Comparison of the diagnostic accuracy of HE4 with CA125 and validation of the ROMA index in differentiating malignant and benign epithelial ovarian tumours among patients in Lagos, Nigeria
Khadijah Adebisi Shittu1, Kabiru Afolarin Rabiu2, Oluwarotimi Ireti Akinola2, Saheed Bolaji Ahmed3 and Adeniyi Abiodun Adewunmi2
1Department of Obstetrics and Gynaecology, Lagos State University Teaching Hospital, Ikeja 100271, Lagos State, Nigeria
2Department of Obstetrics and Gynaecology, Lagos State University College of Medicine, Ikeja 100271, Lagos State, Nigeria
3Department of Community Medicine, University College Hospital, Ibadan 200212, Oyo State, Nigeria
Abstract
This prospective cross-sectional study compared the diagnostic accuracy of human epididymal protein 4 (HE4) with cancer antigen 125 (CA 125) and validates the risk of malignancy algorithm (ROMA) in differentiating benign from malignant ovarian tumours. The study population included 112 women with an ultrasound diagnosis of an adnexal mass, out of whom 49 women had a diagnosis of ovarian cancer following optimal debulking surgery, and 63 women had a diagnosis of benign ovarian tumour. All diagnosis was confirmed by histopathological analysis. Serum HE4 and CA 125 were assessed preoperatively according to the manufacturer’s instructions. CA 125 and HE4 cut-offs were 35 U/mL and 70 pM/L respectively. Serum CA 125 and HE4 were significantly higher in ovarian cancer patients compared to those with benign ovarian tumours (p < 0.001 and p < 0.000, respectively). HE4 had higher sensitivity (77.5% versus 69.4%), specificity (96.8% versus 82.5%), positive predictive value (PPV) (95% versus 75.6%) and negative predictive value (84.7% versus 77.6%) than CA 125. When the two markers were combined with each other in the ROMA index, Specificity and PPV reached 100% each. In the receiver operative characteristics analysis, the area under the curve for CA 125 was 0.679 (95% CI 0.566–0.791, p = 0.001), HE4 was 0.845 (95% CI 0.760–0.930, p = 0.000) and ROMA was 0.902 (95% CI 0.851–0.998, p = 0.000) and this was statistically significant (p < 0.001). Conclusively, HE4 performed better than CA 125 in differentiating benign from malignant ovarian tumours and the combination of the two biomarkers improved the detection of ovarian cancer. In addition, the cut off values corresponding to the highest accuracy for CA 125 and HE4 were 126 U/mL and 42 pM/L respectively in this study. The value for CA 125 is much higher while that of HE4 is much lower than the reference values obtained predominantly from the white population.
Keywords: human epididymis protein 4, cancer antigen 125, risk of malignancy algorithm, ovarian cancer
Correspondence to: Kabiru Afolarin Rabiu
Email: kabiru.rabiu@lasucom.edu.ng
Published: 05/07/2023
Received: 06/01/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ovarian cancer is the 8th most common cancer among women and accounts for 3.4% of all cancers in women in 2020 [1]. It is the third most common cancer of the female genital tract yet remains the leading cause of gynaecological malignancy-related mortality worldwide [1]. The incidence has been rising gradually with incidence expected to double by 2024 [2].
Diagnosis of ovarian cancer still poses a great challenge to clinicians because of the intra-abdominal disadvantaged position of the ovary. Also, symptoms are vague in the early stage: many patients present with gastrointestinal symptoms which causes a lot of misdiagnoses [3]. Consequently, most ovarian cancers remain clinically undetected until patients have developed late-stage disease and only a mere 25% of cancers are detected as stage I disease [4]. The development of an ovarian cancer-specific tumour marker for the early detection of the disease has the capacity to improve the survival rate.
The ovarian biomarker cancer antigen 125 (CA 125) has been extensively studied regarding ovarian cancer screening, detection and progression. First described in 1981 by Bast et al [5] by developing a monoclonal antibody against the antigen, a radioimmunoassay was developed in 1983 to detect serum levels using a threshold of 35 u/mL [6]. However, CA 125 as a biomarker has some limitations. It is not elevated in about half of early-stage ovarian cancers and is elevated in many benign gynaecological diseases such as benign ovarian tumours, pelvic inflammatory disease, endometriosis and even physiological conditions like pregnancy and menstruation. It may also be elevated in some medical conditions [7, 8]. Consequently, there have been reports that the sensitivity, specificity, and positive predictive values (PPV) of CA 125 are not very impressive. Moreover, these indices are much better in postmenopausal women than premenopausal women [8]. New biomarkers could therefore contribute to the accuracy of CA 125 and be useful as an adjunct to CA 125.
