Proportion of papillary thyroid microcarcinoma in Kerala, India, over a decade: a retrospective cohort study
Steve Joseph Benny1, Jeffrey Mathew Boby2, Ravindran Chirukandath3, Togy Thomas4, Ambika Vazhuthakat5, Edwin Saji6, Athul Raj Raju6 and Aju Mathew6,7
1Government Medical College, Thrissur 680596, Kerala, India
2Government Medical College, Kozhikode 673008, Kerala, India
3Department of Surgery, Government Medical College, Thrissur 680596, Kerala, India
4Department of Pathology, Government Medical College, Thrissur 680596, Kerala, India
5Department of Pathology, Government Medical College, Kozhikode 673008, Kerala, India
6Kerala Cancer Care, Kochi, Kerala 682024, India
7Department of Oncology, MOSC Medical College, Ernakulam 682311, Kerala, India
Abstract
Background: Overdiagnosis is a phenomenon where an indolent cancer is diagnosed that otherwise would not have caused harm to the patient during their lifetime. The rising incidence of papillary thyroid cancer (PTC) in various regions of the world is attributed to overdiagnosis. In such regions, the rates of papillary thyroid microcarcinoma (PTMC) are also rising. We aimed to study whether a similar pattern of rising PTMC is found in Kerala, a state in India, where there has been a doubling of thyroid cancer incidence over a decade.
Methods: We conducted a retrospective cohort study in two large government medical colleges, which are tertiary referral facilities in the state of Kerala. We collected data on the PTC diagnosis in Kozhikode and Thrissur Government Medical colleges from 2010 to 2020. We analysed our data by age, gender and tumor size.
Results: The incidence of PTC at Kozhikode and Thrissur Government Medical colleges nearly doubled from 2010 to 2020. The overall proportion of PTMC in these specimens was 18.9%. The proportion of PTMC only marginally increased from 14.7 to 17.9 during the period. Of the total incidence of microcarcinomas, 64% were reported in individuals less than 45 years of age.
Conclusion: The rise in the number of PTCs diagnosed in the government-run public healthcare centres in Kerala state in India is unlikely to be due to overdiagnosis since there was no disproportionate rise in rates of PTMCs. The patients that these hospitals cater to may be less likely to show healthcare-seeking behavior or ease of healthcare access which is closely associated with the problem of overdiagnosis.
Keywords: papillary thyroid microcarcinoma, overdiagnosis, thyroidectomy, cancer incidence, Kerala
Correspondence to: Aju Mathew
Email: cancerkerala@gmail.com
Published: 04/05/2023
Received: 28/11/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Thyroid cancer (TC) is the most prevalent endocrine malignancy accounting for 3.3% of all the neoplasms reported worldwide in the year 2018 [1]. Papillary thyroid cancers (PTC) account for >90% of TCs, followed by follicular thyroid carcinoma (4.5%) and Hurthle cell carcinomas (1.8%). In recent decades there has been a dramatic surge in the incidence of PTC all over the world. By 2030, it will be the fourth most prevalent cancer globally [2].
Overdiagnosis is defined as the detection of either non-progressive cancers or very slow-growing cancers such that individuals die from something else before the cancer ever causes symptoms. The rise in TC diagnosis limited to early-stage indolent variety coupled with a stable mortality rate suggests an overdiagnosis of this malignancy. The rising incidence of PTC in various regions of the world is attributed to overdiagnosis. In such regions, the rates of papillary thyroid microcarcinoma (PTMC) are also rising. PTMC is a subtype of papillary thyroid tumor defined as ≤10 mm in diameter. PTMC is classified as non-incidental or incidental. Incidental PTMC is commonly diagnosed on histopathological examination following thyroid surgery for benign thyroid disease. However, some nodules ≥10 mm may also be diagnosed incidentally as they may be unpalpable depending on their location, lack of symptoms or patient factors like size or girth of neck. Non-incidental PTMC is usually diagnosed based on fine-needle aspiration biopsy and local or distant metastasis. The cancer-specific survival rate of micropapillary TC is about 100% as observed from studies [3–7].
