Potential clinical utility of plasma D-dimer levels among women with cervical cancer in Lagos, Nigeria
Lucky E Tietie1, Kehinde S Okunade1,2, Adaiah P SoibI-Harry2, Sarah O John-Olabode3 and Rose I Anorlu1,2
1Oncology and Pathological Studies Unit, Lagos University Teaching Hospital, Lagos 102215, Nigeria
2Department of Obstetrics & Gynaecology, College of Medicine, University of Lagos, PMB 12003, Lagos, Nigeria
3Department of Haematology and Blood Transfusion, College of Medicine, University of Lagos, PMB 12003, Lagos, Nigeria
Abstract
The link between plasma D-dimer levels and underlying malignancy has been established. How this translates in clinical practice as a marker of detection and prognosis of cervical cancer (CC) is still unknown. This study compared the plasma D-dimer levels in women with and without CC and assessed the associations between plasma D-dimer levels and the stage and grade of CC. It was a comparative cross-sectional study of 65 women with histological diagnosis of CC and an equal number of age-matched cancer-free women enrolled at the University Teaching Hospital in Lagos, Nigeria. Participants’ sociodemographic and clinical data as well as venous blood samples for estimation of plasma D-dimer were collected for statistical analyses. A receiver operating characteristic (ROC) analysis is performed to select the cut-off value of plasma D-dimer for differentiating CC from non-cancer. There was a statistically significant difference in the median levels of plasma D-dimer of women with CC and their cancer-free comparison groups (3,120 (1,189–4,515) versus 210 (125–350) ng/mL; p = 0.001). A plasma D-dimer value of 543 ng/mL was chosen in a ROC analysis as the discriminatory cut-off to differentiate CC from non-cancer. There were significant associations between plasma D-dimer levels and the International Federation of Gynaecology and Obstetrics stage (p = 0.001) or grade (p = 0.001) of CC. The study, therefore, demonstrated the potential clinical usefulness of plasma D-dimer as a diagnostic and prognostic marker of CC.
Keywords: FIGO stage, cervical malignancy, marker, ROC, Lagos
Correspondence to: Kehinde S Okunade
Email: sokunade@unilag.edu.ng
Published: 30/01/2023
Received: 09/10/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cervical cancer (CC) constitutes a significant public health burden in many resource-limited countries [1]. In 2020, it accounted globally for an estimated 604,000 new cases and 342,000 deaths with Africa bearing the largest burden of the disease [2]. Nigeria accounts for 14,943 new cases and 10,403 cancer-related deaths annually [3]. Many biomarkers have been previously used to screen for CC and assess the risk of recurrence after treatment. However, these biomarkers are not sufficient to predict prognosis accurately and their clinical usefulness is still being debated [4]. Therefore, the search for a more reliable marker of early detection and prognostic monitoring of CC continues. Serum tumour biomarkers have been shown to play several roles in cancer management including early detection through screening, diagnostic confirmation, prognostication, monitoring and response to specific treatments [5].
Activation of clotting is common in cancers; hence, there is a high incidence of thrombosis in metastatic, fast-growing, biologically aggressive cancer [6]. This activation also leads to the generation of D-dimer through the degradation of cross-linked fibrin resulting from the proteolytic actions of plasmin [6]. D-dimer is an exceptional marker of fibrin degradation that is formed because of the sequential activation of thrombin, factor XIIIa and plasmin in the coagulation cascade [7]. Elevated levels signal the occurrence of hyperfibrinolysis, and its clinical use is well established in excluding the diagnosis of venous thromboembolism (VTE), for making a diagnosis of acute aortic dissection and for risk stratification of patients for VTE recurrence [7]. Coagulation indexes including plasma D-dimer may be useful as survival biomarkers for several solid malignancies including CC [8]. High pretreatment plasma levels of D-dimer are frequently detected in patients with CC [9, 10], however, the prognostic significance of this finding is still largely unknown. Furthermore, there are still limited data available to show the association between plasma D-dimer levels and invasive CC among Nigerian women. This current study, therefore, evaluated the clinical significance of D-dimer levels in women with CC by comparing the plasma D-dimer levels of women with CC with that of a comparative cancer-free group of women and, in addition, determined the associations between plasma D-dimer levels and the clinical markers of prognosis such as the stage and grade of CC.
