association with daily energy requirements (25–30 kcal/kg BW) and excessive caloric intake. Third, we believe our results from this study may spur other research in the study of body composition, energy expenditure and well-being of the Nigerian breast cancer patient.
Conclusion
In conclusion, the breast cancer patients in this study have excess fat. The caloric intake of breast cancer patients was significantly higher than their TEE. The importance of this is the need to set up a nutritional programme for breast cancer patients and monitor their calorie intake. This can help in the reduction of excess fats and help promote healthy lifestyles.
Acknowledgments
The authors would like to thank all the patients who have generously given their time to be involved in this study and the resident doctors of the surgical oncology clinics especially Dr Fashiku and Dr Aremu for their contribution towards patient selection.
Conflicts of interest
No potential conflict of interest was reported by the authors.
Funding
The researchers did not receive any external funding for this work.
Data availability statement
The data that support the findings of this study are available from the corresponding author, (OI), upon reasonable request.
Author contributions
OI was involved in the design of the study, data analysis and writing of the first draft manuscript. TS was involved in data analysis, study coordination and correction of the draft. SO, HO and OA were involved in the design of the study, coordination and corrections of the draft manuscript. All authors read and approved the final manuscript.
References
1. Siegel RL, Miller KD, and Jemal A (2019) Cancer statistics, 2019 CA Cancer J Clin 69(1) 7–34 https://doi.org/10.3322/caac.21551 PMID: 30620402
2. Abdus-salam AA, Ogunnorin BO, and Adenipekun AA, et al (2019) Percentage body fat in breast cancer patients at the University College Hospital, Ibadan: a case-control study Niger J Med 28(1) 237–247 https://doi.org/10.4103/1115-2613.278592
3. Black E and Richmond R (2019) Improving early detection of breast cancer in sub-Saharan Africa: why mammography may not be the way forward Global Health 15(1) 3 https://doi.org/10.1186/s12992-018-0446-6 PMID: 30621753 PMCID: 6325810
4. Youlden DR, Cramb SM, and Dunn NAM, et al (2012) The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality Cancer Epidemiol 36 237–248 https://doi.org/10.1016/j.canep.2012.02.007 PMID: 22459198
5. Azubuike SO, Muirhead C, and Hayes L, et al (2018) Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review World J Surg Oncol 16(1) 63 https://doi.org/10.1186/s12957-018-1345-2 PMID: 29566711 PMCID: 5863808
6. Laurence G, Ursula GK, and Sabine BM, et al (2006) Body composition changes in breast cancer patients during curative radiation therapy e-SPEN Eur e-J Clin Nutr Metab 1 2–8 https://doi.org/10.1016/j.eclnm.2006.07.005
7. James FR, Wootton S, and Jackson A, et al (2015) Obesity in breast cancer – what is the risk factor? Eur J Cancer 51(6) 705–720 https://doi.org/10.1016/j.ejca.2015.01.057 PMID: 25747851
8. Pan SY and DesMeules M (2009) Energy intake, physical activity, energy balance, and cancer: epidemiologic evidence Methods Mol Biol 472 191–215 https://doi.org/10.1007/978-1-60327-492-0_8
9. Zhang FF, Esther John, and Knight JA, et al (2013) Total energy intake and breast cancer risk in sisters: the breast cancer family registry Breast Cancer Res Treat 137(2) 541–551 https://doi.org/10.1007/s10549-012-2342-8
10. Calle EE, Rodriguez C, and Walker-Thurmond K, et al (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults N Engl J Med 348 1625–1638 https://doi.org/10.1056/NEJMoa021423 PMID: 12711737