Human epididymis protein 4 (HE4) is a glycoprotein encoded by the whey acidic four-disulfide core domain protein 2 (WFDC2) gene. Studies demonstrated the presence of HE4 in the female genitourinary tract, the respiratory tract, the renal epithelium, and the salivary ducts. Despite its wide distribution, it is over-expressed only in pathological tissue, and it has been reported to demonstrate good sensitivity and specificity in detecting ovarian cancer [9].
The risk of ovarian malignancy algorithm (ROMA) takes into consideration the concentration of the two biomarkers and the patient's menopausal status to generate a score, which translates to a high or low likelihood of finding a malignancy based on established cutoffs [10]. Women with ROMA scores above the cutoff have an increased risk of ovarian cancer and should be referred to a gynaecological oncologist prior to surgery [10].
Previous studies done on the role of HE4 in discriminating benign from malignant ovarian tumours have reported conflicting reports [11–14]. Furthermore, these studies were notably done in the white population. Also, we do not know what constitutes normal and abnormal levels of HE4 in blacks. Hence, there is a need for more studies on the subject matter especially in the black population.
This study therefore aims to compare the diagnostic characteristics of HE4 and CA 125 in malignant and benign epithelial ovarian tumours, and to validate the ROMA index in discriminating epithelial ovarian cancer from benign ovarian tumours in Lagos, Nigeria.
Methods
Study design
This was a prospective cross-sectional study.
Study population
The study included 112 patients who presented with ovarian masses at the Lagos State University Teaching Hospital between March 1, 2020, and December 31, 2020. All patients underwent imaging by pelvic ultrasound scan to document the presence of an ovarian mass. Women with a previous history of bilateral oophorectomy, chemotherapy, radiotherapy, or hormonal therapy for other malignancies were excluded from the study.
Serum samples and marker assays
Phlebotomy was done before the commencement of any medications. 10 mL of venous blood samples were obtained from all the patients following an overnight fast before surgery. Thereafter, centrifugation was done at 2,500 rpm for 10 minutes. The serum was then withdrawn and frozen at −20°C until analysis. Serum samples collected from all the patients who met the inclusion criteria were analysed for both CA 125 and HE4.
CA 125 analysis was done on The Abbott Axsym system (Abbott Diagnostics Division, Chicago) based on Micro Particle Enzyme Immunoassay technology.
HE4 assay was done on the fully automated ARCHITECT instrument (Abbott Diagnostics Division, Chicago) based on Electro-Chemi Luminescent micro particle Immunoassay.
Cut-off values was set according to the indications of the manufacturer at 35 IU/L for CA 125 while for HE4 at ≤70 pmol/L.
ROMA is a predictive probability algorithm that classifies women with ovarian mass as being at high or low risk of ovarian cancer based on the combination of serum values of CA 125, HE4 and menopausal status.
ROMA classifies patients as being at a low or at a high risk for malignant disease using the following algorithms:
Premenopausal: predictive index (PI) = −12.0 + (2.38 × LN(HE4)) + (0.0626 × LN(CA 125))
Postmenopausal: PI = −8.09 + (1.04 × LN(HE4)) + (0.732 × LN(CA 125))
Predicted probability: (PP) = 100 × exp (PI)/(1 + exp (PI))
HE4 and CA 125 values were input into the ROMA calculator software with automatic calculation of the corresponding ROMA index [15]. According to the manufacturer’s insert, the following thresholds were selected for ROMA predictive probability:
Pre-menopausal women:
* PP ≥11.4% = high risk of finding ovarian cancer
* PP <11.4% = low risk of finding ovarian cancer
Post-menopausal women:
* PP ≥29.9% = high risk of finding ovarian cancer
* PP <29.9% = low risk of finding ovarian cancer
Patients
The patients recruited had surgical removal of the ovarian mass and diagnosis was confirmed from histological report of the removed specimen. Patients with suspected ovarian malignancy had staging laparotomy done. The surgeries were done by the gynaecology oncology team led by a consultant gynaecologist. The surgical procedures in benign cases included resection of the cyst or unilateral/bilateral oophorectomy, and in the malignant cases total abdominal hysterectomy, bilateral salpingo-oophorectomy, infracolic omentectomy and pelvic and para-aortic lymphadenectomy when indicated. Cytologic analysis of ascitic fluid or when absent of peritoneal washing was performed. Optimal debulking is defined as residual tumour less than or equal to 1 cm in maximum diameter. Tumour was staged according to 2009 International federation of gynaecology and obstetrics staging system and histologically defined according to WHO classification.