Materials and methods
Study design and setting
The study was a comparative cross-sectional study conducted among women diagnosed with CC and their healthy cancer-free counterparts enrolled at the gynaecological outpatient and radiation oncology clinics of the University Teaching Hospital in Lagos, Nigeria. The hospital is the teaching hospital of a College of Medicine that serves mainly as a referral centre for other government-owned and private hospitals in the state, and its environs.
Study population and sample size determination
The study population included treatment-naïve (yet to commence any form of treatment) women with histologically diagnosed CC enrolled at the gynaecological outpatient and radiation oncology clinics and their age-matched comparison group comprising cancer-free women attending the gynaecological outpatient clinics for infertility treatment. All women with obesity (body mass index up to 30.0 kg/m2), those with major medical conditions such as hypertension, diabetes, liver or renal diseases, previous or concomitant history of cancer, haematological diseases, coagulation disorders or women on anticoagulants were excluded from this study. The sample size for each study group (n = 65) was estimated using the formula for comparison of two independent groups [11] to achieve power (1–Zβ) of 80% (0.842) at a type 1 error (Zα) rate of 5% (1.96) with 95% confidence level and effect size of 0.50 while adjusting for a non-response rate of 20%.
Participant enrolment and data collection
Eligible participants for the study were enrolled by consecutive sampling until the required sample size was achieved. The purpose and procedures of the study were explained to all participants and their informed consent was obtained before enrolment in the study. A structured interviewer-administered questionnaire and patients’ medical records were used to obtain information such as socio-demographic characteristics, medical and reproductive history, details of cancer diagnosis, as well as the revised 2019 International Federation of Gynaecology and Obstetrics (FIGO) clinicopathologic stage (stage I to IV) [12] and the traditional Broders’ grading system of squamous cell carcinoma (SCC) [13] characterised by squamous differentiation – well-differentiated (low grade, G1), moderately differentiated (intermediate grade, G2), poorly differentiated (high grade, G3) and undifferentiated (high grade, G4) tumour [13]. Following this, about three millilitres (3 mL) of whole blood were collected from each participant’s antecubital vein, dispensed into a trisodium citrate anticoagulated vacutainer bottle labelled with the participant’s identification (ID) code, and then transported within 30 minutes to the departmental research laboratory where the specimen was centrifuged (at 1500 × g for 15 minutes) within 1 hour to avoid degradation. Following this, about 1 mL of platelet-poor plasma was extracted and stored at minus 20°C in cryogenic vials until laboratory analysis.
Laboratory analysis
Plasma D-dimer concentration was determined using the fluorescence immunoassay rapid quantitative test that uses a sandwich immuno-detection method with an assay working range of 50~10,000 ng/mL and a detection limit of 50 ng/mL. About 10 µL of the plasma was drawn using a plastic micropipette for transfer and mixing in a detection buffer tube labelled with the participant’s ID code. Approximately 75 µL of the buffered mixture is then transferred in a micropipette into the sample well of the test cartridge. The fluorescence-labelled detector D-dimer antibodies on the sample pad bind to D-dimer antigens in the buffered blood specimen in the test cartridge to form immune complexes in a quick test mode. The complexes migrate by capillary action and the migrating complexes are then immobilised and captured on the nitrocellulose matrix of the test strip. Therefore, the more D-dimer antigens in the blood specimen, the more complexes that are accumulated and captured on the test strip and thus signal intensity of fluorescence of detector antibodies reflects the amount of D-dimer captured. Quality control was ensured through a built-in control to ensure accuracy and by minimising false positive results using a specific 3B6/22 monoclonal antibody reagent.
Statistical analysis
Data were analysed using IBM SPSS statistical software version 28.0 for Windows (Armonk, New York). The normality of continuous data was tested using the Kolmogorov–Smirnov test with Lilliefors’ significance correction. Participants’ demographic and clinical data were summarised in the descriptive statistics as mean (± standard deviation (SD)) for continuous variables and frequency (and percentages) for categorical variables. Univariate analyses were conducted between the participants’ characteristics and the plasma D-dimer categories of the case and comparison groups. Associations between continuous variables were tested using the independent sample t-test (normal distribution) or Mann–Whitney U and Kruskal Wallis test (skewed data), whereas categorical variables were compared using Pearson’s χ2 or Fisher’s exact test. We performed a receiver operating characteristic (ROC) analysis to select the best discriminating cut-off value of plasma D-dimer that differentiated CC from non-cancer based on optimal sensitivity and specificity. We then tested the associations between plasma D-dimer levels and CC histological prognostic factors such as the type, stage and grade of the disease. Post-hoc analyses were performed to test the difference in the median levels of D-dimer between the different categories of participants in the CC group based on the FIGO stage and histological grade of the disease with significance values adjusted by the Bonferroni correction for multiple tests. Statistical significance was set at p < 0.05.