India has one of the lowest average TC incidence rates in the world. However, the state of Kerala has an age-adjusted TC incidence rate of 13.3, ranking it eighth among the most affected regions of the world [8]. The state of Kerala has an outstanding profile in educational and health care standards compared with the other states of India and is on par with some of the high-income countries [9–12]. We aimed to study whether a similar pattern of rising PTMC is found in Kerala, like in other affluent regions of the world where the rising TC incidence is due to overdiagnosis.
Materials and methods
We conducted a retrospective cohort study in two large government medical colleges in Kerala – Kozhikode and Thrissur. These institutions are considered tertiary referral facilities in the public sector. We included patients who were diagnosed with PTC between January 2010 and December 2020. All patients must have undergone a type of thyroid surgery. Institutional ethics approvals were obtained from both medical colleges.
Data on patient demographics and histopathological features of PTC were collected from medical records. Data were then analysed to determine the proportion of PTMC among the PTCs diagnosed during the study period. We analysed our results by categories of age at diagnosis, gender and size of the tumor.
Statistical analysis
Descriptive analysis was used for summary statistical analyses. Data on patient demographics and clinicopathological features were reported as absolute numbers and as well as percentages.
Results
1,805 patients with PTCs were diagnosed during the study period (1,422 females and 383 males). The incidence of PTMC was 18.9%.
Proportion of PTMC in different age groups
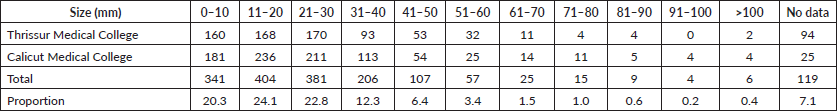
When analysed by 10-year age groups, most of the patients with PTC were in the age group of 36–45 (27.1%) (Figure 1). However, the highest proportion of PTMC (23.2%) was noted in the age group of 46–55. 63.9% were reported in individuals less than 45 years of age.
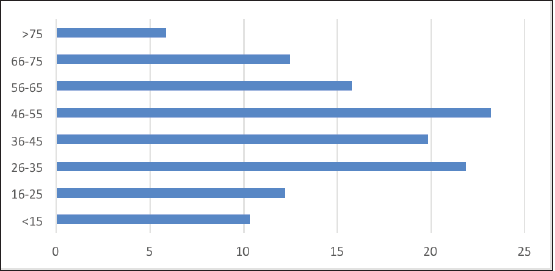
Figure 1. Proportion of PTMCs by age.
Proportion of PTMC in males compared to females
The incidence of PTMC in men (16.9%) was slightly lower than in women (19.4%) (Figure 2). Also, while the peak incidence age group in men corresponded to that seen overall (46–55 years); among women, the peak incidence of PTMC was found to be in a younger age group of 26–35.
Size of the tumor
The highest proportion of PTCs was in the category of 11–20 mm (24.13%) followed by 21–30 mm (22.76%) and ≤10 mm (20.37%) (Figure 3). Excluding those tumors where size was not mentioned, the incidence of PTMC was 18.9% in this study cohort. 45% of all PTC in the study was below palpable size (2 cm).
Numbers of PTMCs and PTCs and their proportion throughout the study
The number of PTCs decreased slightly from 116 in 2010 to 93 in 2012 but then rose sharply to 174 in 2012. The number of PTCs then gradually rose to 217 over the next 7 years (Figure 4). However, in 2020, far fewer PTCs were diagnosed, likely due to COVID19. The number of PTCs showed a moderate upward trend over the decade (Figure 4). On the other hand, the number of PTMCs saw wide fluctuations with minimal absolute change during the period of the study. Consequently, the proportion of PTMCs demonstrated a moderate downward trend during the study period (Figure 5).
Discussion
Previous studies [1, 13] suggested the following points as supporting TC overdiagnosis: (i) substantial increase in incidence but with a large variability among and within countries; (ii) the mortality trends remained stable; (iii) the increase involved mostly small papillary subtypes; (iv) young or middle-age adults mostly affected. The variability of incidence of TC in the different states combined with stable mortality rates has well been demonstrated in other similar studies done in the region [1, 8]. In this study, we aimed to find out whether the increase in the diagnosis of PTC was closely associated with a rise in PTMCs.