Data processing and analysis
Data was collated daily, checked for completeness and all errors corrected immediately. The data was entered and analysed using statistical package for social sciences version 22. Descriptive statistics, tables, charts and graphs were used to summarise variables. Uniformly distributed data were summarized using the mean and SD, while data that are not uniformly distributed were summarised using median and inter quartile range. Continuous variables were compared using the student T test. Categorical variables were compared using the chi square test. The cut off point for CA 125, HE4 and ROMA that will probably distinguish between benign and malignant ovarian tumours was determined using the area under the receiver operator characteristic (ROC). Sensitivity, specificity, PPV, negative predictive values (NPV) were also calculated. The level of statistical significance was set at 5%.
Ethical considerations
An ethical approval was obtained from the Health Research Ethics Committee of the Lagos State University Teaching Hospital before the recruitment of participants.
Results
Table 1 shows the socio-demographic and gynaecological characteristics of the study participants. Approximately 43% of the study participants were aged 40–59 years while only 15.4% of them were aged <20 years. The mean age was 42.54 ± 13.75 years. Fifty (44.6%) of them had skilled occupation while 45.5% had tertiary education. Of all the study participants, 14(12.5%) had a positive family history of breast cancer, while 11(9.8%) had a family history of ovarian cancer. Seventy-three (65.2%) of the participants were premenopausal while 39(34.8%) were post-menopausal. The modal parity group was 1–4 with a percentage of 42%.
The serum levels of HE4, CA 125 and calculated ROMA index were all significantly higher in the epithelial ovarian cancer group than those in the benign ovarian disease group and this was statistically significant (p-value = 0.000, 0.001 and 0.000, respectively). This is depicted in Table 2.
Table 3 shows the sensitivity, specificity, positive and NPV of CA 125, HE4 and ROMA index. HE4 had higher sensitivity and specificity than CA 125 (77.5% versus 69.4% and 96.8% versus 82.5%, respectively). Also, the PPV and NPV of HE4 were higher than CA 125 (95% versus 75.6% and 84.7% versus 77.6%, respectively). Specificity and PPV were increased to 100% each when the two markers were combined with each other in the ROMA index.
Table 4 and Figure 1 depicts the area under the curve (AUC) for the ROC of CA 125, HE4 and ROMA index in the diagnosis of epithelial ovarian cancer. The AUC for HE4 was 0.845 (95% CI 0.760–0.930, p = 0.000). This shows that it is a good test for differentiation of malignant from benign epithelial ovarian tumours, while that of CA 125: 0.679 (95% CI 0.566–0.791, p = 0.001) shows that it is a fair test for differentiation of malignant from benign epithelial ovarian tumours. Additionally, the AUC for the combination of the two serum markers (ROMA) was 0.902 (95% CI 0.851–0.998, p = 0.000) which shows that it is an excellent test for differentiation of malignant from benign epithelial ovarian tumours.
Table 5 shows the cut off values for CA 125, HE4 and ROMA index obtained from this study, above which an epithelial ovarian tumour is likely to be malignant.
Discussion
Ovarian cancer is an enigmatic disease characterised by late presentation and advanced disease. at presentation. Diagnosis of ovarian cancer still poses a great challenge to clinicians because of the intra-abdominal disadvantaged position of the ovary. Also, symptoms are vague and non-specific in the early stage of the disease, and it is often difficult to make a diagnosis of benign or malignant ovarian tumour pre-operatively [16].
Table 1. Distribution of socio-demographic and gynaecologic characteristics of the study participants.
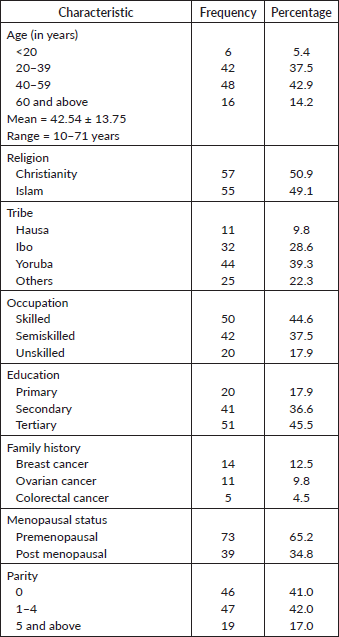
Table 2. Serum levels of HE4, CA125 and ROMA index among patients with benign and malignant ovarian tumours.