Statement of ethics
This study protocol was reviewed and approved by the Health Research Ethics Committee of the Lagos University Teaching Hospital with approval number ADM/DCST/HREC/APP/2443 before participants’ enrolment in the study. Ethical principles according to the World Medical Association Declaration of Helsinki were applied throughout the study. The participants were counselled, read and signed an informed consent form before their enrolment in the study. Strict adherence to the privacy and confidentiality of participants’ information was ensured during and after the conduct of the study.
Results
The mean age of the participants in the CC group (52.8 ± 11.4 years) was not statistically different from that of their cancer-free comparison group (49.3 ± 13.6 years), p = 0.120. There were statistically significant differences in parity (p = 0.001), tribe (p = 0.016), educational level (p = 0.001), occupation (p = 0.001) and marital status (p = 0.047) between the two groups of participants. There were no differences in the age at coitarche (p = 0.129), the number of lifetime sex partners (p = 0.283), use of oral contraceptive pill (OCP) (p = 0.840) and human immunodeficiency virus (HIV) serostatus (p = 0.380) between the two groups of participants (Table 1).
Table 1. Characteristics of participants in CC and cancer-free groupsa.
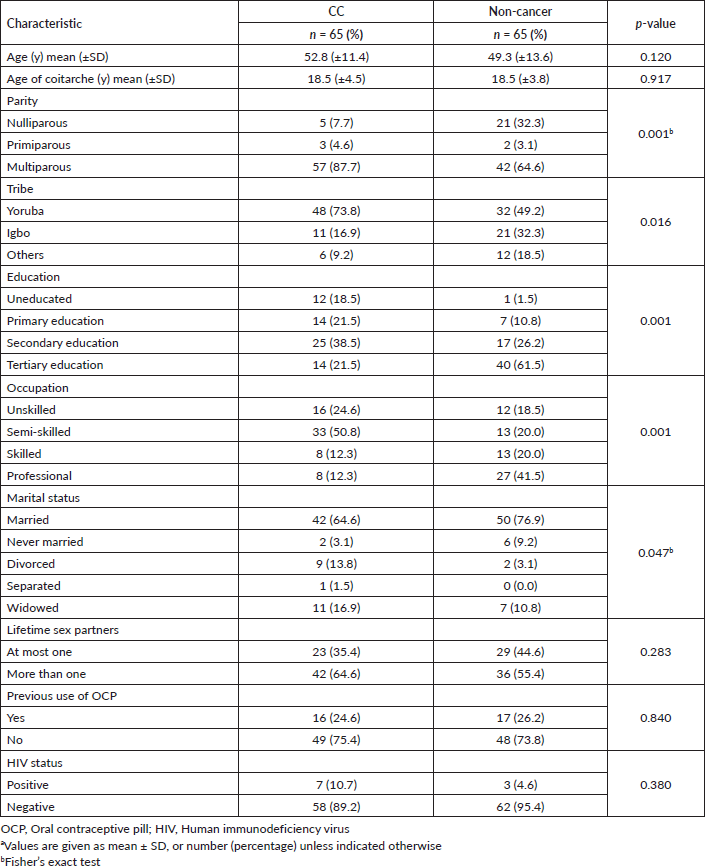
Figure 1. Median plasma levels in women with CC (3,120 (1,189–4,515) ng/mL) versus cancer-free women (210 (125–350) ng/mL), p = 0.001.
As shown in Figure 1, there was a statistically significant difference in the plasma D-dimer levels between women who had CC, 3,120 (1,189–4,515) ng/mL, and that of their cancer-free counterparts, 210 (125–350) ng/mL, p = 0.001. Based on the ROC analysis, the best discriminating cut-off value of plasma D-dimer was 543 ng/mL at an area under the ROC curve (AUROC) of 0.986 (95% confidence interval (CI) 0.972, 0.997), p = 0.001 corresponding to a CC detection sensitivity of 95.4% and specificity of 92.3% (Figure 2).