11. Caan BJ, Cespedes Feliciano EM, and Kroenke CH (2018) The importance of body composition in explaining the overweight paradox in cancer Cancer Res 78 1906–1912 https://doi.org/10.1158/0008-5472.CAN-17-3287 PMID: 29654153 PMCID: 5901895
12. Dirx MJ, Zeegers MP, and Dagnelie PC, et al (2003) Energy restriction and the risk of spontaneous mammary tumors in mice: a meta-analysis Int J Cancer 106 766–770 https://doi.org/10.1002/ijc.11277 PMID: 12866038
13. Pitts GC (1984) Body composition in the rat: interactions of exercise, age, sex, and diet Am J Physiol 246 R495–R501 PMID: 6720924
14. Fonseca DC, Sala P, and Ferreira B, et al (2018) Bodyweight control and energy expenditure Clin Nutr Exp 20 55–59 https://doi.org/10.1016/j.yclnex.2018.04.001
15. Nielsen S, Hensrud D, and Romanski S, et al (2000) Body composition and resting energy expenditure in humans: role of fat, fat-free mass and extracellular fluid Int J Obes 24 1153–1157 https://doi.org/10.1038/sj.ijo.0801317
16. VanItallie TB, Yang UM, and Heymsfield SB, et al (1990) Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status Am J Clin Nutr 52 953–959 https://doi.org/10.1093/ajcn/52.6.953 PMID: 2239792
17. Purcell SA, Wallengren O, and Baracos VE, et al (2020) Determinants of change in resting energy expenditure in patients with stage III/IV colorectal cancer Clin Nutr 39 134–140 https://doi.org/10.1016/j.clnu.2018.12.038
18. Deurenberg P, Weststrate JA, and Seidell JC (1991) Body mass index as a measure of body fatness: age- and sex-specific prediction formulas Br J Nutr 65 105–114 https://doi.org/10.1079/BJN19910073 PMID: 2043597
19. Khor SM and Mohd BB (2011) Assessing the resting energy expenditure of cancer patients in the Penang General Hospital Malays J Nutr17(1) 43–53 PMID: 22135864
20. Nigerian Food Composition Table. Version 1.0, Sept. 2017 (2017) (Pretoria: University of Pretoria)
21. Ntekim A, Oluwasanu M, and Odukoya O (2022) Breast cancer in adolescents and young adults less than 40 years of age in Nigeria: a retrospective analysis Int J reast Cancer 2022 9943247 https://doi.org/10.1155/2022/9943247
22. Iyengar NM, Arthur R, and Manson JE, et al (2019) Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a randomized clinical trial and observational study JAMA Oncol 5(2) 155–163 https://doi.org/10.1001/jamaoncol.2018.5327 PMCID: 6439554
23. Rohan TE, Heo M, and Choi L, et al (2013) Body fat and breast cancer risk in postmenopausal women: a longitudinal study J Cancer Epidemiol 2013 754–815 https://doi.org/10.1155/2013/754815
24. Peltz G, Aguirre MT, and Sanderson M, et al (2010) The role of fat mass index in determining obesity Am J Hum Biol 22(5) 639–647 https://doi.org/10.1002/ajhb.21056 PMID: 20737611 PMCID: 2929934
25. Jeong SM, Lee DH, and Rezende LFM, et al (2023) Different correlation of body mass index with body fatness and obesity-related biomarker according to age, sex and race-ethnicity Sci Rep 13 3472
26. Akinyemiju T, Jones K, and Gupta A, et al (2021) H3 Africa Kidney Research Network; Daramola A. Association of body composition with odds of breast cancer by molecular subtype: analysis of the mechanisms for established and novel risk factors for breast cancer in Nigerian women (MEND) study BMC Cancer 21(1) 1051 https://doi.org/10.1186/s12885-021-08775-8 PMID: 34563146 PMCID: 8464100
27. Bouchard C (2007) BMI, fat mass, abdominal adiposity and visceral fat: where is the “beef?” Int J Obes (Lond) 31(10) 1552–1553 https://doi.org/10.1038/sj.ijo.0803653 PMID: 17549092
