Metaplastic carcinoma of the breast: real-world outcome from a tertiary cancer centre in India
Ananthi Balasubramanian1a, Priya Iyer1, Rama Ranganathan2, Kanchan Murhekar3, Manikandan Dhanushkodi4, Selvaluxmy Ganesarajah1, Sridevi Velusami5 and Arvind Krishnamurthy5
1Department of Radiation Oncology, Cancer Institute (WIA), Chennai 600 036, India
2Department of Epidemiology and Cancer Registry, Cancer Institute (WIA), Chennai 600 036, India
3Department of Oncopathology, Cancer Institute (WIA), Chennai 600 036, India
4Department of Medical Oncology, Cancer Institute (WIA), Chennai 600 036, India
5Department of Surgical Oncology, Cancer Institute (WIA), Chennai 600 036, India
ahttps://orcid.org/0000-0001-7107-8145
Abstract
Metaplastic carcinoma (MPC) is a rare subgroup of breast tumours accounting for <5% of all invasive breast cancers. Histologically confirmed 40 MPC from January 2001 to December 2018 were identified from our electronic database: stage I 2.5% (n = 1), stage II 40% (n = 16), stage III 45% (n = 18) and stage IV 12.5% (n = 5). The mean tumour size was 6 cm, node-negative in 60%, and hormone receptor-negative in 75%. Among the 35 non-metastatic patients, 17 (48.6%) received initial neoadjuvant treatment (NAT), followed by surgery, and only 1 had a complete pathological response. At a median follow-up of 60 months, 17% (n = 6) had a recurrence. All six of them had lung metastasis. The 5-year overall survival (OS) and disease-free survival were 64.4% and 66.3%, respectively. Age more than 46 years (p = 0.027), tumour size more than 5 cm (p = 0.037), and nodal positivity (p = 0.001) were predictors of OS. In node-positive patients, the 5-year OS in those who underwent initial surgery was 80% and after NAT was 21.4% (p = 0.069). In node-negative patients, the 5-year OS after initial surgery was 83.3% and after NAT was 90% (p = 0.380). A statistical significance could not be demonstrated due to the small number of patients. Due to chemoresistance, the concept of initial NAT in MPC of the breast is a subject to be studied in the future. Upfront surgery should be considered for operable diseases (including stage III), followed by a decision on adjuvant therapy. Optimal treatment and effective systemic therapy regimens are yet to be defined.
Keywords: metaplastic carcinoma, chemoresistance, receptor negative, triple negative, node-positive
Correspondence to: Ananthi Balasubramanian
Email: ananthib@ymail.com and b.ananthi@cancerinstitutewia.org
Published: 14/07/2022
Received: 08/05/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Metaplastic carcinoma (MPC) is a rare subgroup of breast tumours accounting for <5% of all invasive breast cancers. MPC consists of a group of heterogeneous elements. The neoplastic epithelium is differentiated into squamous cells and/or mesenchymal-looking elements. According to the World Health Organisation classification of breast tumours 2012 [1], MPC is classified as a low-grade adenosquamous carcinoma, fibromatosis-like MPC, squamous cell carcinoma, spindle cell carcinoma and carcinoma with mesenchymal differentiation. Carcinoma with mesenchymal differentiation includes chondroid, osseous, rhabdomyoid and even neuroglial differentiation with carcinomatous areas. Usually, a mixture of different elements is demonstrated in MPC with or without ductal carcinoma. Most of them are triple-negative, and the chemotherapy regimens used to treat MPC are the same as invasive breast cancer. In MPC, the standard protocols have different responses. They are associated with a poor prognosis [2–4] due to their chemoresistance [5–7] when compared with invasive ductal carcinoma (IDC) or triple-negative ductal carcinoma [8, 9]. These tumours tend to relapse in the lungs and central nervous system. Whether MPC has a worse prognosis than IDC is yet to be defined, and effective chemotherapy regimens in this subtype are the need of the hour. We conducted this retrospective study to determine the patient and tumour characteristics, response to standard treatment regimens, patterns of recurrence and survival outcomes in MPC over 17 years from a single institution.