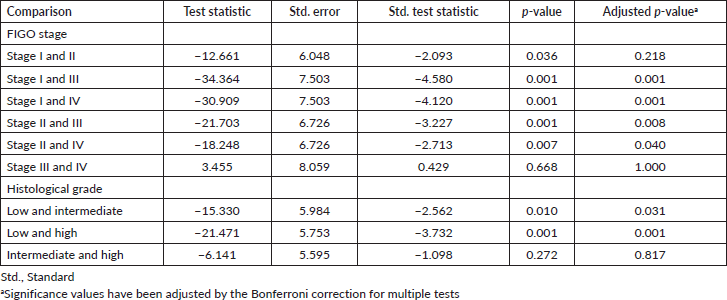
Of the 65 women with CC, the majority had SCC (n = 58, 89.2%), early stage I and II (n = 44, 67.7%) and histological grade 3 disease (n = 25, 38.5%) (Table 2). There were statistically significant associations between plasma D-dimer levels and FIGO stage (p = 0.001) and histological grade of CC (p = 0.001). Following adjustment by the Bonferroni correction for multiple tests, the post-hoc analyses revealed significant differences in the plasma D-dimer levels between stage I and III (p = 0.001), stage I and IV (p = 0.001), stage II and III (p = 0.008) and stage II and IV (p = 0.040). In addition, there were statistically significant differences in the plasma D-dimer levels between grades 1 and 2 (p = 0.031) and grades 1 and 3 (p = 0.001) (Table 3).
Discussion
The study was conducted to investigate the possible association between plasma D-dimer levels and CC and its histological prognostic markers among women at a teaching hospital in Lagos, Nigeria. The study found statistically significant associations between elevated plasma D-dimer levels and CC as well as the stage and grade of the disease.
The mean age of women with CC in this study (52.8 ± 11.4 years) is in keeping with that of previous studies conducted among similar cohorts of participants in the same clinical setting [14, 15] and that of the finding by Nkyekyer [16] in a 5-year review of gynaecological cancer patients in Korle Bu Teaching Hospital, Accra, Ghana. This also coincides with the peak age range (50–54 years) at which deaths from CC result in most years of life lost and therefore, it is the time during which the implementation of CC screening would likely be most effective in saving more lives [17]. We found a statistically significant difference between multiparity and CC, a finding which further confirmed the historically documented hypothesis that an increasing number of full-term pregnancies is a significant independent risk predictor of CC [18] due to the increased hormone levels and impaired immune response of pregnancies [19] together with the local tissue damage that occurs during vaginal childbirth resulting in cellular oxidative stress and DNA damage leading to Human papillomavirus (HPV) cellular integration and persistence [20].
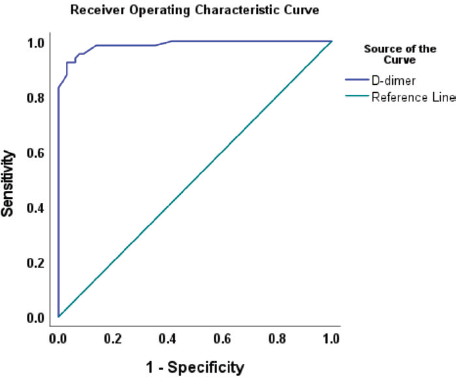
Figure 2. ROC curve showing the best discriminating cut-off value of plasma D-dimer (543 ng/mL) at AUROC curve of 0.986 (95% CI: 0.972, 0.997), p = 0.001.
Table 2. Tumour characteristics and plasma D-dimer levels in participants with CC (n = 65).
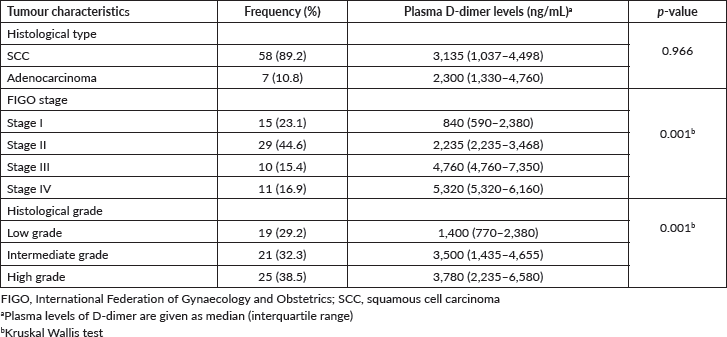
Table 3. Pairwise comparisons of plasma D-dimer levels stratified by FIGO stages and histological grades of CC.