28. Barreira TV, Harrington DM, and Staiano AE, et al (2011) Body adiposity index, body mass index, and body fat in white and black adults JAMA 306(8) 828–830 https://doi.org/10.1001/jama.2011.1189 PMID: 21862743 PMCID: 3951848
29. Zeng Q, Dong SY, and Sun XN, et al (2012) Percent body fat is a better predictor of cardiovascular risk factors than body mass index Braz J Med Biol Res 45(7) 591–600 https://doi.org/10.1590/S0100-879X2012007500059 PMID: 22510779 PMCID: 3854278
30. Lauby-Secretan B, Scoccianti C, and Loomis D, et al (2016) Body fatness and cancer--viewpoint of the IARC Working Group N Engl J Med 375(8) 794–798 https://doi.org/10.1056/NEJMsr1606602 PMID: 27557308 PMCID: 6754861
31. Barberio AM, Alareeki A, and Viner B, et al (2019) Central body fatness is a stronger predictor of cancer risk than overall body size Nat Commun 10(1) 383 https://doi.org/10.1038/s41467-018-08159-w PMCID: 6342989
32. Heitmann B, Erikson H, and Ellsinger BM, et al (2000) Mortality associated with body fat, fat-free mass and body mass index among 60-year-old Swedish men—a 22-year follow-up. The study of men born in 1913 Int J Obes 24 33–37 https://doi.org/10.1038/sj.ijo.0801082
33. Padwal R, Leslie WD, and Lix LM, et al (2016) Relationship among body fat percentage, body mass index, and all-cause mortality: a cohort study Ann Intern Med 164(8) 532–541 https://doi.org/10.7326/M15-1181 PMID: 26954388
34. Renehan AG, Tyson M, and Egger M, et al (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies Lancet 371(9612) 569–578 https://doi.org/10.1016/S0140-6736(08)60269-X PMID: 18280327
35. Zuconi CP, Ceolin Alves AL, and Toulson Davisson Correia MI (2015) Energy expenditure in women with breast cancer Nutrition 31(4) 556–559 https://doi.org/10.1016/j.nut.2014.05.009
36. Vazeille C, Jouinot A, and Durand JP, et al (2017) Relation between hypermetabolism, cachexia, and survival in cancer patients: a prospective study in 390 cancer patients before initiation of anticancer therapy Am J Clin Nutr 105 1139–1147 https://doi.org/10.3945/ajcn.116.140434 PMID: 28356274
37. WCRF/AICR (2017) Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Breast Cancer (World Cancer Research Fund/American Institute for Cancer Research) http://www.aicr.org/continuous-update-project/reports/breast-cancerreport-2017.pdf
38. Lope V, Martín M, and Castelló A, et al (2019) Overeating, caloric restriction and breast cancer risk by pathologic subtype: the EPIGEICAM study Sci Rep 9(1) 3904 https://doi.org/10.1038/s41598-019-39346-4 PMID: 30846706 PMCID: 6405854
39. Geisler C, Braun W, and Pourhassan M, et al (2016) Age-dependent changes in resting energy expenditure (REE): insights from detailed body composition analysis in normal and overweight healthy Caucasians Nutrients 8(6) 322 https://doi.org/10.3390/nu8060322 PMID: 27258302 PMCID: 4924163
40. Otemuyiwa IO and Adewusi SRA (2012) Effects of fast food consumption on nutrient intake among Nigerian Elite in Lagos, Nigeria Int J Health Nutr 3(2) 12–19
41. Bandera EV, Qin B, and Lin Y, et al (2021) Association of body mass index, central obesity, and body composition with mortality among black breast cancer survivors JAMA Oncol 7(8) 1–10 https://doi.org/10.1001/jamaoncol.2021.1499 PMID: 34086040 PMCID: 8377573
42. Jung GH, Kim JH, and Chung MS (2020) Changes in weight, body composition, and physical activity among patients with breast cancer under adjuvant chemotherapy Eur J Oncol Nurs 44 101680 https://doi.org/10.1016/j.ejon.2019.101680
43. Bradshaw PT, Ibrahim JG, and Stevens J, et al (2012) Post diagnosis change in bodyweight and survival after breast cancer diagnosis Epidemiology 23 320–327 https://doi.org/10.1097/EDE.0b013e31824596a1 PMID: 22317813 PMCID: 3586208