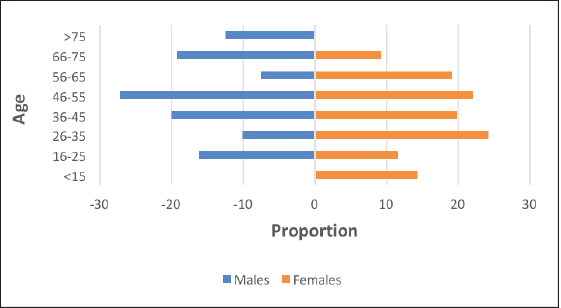
Figure 2. Age distribution of PTMC.
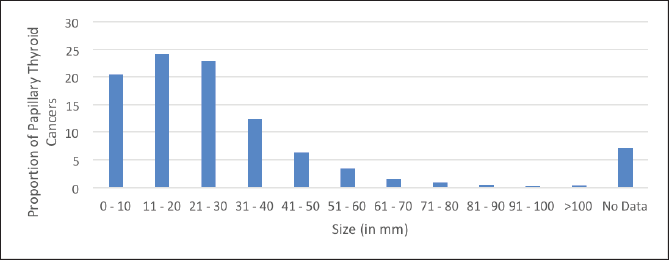
Figure 3. Proportion of PTCs by tumour size.
Although we found a significant rise in the incidence of PTCs, we did not find a proportionate rise in PTMCs in the centres. Mathai et al [14] performed a study to assess the rising trend of papillary microcarcinomas in a single institution in the Thiruvananthapuram district of Kerala on a population very similar to ours, albeit in a private healthcare setting. They found the frequency of microcarcinomas in their studies to be 20.9% which was very similar to the 18.9% seen in our study. Other studies by Lam et al [15], Kaliszewski et al [16] and Girardi et al [17] found higher frequencies of microcarcinomas at 27.8%, 39.7% and 42.1%, respectively. However, John et al [18] and Gürleyik et al [19] observed a lower incidence of 7.2% and 9.4% in their studies of thyroidectomy specimens.
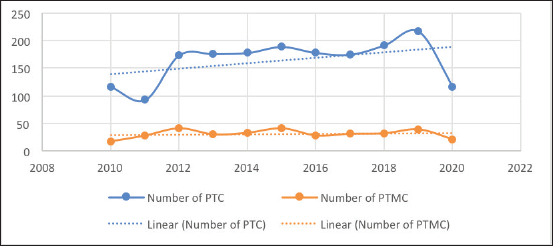
Figure 4. Number of PTC and PTMCs over the study period.
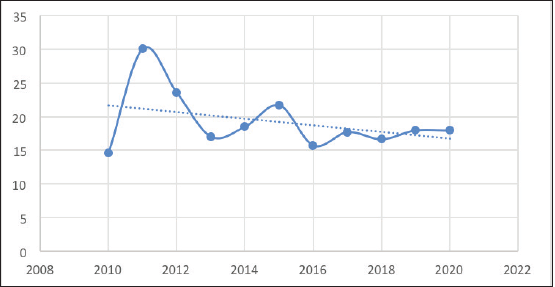
Figure 5. Proportion of PTMCs over the study period.
In their study, Mathai et al [14] observed an increase in PTC incidence accompanied by a significant increase in the frequency of microcarcinomas (they do not provide the data to support their assertion). Kaliszweski et al [16] and Jung et al [20] also reported a significant increase in the proportion of PTMC over the years. Abboud et al [21] found that the frequency of microcarcinomas remained stable for 11 years and that the increase in PTC was independent of microcarcinomas. Vlassopoulou et al [22] in a 30-year study also observed a stable frequency of microcarcinoma.
Interestingly, while the absolute numbers of both PTC and PTMC decreased in 2020, presumably due to the effects of the COVID-19 pandemic, the proportion of PTMCs remained similar to that of the previous years. If overdiagnosis played a role in the rise in PTC numbers in our study, there would have been a disproportionate fall in the number of PTMCs diagnosed compared to the PTCs, especially during COVID19, where a lot of avoidable medical and surgical procedures were delayed.