There is increasing evidence to suggest that thrombotic episodes may occur months or years long before the diagnosis of cancer [21, 22]. Tumour cells are known to activate the clotting-fibrinolytic system, releasing various fibrinolytic markers and haemostatic factors. These in turn stimulate vascular endothelial cell proliferation and promote neo-angiogenesis necessary for tumour growth [23]. This thus suggests the possible role of thrombotic markers such as plasma D-dimer as potential markers of occult malignancies. The finding of significantly higher median plasma D-dimer levels in women with CC compared with cancer-free women in the current study further corroborates this important theory. This is also in similarity to the finding from the study by Vahid et al [24] where higher plasma D-dimer levels were found in malignant gynaecological diseases of the cervix, uterus and ovaries compared to benign lesions and that of a prospective study conducted by Luo et al [23] among 296 patients with CC in Guangdong, China. The CC detection sensitivity and specificity of 95.4% and 92.3%, respectively, for the D-dimer assay used in our study suggest its utility as a potential low-cost and convenient diagnostic tool for CC among women in resource-limited settings.
As clotting activation occurs most commonly in cancers, there is a high incidence of thrombosis in metastatic, fast-growing, biologically aggressive cancer with associated poor prognosis [25]. Elevated plasma D-dimer levels, independent of VTE episodes, have been correlated with poorer prognosis of solid gynaecological tumours such as ovarian [21, 26], cervical [23, 27], and endometrial cancer [28]. Preoperative D-dimer has been shown to be an effective prognostic predictor in women with CC [8, 23, 24]. Our current study found significant associations between plasma D-dimer levels and the FIGO stage and grade of CC in close similarity to these previous studies by Li et al [8], Luo et al [23]and Vahid et al [24]. This also corroborates the findings from the study by Nakamura et al [27] where pre-operative D-dimer measurement was suggested as a potential biomarker of CC prognosis. In the study by Li et al [8], the prognosis of women with CC was poorer if their D-dimer levels were greater than 685 ng/mL which is quite lower than the detection values of greater than 2,235 ng/mL and 3,500 ng/mL for advanced tumour stage (stage III and IV) and poorly differentiated and undifferentiated grade of the disease (high grade), respectively. Although the tumour grade of cervical SCC and adenocarcinoma is regularly included in histopathology reports, at present, there is no grading system that has achieved universal acceptance thus limiting the prognostic value of tumour grade on CC [29]. This is because there are considerable inter-observer variations in the grading of SCC which `subjectively’ depends on how easy it is to recognise the characteristics of squamous epithelium, pleomorphism and mitotic activity [30]. Therefore, as tumour grade is no longer taken into consideration by most guideline recommendations for the management of CC [29, 31, 32], the finding of an association between D-dimer levels and disease grade as reported in this study should be carefully interpreted until future studies are conducted using a standard consensus grading system. Furthermore, the plasma D-dimer levels are not affected by the histological type of CC in this study, thus suggesting its use as a convenient tool for predicting disease prognosis irrespective of the tumour type. A few limitations in our study include the cross-sectional design which made it difficult to infer any causal inferences; the inability to collect accurate historical data on VTE which could confound our findings [33]; and the limited number of participants’ data available for the subgroup analyses.
Conclusion
We found significant associations between elevated plasma D-dimer levels and CC, and the stage and grade of the disease. The study demonstrated the potential clinical usefulness of plasma D-dimer as a diagnostic and prognostic predictor of CC. However, more reliable evidence should be obtained from future longitudinal studies that will evaluate the longitudinal changes in plasma D-dimer and their relationships to the survival indicators in women with cancerous lesions of the cervix.
Acknowledgments
The authors’ thank all the participating women and staff of the gynaecology and radiation oncology outpatient clinics of the hospital who have contributed to this study.
Conflicts of interest
The authors declare that they have no competing interests.