Table 3. Sensitivity, specificity, positive and NPV for CA125, HE4 and ROMA index.
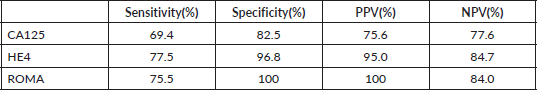
Table 4. Comparison of AUC from the ROC curve analysis for serum CA125, HE4 and ROMA levels.
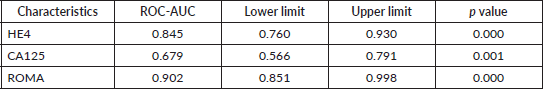
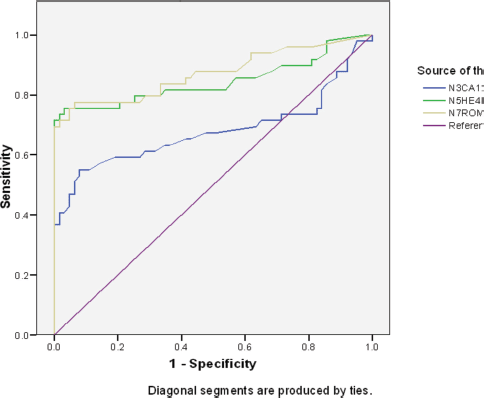
Figure 1. ROC curve of CA125, HE4 and ROMA index.
Table 5. Cut off points for the analytes for making a diagnosis of ovarian cancer.

CA 125 has been used universally in the diagnosis of ovarian cancer while HE4 and ROMA index have been tested mainly in the white population. Considering that what constitute normal findings in white population may be different from what obtains in blacks, this prospective cross-sectional study was therefore carried out to compare the accuracy of HE4 with CA 125, and the risk stratification tool: ROMA, to differentiate benign from malignant epithelial ovarian tumours prior to surgical intervention and therefore aid appropriate referrals.
This study found a significant difference between benign and malignant epithelial ovarian tumours with respect to HE4 and ROMA levels, hence, the study suggests a possible role for the use of HE4 and ROMA index as a diagnostic marker for detecting epithelial ovarian cancer.
This study revealed that serum HE4 levels were higher in the epithelial ovarian cancer group as compared with the benign ovarian disease group and this finding was statistically significant. This finding is consistent with the findings of a previous study by Montagnana et al [17] in Verona, Italy. In this prospective observational study, it was reported that the mean serum level of HE4 was significantly higher than those in the benign ovarian tumour group. several other studies also reported similar findings [10, 12, 13, 18].
In this study, HE4 had a higher sensitivity, specificity, PPV, and NPV when compared with CA 125. This finding agrees with that of Hamed et al [19] in Egypt who reported a similar higher sensitivity (90% versus 83.3%), specificity (95% versus 85%), PPV (93.1% versus 80.7%) and NPV (92.7% versus 87.2%) of HE4 when compared to CA 125. However, Zhang et al [20] in China and Pitta et al [21] in Brazil reported CA 125 to have a higher sensitivity, specificity, positive and NPV than HE4.
The limitation of the use of a single tumour marker in making a diagnosis of ovarian cancer has been addressed in several clinical scenarios, hence the need to switch to a multi-marker approach in clinical practice to achieve better diagnostic accuracy. Thus, when both tumour markers were combined in the ROMA predictive probability, higher specificity and PPV were obtained. Anastasi et al [22] in their study also reported a specificity and PPV of 100% for ROMA. Higher specificity means that it is likely to predict the absence of ovarian cancer better than either tumour marker alone. Improvement in PPV has the resultant effect of reducing inappropriate referrals and its associated cost and waste of time. It also reduces the number of midline laparotomies which is still the standard of care in suspected ovarian cancer, thereby affording patients the use of more cosmesis-appealing incisions.