Our findings deviate from the generally accepted ideas of overdiagnosis as it related to PTMCs – in regions with overdiagnosis, an increasing incidence of PTMC could also be observed. But, on further thought, it does support the hypothesis that the increase in the incidence of PTCs in Kerala state in India is from the phenomenon of overdiagnosis. Our study revealed a higher proportion of patients in the 11–20 mm and 21–30 mm size categories compared to tumors less than 10 mm perhaps as a result of the introduction of ultrasonographic guidelines in the last decade and their implementation for the management of thyroid nodules. A study conducted among Japanese patients also recommends close observation for PTMCs unless it shows features of tumor progression [23]. The rising incidence of TC has been strongly linked to increased access to a standard health care system with national screening programs for the public and in regions with higher literacy and socioeconomic levels [13, 24–26]. PTC overdiagnosis arises from greater health-seeking behavior and easier access to healthcare. Our study population does not fit such a group of individuals – our data arose from two large government-run hospitals in Kerala which serve the relatively lower socioeconomic strata of the population. These state-run institutions are bound by economic and manpower constraints, thereby maximum utilisation of limited resources is prioritised. This supports the notion that overdiagnosis is a phenomenon in affluent societies with greater access and utilisation of healthcare.
Therefore, a similar study in large private centres may be needed to test our hypothesis. But it also brings up the issue of the significant increase in the incidence of PTCs over a decade. If there is no health-seeking behavior or ease of healthcare access, without a proportional rise in PTMCs, why did PTC rates increase substantially over time? Such contradictory findings just point to the fact that the high incidence of TC may not all be because of overdiagnosis, and further research into various risk factors is emergently needed. A large prospective multicentre cohort study is also an urgent area of unmet need in Kerala.
Our study has a few limitations. First, we do not have data on patients being treated in non-governmental institutions in Kerala. Second, a detailed analysis of the histopathological samples was not available. This might have helped to rule out possible chances of misdiagnosis. Third, being a retrospective study, the lymph node status of our patients could not be analysed. And finally, we do not have the mortality data for our study population.
Conclusion
The rise in the number of PTCs diagnosed in the centers under study from 2010 to 2020 is unlikely to be due to overdiagnosis. Although the state of Kerala experiences an overdiagnosis of TCs, it may not be as prevalent in government-run hospitals like the ones in our study that are economically constrained. The patients that our hospitals cater to may be less likely to show healthcare-seeking behavior which is a prerequisite to the problem of overdiagnosis. This supports the notion that overdiagnosis is a phenomenon in affluent societies. A similar study, conducted on people of diverse backgrounds using both public and private healthcare services would be helpful to understand the plausibility of overdiagnosis causing the profound rise in the incidence of TC in Kerala.
Conflicts of interest
The authors declare no conflict of interest.
Funding
This research received no external funding.
References
1. Panato C, Vaccarella S, and Dal Maso L, et al (2020) Thyroid cancer incidence in India between 2006 and 2014 and impact of overdiagnosis J Clin Endocrinol Metab 105 2507–2514 https://doi.org/10.1210/clinem/dgaa192 PMID: 32297630 PMCID: 7947989
2. Jegerlehner S, Bulliard JL, and Aujesky D, et al (2017) Overdiagnosis and overtreatment of thyroid cancer: a population-based temporal trend study PLoS One 12 e0179387 https://doi.org/10.1371/journal.pone.0179387 PMID: 28614405 PMCID: 5470703
3. Morris LGT, Sikora AG, and Tosteson TD, et al (2013) The increasing incidence of thyroid cancer: the influence of access to care Thyroid 23 885–891 https://doi.org/10.1089/thy.2013.0045 PMID: 23517343 PMCID: 3704124
4. Mazzaferri EL (1993) Management of a solitary thyroid nodule N Engl J Med 328 553–559 https://doi.org/10.1056/NEJM199302253280807 PMID: 8426623
5. Haugen BR, Alexander EK, and Bible KC, et al (2015) American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer Thyroid 26 1–133 https://doi.org/10.1089/thy.2015.0020