Funding Information
The funding for this work was mostly provided by the first author (LET) as part of the requirements for the award of his postgraduate fellowship certificate in Obstetrics and Gynaecology. The work was also supported in part by the National Cancer Institute and Fogarty International Center of the National Institutes of Health under Award Numbers K43TW011930, D43TW010934 and D43TW010543. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, Fogarty International Center or the National Institutes of Health.
Data availability statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
1. Okunade KS, Adejimi AA, and John-Olabode SO, et al (2022) An overview of HPV screening tests to improve access to cervical cancer screening amongst underserved populations: from development to implementation Risk Manag Healthc Policy [Internet] 15 1823–1830 https://doi.org/10.2147/RMHP.S296914 PMID: 36176779 PMCID: 9514784
2. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
3. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, and Dicker D, et al (2013) The global burden of cancer 2013 JAMA Oncol 1(4) 505–527
4. Kim BG (2013) Squamous cell carcinoma antigen in cervical cancer and beyond J Gynecol Oncol 24(4) 291 https://doi.org/10.3802/jgo.2013.24.4.291 PMID: 24167661 PMCID: 3805906
5. Güzel C, van Sten-Van’t Hoff J, and de Kok IMCM, et al (2021)Molecular markers for cervical cancer screening Exp Rev Proteomics [https://www.tandfonline.com/action/journalInformation?journalCode=ieru20] Date accessed: 24/09/22
6. de Buyzere M, Philippé J, and Duprez D, et al (1993) Coagulation system activation and increase of D-dimer levels in peripheral arterial occlusive disease Am J Hematol 43(2) 91–94 https://doi.org/10.1002/ajh.2830430204 PMID: 8342557
7. Gadducci A, Barsotti C, and Cosio S, et al (2011) Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: a review of the literature Gynecol Endocrinol 27(8) 597–604 https://doi.org/10.3109/09513590.2011.558953 PMID: 21438669
8. Li B, Shou Y, and Zhu H (2021) Predictive value of hemoglobin, platelets, and D-dimer for the survival of patients with stage IA1 to IIA2 cervical cancer: a retrospective study J Int Med Res 49(12) 030006052110610
9. Xu L, He F, and Wang H, et al (2017) A high plasma D-dimer level predicts poor prognosis in gynecological tumors in East Asia area: a systematic review and meta-analysis Oncotarget 8(31) 51551–51558 https://doi.org/10.18632/oncotarget.17936 PMID: 28881667 PMCID: 5584268
10. Li W, Tang Y, and Song Y, et al (2018) Prognostic role of pretreatment plasma D-dimer in patients with solid tumors: a systematic review and meta-analysis Cell Physiol Biochem 45(4) 1663–1676 https://doi.org/10.1159/000487734 PMID: 29490291
11. Wang X and Ji X (2020) Sample size estimation in clinical research Chest 158(1) S12–S20 https://doi.org/10.1016/j.chest.2020.03.010
12. Bhatla N, Berek JS, and Cuello Fredes M, et al (2019) Revised FIGO staging for carcinoma of the cervix uteri Int J Gynaecol Obstet 145(1) 129–135 https://doi.org/10.1002/ijgo.12749 PMID: 30656645
13. Wright JR (2020) Albert C. Broders, tumor grading, and the origin of the long road to personalized cancer care Cancer Med 9 4490–4494 https://doi.org/10.1002/cam4.3112 PMID: 32378792 PMCID: 7333853
14. Offor JO, Okunade KS, and Iwalokun BA, et al (2021) Evaluation of oxidative markers in women with invasive cervical cancer in Lagos, Nigeria Ecancermedicalscience 15 1266 https://doi.org/10.3332/ecancer.2021.1266 PMID: 34567251 PMCID: 8426022
15. Sekumade A, Okunade K, and Olorunfemi G, et al (2019) Association between serum folate level and invasive cervical cancer at a university teaching hospital in South-West Nigeria J Cancer Res Pract 6(4) 179 https://doi.org/10.4103/JCRP.JCRP_24_19