The diagnostic accuracy of CA 125, HE4 and by extension the ROMA index in differentiating benign from malignant ovarian tumours were verified using the ROC analysis. The resultant AUC values of CA 125, HE4 and ROMA show that CA 125 is a fair test, HE4 is a good test while ROMA is an excellent test for distinguishing malignant from benign ovarian tumours. Thus, ROMA performed better than either tumour marker alone. This means that HE4 and ROMA improved the diagnosis of ovarian cancer when compared to the current standard in this environment, CA 125. This is similar to the results of Moore et al [23] who found that a combination of CA 125 and HE4 performed better than CA 125 alone. However, a study done by Gorp et al [24] in Belgium reported that neither HE4 nor ROMA performed better than CA 125 in detecting ovarian cancer. It is therefore highly desirable that ROMA be used to evaluate ovarian cancer pre-operatively, but in our environment which is a developing country with limited resources, even though ROMA is desirable, where patients cannot afford the two tests, HE4 alone may be used in place of CA 125 because it has a better diagnostic accuracy.
This study also aimed to find the cut off points for the tumour markers. The cut off values corresponding to the highest accuracy for CA 125, HE4 and ROMA were 126 U/mL, 42 pM/L and 16.7% respectively, although these values are different from what obtained in other studies [11, 23], they were established at the point on the ROC curve where the least false negative and false positive results existed. Probably, the difference is because of inherent racial differences in the population studied. Notably, significantly higher than the cut-off used in clinical practice is the cut-off value of CA 125 obtained from this study. This may be one of the reasons why there is a lot of misdiagnoses of ovarian cancer in our environment as CA 125 is the major tumour marker used in diagnosing epithelial ovarian cancer, and the reference value was obtained following studies done mainly in the white population [7, 13, 21, 25]. A lot of patients with CA 125 greater than 35 U/mL end up not having malignant disease after histological examination. However, it is difficult to generalise this finding amongst the Nigerian, and at large, the black population mainly because of the small sample size, and the uni-centre nature of this study, hence there may be needed for larger scale studies mainly in the black population to determine what constitute the cut off point for CA 125 in the diagnosis of ovarian cancer.
This study has some limitations, it is an institution-based study with a relatively small sample size which may not be representative of the general population, thus a larger scale multicentred study is required to further validate the findings of this study.
Conclusion
HE4 demonstrated better diagnostic accuracy than CA 125 as a tumour marker for differentiating benign from malignant ovarian tumours. It demonstrated better sensitivity, specificity, positive and NPV. HE4 improves the utility of CA 125 and the combination of the two biomarkers in the ROMA index further improved the diagnostic accuracy. The use of this combination may help in the discrimination of benign from malignant ovarian tumours as compared with the use of either tumour marker alone, thus facilitating appropriate referrals for definitive care. In addition, the cut off values corresponding to the highest accuracy for CA 125 and HE4 were 126 U/mL and 42 pM/L respectively in this study. The value for CA 125 is much higher while that of HE4 is much lower than the reference values obtained predominantly from the white population, probably due to inherent racial differences.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
None.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Huang J, Chan W, and Ngai C, et al (2022) Worldwide burden, risk factors, and temporal trends of ovarian cancer: a global study Cancers 14 2230 https://doi.org/10.3390/cancers14092230 PMID: 35565359 PMCID: 9102475
3. Rabiu KA, Akinola OI, and Adewunmi AA, et al (2013) Delays in presentation and management of ovarian cancer in Lagos, Nigeria J Obstet Gynaecol (Lahore) 33(3) 305–308 https://doi.org/10.3109/01443615.2012.753417
4. Smits S, Simon AE, and Forbes LJ, et al (2014) Ovarian cancer symptom awareness and anticipated delayed presentation in a population sample BMC Cancer 14 171 https://doi.org/10.1186/1471-2407-14-171 PMID: 24612526 PMCID: 3975332