6. Harach H, Franssila KO, and Wasenius VM (1985) Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study Cancer 56 https://doi.org/10.1002/1097-0142(19850801)56:3<531::AID-CNCR2820560321>3.0.CO;2-3
7. Furuya-Kanamori L, Bell KJL, and Clark J, et al (2016) Prevalence of differentiated thyroid cancer in autopsy studies over six decades: a meta-analysis J Clin Oncol 34 https://doi.org/10.1200/JCO.2016.67.7419 PMID: 27601555
8. Mathew IE and Mathew A (2017) Rising thyroid cancer incidence in Southern India: an epidemic of overdiagnosis? J Endocr Soc 1 480–487 https://doi.org/10.1210/js.2017-00097 PMID: 29264503 PMCID: 5686600
9. Papanicolas I, Woskie LR, and Jha AK (2018) Health care spending in the United States and other high-income countries JAMA 319 1024–1039 https://doi.org/10.1001/jama.2018.1150 PMID: 29536101
10. Nath I (1998) India Lancet 351 1265–1275 https://doi.org/10.1016/S0140-6736(98)03010-4
11. Smith RD and Mallath MK (2019) History of the growing burden of cancer in India: from antiquity to the 21st century J Glob Oncol 5 1–15 PMID: 31373840 PMCID: 7010436
12. Reitzel LR, Nguyen N, and Li N, et al (2014) Trends in thyroid cancer incidence in Texas from 1995 to 2008 by socioeconomic status and race/ethnicity Thyroid 24 556–567 https://doi.org/10.1089/thy.2013.0284 PMCID: 3949437
13. Ahn HS, Kim HJ, and Welch HG (2014) Korea’s thyroid-cancer “epidemic”--screening and overdiagnosis N Engl J Med 371 1765–1767 https://doi.org/10.1056/NEJMp1409841 PMID: 25372084
14. Mathai AM, Preetha K, and Valsala Devi S, et al (2019) Analysis of malignant thyroid neoplasms with a striking rise of papillary microcarcinoma in an endemic goiter region Indian J Otolaryngol Head Neck Surg 71 121–130 https://doi.org/10.1007/s12070-017-1156-8 PMID: 31741946 PMCID: 6848467
15. Lam AKY, Lo CY, and Lam KSL (2005) Papillary carcinoma of thyroid: a 30-yr clinicopathological review of the histological variants Endocr Pathol 16 323–330 https://doi.org/10.1385/EP:16:4:323
16. Kaliszewski K, Zubkiewicz-Kucharska A, and Kiełb P, et al (2018) Comparison of the prevalence of incidental and non-incidental papillary thyroid microcarcinoma during 2008-2016: a single-center experience World J Surg Oncol 16 202 https://doi.org/10.1186/s12957-018-1501-8
17. Girardi FM, Barra MB, and Zettler CG (2013) Variants of papillary thyroid carcinoma: association with histopathological prognostic factors Braz J Otorhinolaryngol 79 738–744 https://doi.org/10.5935/1808-8694.20130135
18. John AM, Jacob PM, and Oommen R, et al (2014) Our experience with papillary thyroid microcancer Indian J Endocrinol Metab 18 410–413 https://doi.org/10.4103/2230-8210.131211 PMID: 24944940 PMCID: 4056144
19. Gürleyik E, Gurleyik G, and Karapolat B, et al (2016) Incidental papillary thyroid microcarcinoma in an endemic goiter area J Thyroid Res 2016 1–6 https://doi.org/10.1155/2016/1784397
20. Jung HS, Jeon MJ, and Song DE, et al (2014) Time trends analysis of characteristics of patients with thyroid cancer in a single medical center J Korean Thyroid Assoc 7 159 https://doi.org/10.11106/cet.2014.7.2.159
21. Abboud B, Sader Ghorra C, and Rassy M, et al (2015) Epidemiological study of thyroid pathology in a university hospital Acta Chir Belg 115 414–417 https://doi.org/10.1080/00015458.2015.11681143
22. Vlassopoulou V, Vryonidou A, and Paschou SA, et al (2016) No considerable changes in papillary thyroid microcarcinoma characteristics over a 30-year time period BMC Res Notes 9 252 https://doi.org/10.1186/s13104-016-2018-2 PMID: 27129971 PMCID: 4850716
23. Ito Y, Miyauchi A, and Inoue H, et al (2010) An observational trial for papillary thyroid microcarcinoma in Japanese patients World J Surg 34 28–35
24. Hall SF, Irish J, and Groome P, et al (2014) Access, excess, and overdiagnosis: the case for thyroid cancer Cancer Med 3 154–161 https://doi.org/10.1002/cam4.184 PMID: 24408145 PMCID: 3930400
25. Udelsman R and Zhang Y (2014) The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds Thyroid 24 472–479 https://doi.org/10.1089/thy.2013.0257 PMCID: 3949447
26. Altekruse S, Das A, and Cho H, et al (2015) Do US thyroid cancer incidence rates increase with socioeconomic status among people with health insurance? an observational study using SEER population-based data BMJ Open 5 e009843 https://doi.org/10.1136/bmjopen-2015-009843 PMID: 26644126 PMCID: 4679945
Supplementary information
Table 1. Total number of micropapillary carcinomas diagnosed among papillary thyroid carcinomas in Thrissur and Calicut medical colleges from 2010 to 2020.