Materials and methods
The Institutional Ethics Committee of Cancer Institute (WIA), India, approved this study (Ethics Committee approval number IEC/2021/March 02). Women (age >18 years) diagnosed with metaplastic breast cancer from January 2001 to December 2018 were included in this analysis. The clinical data and treatment details were retrieved from the electronic medical record (EMR) by coding the diagnosis as metaplastic breast cancer. Patients whose treatment details were not available in the EMR were collected from the patients’ case records. Those who defaulted for follow-up were contacted by phone, and information regarding their status was obtained. The details included for demographic analysis were age, initial diagnostic procedure, histopathological subtype, clinical stage, including hormone receptor status, and HER2/neu status. The data captured included details of treatment received, including surgery, nodal involvement, chemotherapy, and radiotherapy details, including response (both clinical and pathological) to neoadjuvant treatment (NAT), details of recurrence as local, systemic or both and survival. Hormone receptor positivity (estrogen receptor (ER) and progesterone receptor (PR)) was defined as any score of 1% and above. HER2/neu positivity was taken as immunohistochemistry 3+ or if fluorescence in-situ hybridisation (FISH) was positive. All patients were treated after discussion in a multidisciplinary tumour board. Early stage (stages I and II) underwent surgery, followed by a decision on adjuvant therapy. Locally advanced stage (stage III) underwent NAT and was reassessed for surgery. Advanced metastatic stage (stage IV) received palliative therapy.
Statistical analysis
All data were analysed with IBM SPSS statistics, version 22 (SPSS Inc., Chicago, IL). Demographic and clinicopathologic features were summarised by using descriptive statistics. Overall survival (OS) was defined as the time from diagnosis to death due to any cause or last follow-up date. Disease-free survival (DFS) was defined as the time from diagnosis to recurrence (local, distant or both) or breast cancer-related event or death. Time to recurrence was defined as the time from diagnosis to the first recurrence. Progression-free survival (PFS) was defined as the time from the date of recurrence in non-metastatic patients or from diagnosis in metastatic patients until death or the date of censoring. The objective response rate (ORR) was defined as the ratio of partial and complete responses to treatment. Survival was estimated by Kaplan–Meier’s method, and log-rank test was used to compare prognostic factors. All statistical tests were two-sided, and a p-value of <0.05 was considered significant.
Results
Around 12,127 patients were diagnosed with breast cancer from January 2001 to December 2018 at Cancer Institute (WIA), Chennai, India. Among them, 40 patients (<1%) with metaplastic breast cancer were included in this analysis. Out of the 40, non-metastatic patients were 35 and metastatic patients were 5. The tumour, patient and treatment characteristics are shown in Table 1. The median age at presentation was 47 years (32–90 years). The mean size of the tumour was 6 cm (range = 2–16 cm). Majority of the patients presented with stage III (45%), followed by stage II (40%). Most of them were T2 (40%), T3 (27.5%) and T4 (27.5%). The lymph node status was positive in 40%. At presentation, five patients (12.5%) were diagnosed to have stage IV, with metastasis to lungs (n = 3), bone (n = 1) and brain (n = 1). The median follow-up duration was 60 months (5–161 months). The common pathological variant was mesenchymal differentiation in 21 (52.5%) and was also the common variant in ≤46 years (p = 0.03). The most common pathological diagnostic method was trucut biopsy in 65% and excision biopsy in 35%. Hormone receptors (ER and PR) were negative in 75% and triple negative in 37.5%.
Out of the 35 non-metastatic patients, 51.4% (n = 18) underwent upfront surgery, followed by adjuvant treatments, compared to 48.6% (n = 17) who received NAT. None of the HER2/neu-positive patients (n = 4) received trastuzumab. The responses observed to NAT (n = 17) were complete pathological response in one (5.9%), partial response in four (23.5%), static response in nine (53%) and progressive disease in three (17.6%). Out of the 35 non-metastatic patients, 91.4% (n = 32) underwent surgery, i.e., modified radical mastectomy in 30 and breast conservation surgery in 2, and 8.6% (n = 3) did not undergo surgery. Radiotherapy was administered in 68.6% (n = 24). In stage IV, one patient underwent a palliative mastectomy.
Table 1. Clinicopathological characteristics of the 40 patients with MPC.

Failure patterns and survival
For non-metastatic patients (n = 35), the median duration of follow-up was 71 months. Between 8 and 50 months, 17% developed systemic recurrences (n = 6). All six had lung metastasis (Table 2). Three of those who received NAT did not undergo planned surgery. During NAT, one had brain metastasis and the other two had local progressive disease only. One of the patients with local progressive disease considered inoperable had an initial biopsy of infiltrating breast carcinoma. A repeat biopsy was done because of local progression after NAT, which confirmed squamous cell carcinoma. The other patient was elderly on hormones and was inoperable due to local progressive disease. The median time to recurrence/progression was 15 months. The estimated OS at 5 years was 74.9% and DFS was 76%.