5. Bast RC, Feeney M, and Lazarus H, et al (1981) Reactivity of a monoclonal antibody with human ovarian carcinoma J Clin Investig 68(5) 1331–1337 https://doi.org/10.1172/JCI110380 PMID: 7028788 PMCID: 370929
6. Bast RC, Klug TL, and John ES, et al (1983) A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer N Eng J Med 309(15) 883–887 https://doi.org/10.1056/NEJM198310133091503
7. Clarke-Pearson DL (2009) Clinical practice. Screening for ovarian cancer N Eng J Med 361(2) 170–177 https://doi.org/10.1056/NEJMcp0901926
8. Charkhchi P, Cybulski C, and Gronwald J, et al (2020) CA125 and ovarian cancer: a comprehensive review Cancers 12 3730 [doi:10.3390/cancers12123730] https://doi.org/10.3390/cancers12123730 PMID: 33322519 PMCID: 7763876
9. Ferraro S, Braga F, and Lanzoni M, et al (2013) Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review J Clin Pathol 66 273–281 https://doi.org/10.1136/jclinpath-2012-201031 PMID: 23426716
10. Wei S, Hui L, and Bei Z (2016) The diagnostic value of serum HE4 and CA-125 and ROMA index in ovarian cancer Biomed Rep 5 41–44 https://doi.org/10.3892/br.2016.682 PMID: 27347403 PMCID: 4906902
11. Chang X, Ye X, and Dong L, et al (2011) Human epididymis protein 4 (HE4) as a serum tumor biomarker in patients with ovarian carcinoma Int J Gynecol Cancer 21(5) 852–858 https://doi.org/10.1097/IGC.0b013e31821a3726 PMID: 21633297
12. Partheen K, Kristjansdottir B, and Sundfeldt K (2011) Evaluation of ovarian cancer biomarkers HE4 and CA-125 in women presenting with a suspicious cystic ovarian mass J Gynecol Oncol 22(4) 244–252 https://doi.org/10.3802/jgo.2011.22.4.244
13. Moore R, Miller M, and Steinhoff M, et al (2012) Serum HE4 levels are less frequently elevated than CA125 in women with benign gynecologic disorders Am J Obstet Gynecol 206(4) 351–358 https://doi.org/10.1016/j.ajog.2011.12.029
14. Lin J, Qin J, and Sangvatanakul V (2013) Human epididymis protein 4 for differential diagnosis between benign gynecologic disease and ovarian cancer: a systematic review and meta-analysis J Obstet Gynecol Reprod Biol 167(1) 81–85 https://doi.org/10.1016/j.ejogrb.2012.10.036
15. ROMATM Calculator-Aid in Assessing the Risk of Ovarian Cancer in Women With a Pelvic Mass [<a href=https://diagnostics.roche.com/global/en/article-listing/roma-calculator.html>roche.com</a>] Date accessed: 16/12/2021
16. Levy L and Purcell K (2007) Premalignant and malignant disorders of the ovaries and oviducts Current Obstetric and Gynecologic Diagnosis and Treatment 10th edn, eds A DeCherney and L Nathan (New York: Lange Medical Books/McGraw Hill) pp 848–858
17. Montagnana M, Danese E, and Ruzzenente O, et al (2011) The ROMA (Risk of ovarian malignancy algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin Chem Lab Med 49(3) 521–525 https://doi.org/10.1515/CCLM.2011.075 PMID: 21288178
18. Nolen B, Velikokhatnaya L, and Marrangoni A, et al (2010) Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass Gynecol Oncol 117(3) 440–445 https://doi.org/10.1016/j.ygyno.2010.02.005 PMID: 20334903 PMCID: 2873171
19. Hamed EO, Ahmed H, and Seddek OB, et al (2013) Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response Diagn pathol 23 8–11
20. Zhang PJ, Wang CX, and Tian YP, et al (2015) Comparison of HE4, CA125 and ROMA diagnostic accuracy: a prospective and multicenter study for Chinese women with epithelial ovarian cancer Medicine (Baltimore) 94(52) 240–250 https://doi.org/10.1097/MD.0000000000002402
21. Pitta D, Sarian LO, and Barreta A, et al (2013) Symptoms, CA125 and HE4 for the pre op prediction of ovarian malignancy in Brazilian women with ovarian masses BMC Cancer 13 423–429 https://doi.org/10.1186/1471-2407-13-423
22. Anastasi E, Granato T, and Falzarano R, et al (2013) The use of HE4, CA125 and CA72-4 biomarkers for differentiating diagnosis between ovarian endometrioma and epithelial ovarian cancer J Ovarian Res 6(1) 44–49 https://doi.org/10.1186/1757-2215-6-44 PMID: 23816286 PMCID: 3701500
23. Moore R, McMeekin D, and Brown A, et al (2009) A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass Gynecol Oncol 112(1) 40–46 https://doi.org/10.1016/j.ygyno.2008.08.031
24. Gorp TV, Cadron I, and Despierre E, et al (2011) HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the risk of ovarian malignancy algorithm Br J Cancer 104(5) 863–870 https://doi.org/10.1038/sj.bjc.6606092 PMID: 21304524 PMCID: 3048204
25. Bast R, Badgwell D, and Lu Z, et al (2005) New tumor markers: CA125 and beyond Int J Gynecol Cancer15(3) 274–281 https://doi.org/10.1111/j.1525-1438.2005.00441.x PMID: 16343244