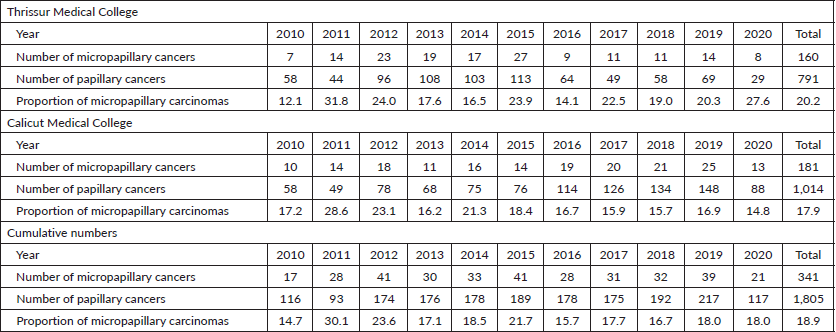
Table 2. Total number of micropapillary carcinomas diagnosed among papillary thyroid carcinomas in Thrissur and Calicut medical colleges from 2010 to 2020 (females).
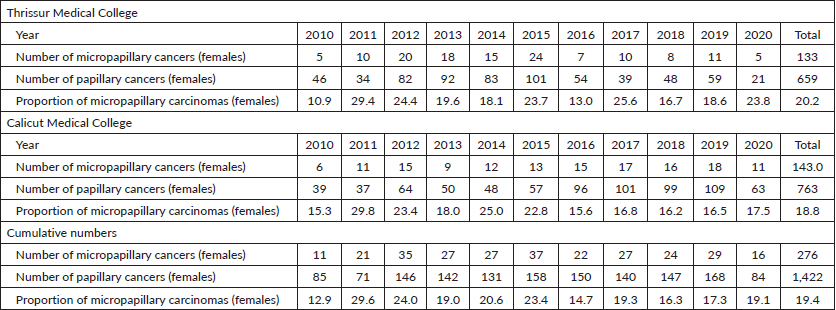
Table 3. Total number of micropapillary carcinomas diagnosed among papillary thyroid carcinomas in Thrissur and Calicut medical colleges from 2010 to 2020 (males).
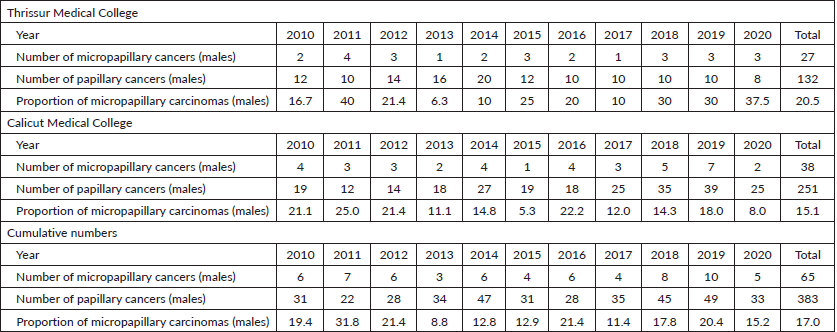
Table 4. Size distribution of PTCs.