16. Nkyekyer K (2009) Pattern of gynaecological cancers in Ghana East Afr Med J 77(10) https://doi.org/10.4314/eamj.v77i10.46708
17. Law MR, Morris JK, and Wald NJ, et al (1999) The importance of age in screening for cancer J Med Screen 6(1) 16–20 https://doi.org/10.1136/jms.6.1.16 PMID: 10321365
18. Okunade KS (2020) Human papillomavirus and cervical cancer J Obstet Gynaecol 40(5) 602–608 https://doi.org/10.1080/01443615.2019.1634030 PMID: 31500479 PMCID: 7062568
19. International Collaboration of Epidemiological Studies of Cervical Cancer (2006) Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies Int J Cancer [Internet] 119 1108–1124 https://doi.org/10.1002/ijc.21953 PMID: 16570271
20. Williams VM, Filippova M, and Soto U, et al (2011) HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress Future Virol 6(1) 45–57 https://doi.org/10.2217/fvl.10.73 PMID: 21318095 PMCID: 3037184
21. Man YN, Wang YN, and Hao J, et al (2015) Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism Int J Gynecol Cancer 25(1) 24–32 https://doi.org/10.1097/IGC.0000000000000303
22. Chen WH, Tang LQ, and Wang FW, et al (2014) Elevated levels of plasma D-dimer predict a worse outcome in patients with nasopharyngeal carcinoma BMC Cancer 14 583 https://doi.org/10.1186/1471-2407-14-583 PMID: 25109220 PMCID: 4242497
23. Luo YL, Chi PD, and Zheng X, et al (2015) Preoperative D-dimers as an independent prognostic marker in cervical carcinoma Tumor Biol 36(11) 8903–8911 https://doi.org/10.1007/s13277-015-3650-5
24. Vahid Dastjerdi M, Ahmari S, and Alipour S, et al (2015) The comparison of plasma D-dimer levels in benign and malignant tumors of cervix, ovary and uterus Int J Hematol Oncol Stem Cell Res 9(3) 107–111 PMID: 26261694 PMCID: 4529676
25. de Buyzere M, Philippé J, and Duprez D, et al (1993) Coagulation system activation and increase of D-dimer levels in peripheral arterial occlusive disease Am J Hematol 43(2) 91–94 https://doi.org/10.1002/ajh.2830430204 PMID: 8342557
26. Sakurai M, Satoh T, and Matsumoto K, et al (2015) High pretreatment plasma D-dimer levels are associated with poor prognosis in patients with ovarian cancer independently of venous thromboembolism and tumor extension Int J Gynecol Cancer 25(4) 593–598 https://doi.org/10.1097/IGC.0000000000000415 PMID: 25756402 PMCID: 4406979
27. Nakamura K, Nakayama K, and Ishikawa M, et al (2016) High pre-treatment plasma D-dimer level as a potential prognostic biomarker for cervical carcinoma Anticancer Res 36(6) 2933–2938 PMID: 27272807
28. Li J, Lin J, and Luo Y, et al (2015) Multivariate analysis of prognostic biomarkers in surgically treated endometrial cancer PLoS One 10(6) e0130640 https://doi.org/10.1371/journal.pone.0130640 PMID: 26107255 PMCID: 4479375
29. McCluggage WG (2018) Towards developing a meaningful grading system for cervical squamous cell carcinoma J Pathol Clin Res 4(2) 81–85 https://doi.org/10.1002/cjp2.98 PMID: 29665326 PMCID: 5903690
30. Graflund M, Sorbe B, and Hussein A, et al (2002) The prognostic value of histopathologic grading parameters and microvessel density in patients with early squamous cell carcinoma of the uterine cervix Int J Gynecol Cancer 12(1) 32–41 https://doi.org/10.1136/ijgc-00009577-200201000-00006 PMID: 11860534
31. Eifel PJ, Fisher CM, and Frederick P, et al (2017) NCCN Guidelines ® Version 1
32. Cibula D, Pötter R, and Planchamp F, et al (2018) The European society of gynaecological oncology/European society for radiotherapy and oncology/European society of pathology guidelines for the management of patients with cervical cancer Virchows Archiv 472(6) 919–936 https://doi.org/10.1007/s00428-018-2362-9 PMID: 29725757
33. Arpaia G, Carpenedo M, and Verga M, et al (2009) D-dimer before chemotherapy might predict venous thromboembolism Blood Coagul Fibrinolysis 20(3) 170–175 https://doi.org/10.1097/MBC.0b013e32831bc2de PMID: 19276795