For metastatic patients (n = 5), the median follow-up duration was 9 months (5–26 months), with lung metastasis in 60% (n = 3), brain metastasis in 20% (n = 1) and bone metastasis in 20% (n = 1). The median PFS was 4 months (3–21 months), and none were alive at the end of 24 months. All received palliative therapy. One patient with an initial biopsy of triple-negative invasive breast cancer had local disease progression with the tumour size reaching 25 cm, and no response at the metastatic site (lungs), despite multiple lines of chemotherapy and palliative mastectomy was performed. The final diagnosis was a chondromyxoid variant of MPC and node-negative.
For the entire group of metaplastic patients (n = 40), at the time of censoring (December 2018), 15 patients had died (n = 15). Five were stage IV at presentation and died due to progressive disease. Out of the 10 non-metastatic patients, 6 died due to recurrent disease in the lungs. Out of the three patients who died during NAT, one died due to brain metastasis and two died due to progressive local disease. One died due to old age. The most common metastatic site was the lungs in 22.5%, brain in 5% and bone in 2.5%. The median PFS was 6 months. The 5-year OS and DFS were 64.4% and 66.3%, respectively.
Predictors of survival among the studied metaplastic patients (n = 40)
The stage was a predictor of OS. The 5-year OS in stages I and II, stage III and stage IV was 87.5%, 60.6% and 0%, respectively (p = 0.00001) (Figure 1). Age more than 46 years (p = 0.027) (Figure 2), tumour size more than 5 cm (p = 0.037) (Figure 3) and nodal positivity (p = 0.001) (Figure 4) were predictors of OS.
Table 2. Details of recurrent MPC.
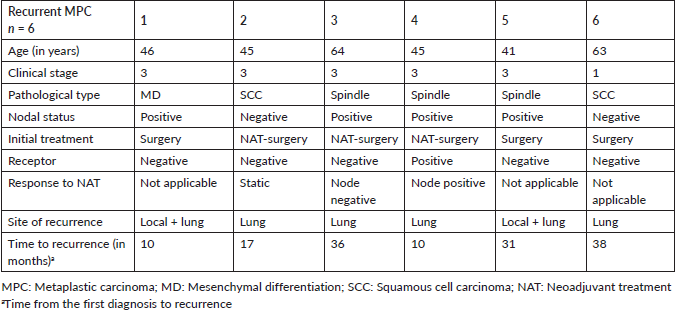
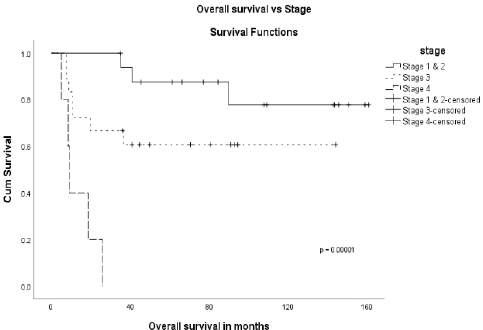
Figure 1. OS versus stage.
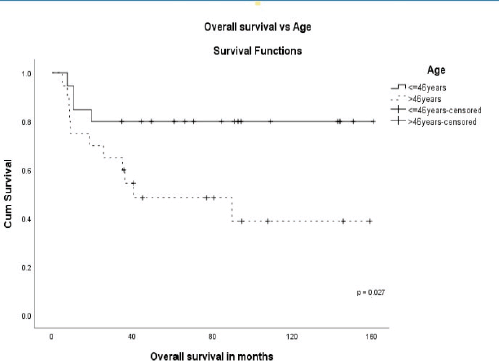
Figure 2. OS versus age.
Figure 3. OS versus tumour size.

Figure 4. OS versus node.
Predictors of survival in the non-metastatic patients (n = 35)
On univariate analysis, there were no statistically significant differences in OS or DFS regarding age, tumour size, receptor status, pathological subtype, and treatments received. During NAT, three had progressive disease. Clinical stage III (p = 0.015), nodal positivity (p = 0.001) and chemotherapy in early stage (p = 0.018) were significant predictors for DFS, and nodal positivity (p = 0.013) for OS (Table 3). Also, both treatment modalities, i.e., either initial surgery or after NAT, in node-positive and negative patients were analysed. In node-positive patients, the 5-year OS in those who underwent initial surgery was 80% and after NAT was 21.4% (p = 0.069) (Figure 5). The 5-year OS after initial surgery was 83.3% in node-negative and after NAT was 90% (p = 0.380) (Figure 6). In node-positive MPT, NAT did not improve DFS or OS, demonstrating that in node-positive patients, NAT does not add to survival benefit due to chemoresistance and variable response to NAT. A statistical significance could not be demonstrated due to the small number of patients.
Discussion
MPC is a rare but aggressive subtype of breast cancer [1–3]. MPC can coexist with ductal carcinoma in-situ (DCIS) or invasive mammary carcinoma. MPC presents with less frequent axillary nodal metastasis compared to IDC [4, 5, 8, 10, 11]. A clinicopathological analysis of 45 patients has reported axillary nodal involvement in 24%, and in some single institutional series, the reported incidence was 24%–28% [9–12]. In our study, axillary nodal involvement was observed in 40%. Also, MPC is more likely to have metastatic disease at presentation [4, 5, 8–10]. In our study, metastasis at presentation was reported in 12.5%. The majority of MPC were triple negative [1, 4, 6, 8, 13–15] and had a worse prognosis than non-metastatic triple-negative breast cancer (TNBC) [1, 4, 6, 8, 16]. Pezzi et al. [17] analysed 892 MPC and compared it with IDC and concluded that MPC presented with fewer T1 tumours (29% versus 65%), more of N0 (78% versus 66%) and fewer estrogen receptor-positive tumours (11% versus 74%). In the literature, HER2/neu expression (score 3+) was reported in 0%–25% [5, 7, 8, 17, 18]. We had 37.5% (n = 15) who were triple negative, receptor-negative in 75% (n = 30), receptor-positive in 25% (n = 10), HER2/neu positive in 10% (n = 4) and HER2/neu unknown status in 42.5% (n = 17). Also, they present with larger tumour sizes (T2, T3) [7–9, 15, 16, 19, 20] and are usually more aggressive than invasive mammary carcinoma [8, 21]. There is no specific characteristic finding in sonography and mammography. Some of the lesions are well defined, solid, cystic and can show benign characteristics [12, 22]. MPC is not a contraindication for breast conservation surgery (BCS), and the survival between BCS and mastectomy showed no difference [20, 23]. Given the larger sizes of tumours reported, most of the patients underwent a mastectomy. In our study group, two patients underwent BCS. Others underwent mastectomy due to the large size of the tumour, and 35% presented after an excision biopsy carried out at a clinic outside the institution. Sang Y et al. [8] reported a 5-year OS of 54.5% in MPC versus 85.1% in IDC and 73.3% in TNBC (p < 0.0001). A multicentric study of 405 patients reported no difference in OS between IDC versus MPC, and spindle cell type showed aggressive behaviour. In our study, out of the seven with spindle cell pathology, two (29%) were metastatic at presentation (Table 1) and three (43%) had recurrences (Table 2) during follow-up, confirming the aggressive nature of spindle cell type. In the early stage, chemotherapy improved survival [24]. In our study, in early-stage 5-year survival in those receiving chemotherapy was 86.7%. The local recurrence rate in large tumours is 35%–62% in the first 2–5 years compared to 17%–20% for IDC of the same tumour size. In our subset, the 5-year OS in tumours >5 cm was 47.1% versus 77.3% in ≤5 cm (p = 0.037). In one of the largest single-institution studies [11], 45 patients with MPC have reported a 5-year OS of 69% at a median follow-up of 28 months (range = 2–138 months). In our series, the 5-year OS was 64.4% at a median follow-up of 60 months (range = 5–161 months).
He et al. [25], in a retrospective population-based study of 1,112 metaplastic cancers, have shown tumours of larger size and less lymph node involvement consistent with our study. Also, they concluded that MPC had a worse prognosis than non-metaplastic triple-negative breast cancer and chemotherapy was not associated with improved survival. Also, as lymph node involvement is less likely, most patients underwent surgical options upfront and received less chemotherapy or radiotherapy than other TNBCs. One meta-analysis confirmed that adjuvant treatment after surgical management may improve 5-year OS in patients with MPC [26]. Our study also shows that NAT did not add to the OS benefit in the node-positive subset. Our analysis also confirms the inadequate response to NAT (Table 1) and initial surgical option in all operable non-metastatic patients should be considered.
Table 3. Univariate analysis of prognostic variables in non-metastatic MPC.
The limitation of our study is the retrospective nature and small number. In our study, the tumours were almost equally staged in clinical stages I and II (n = 17) and stage III (n = 18). Also, irrespective of the tumour’s size, it was observed that 60% had negative axillary nodes, and most of them were receptor-negative (75%). An equal number of patients had undergone initial surgery or NAT, followed by surgery. However, as expected in receptor-negative and triple-negative tumours, the response to neoadjuvant chemotherapy did not increase complete pathological rates as expected. Only one patient had a complete pathological response, and 7 (41%) out of 17 who received NAT continued to be node-positive. Also, the lungs was the common site of recurrence within 15 months in those who had completed planned treatments, explaining the aggressive and chemoresistant nature of the disease (Table 2). Another patient also had brain metastasis during NAT and was considered inoperable. Whether upfront surgery in operable patients will improve survival is a subject to be studied in the future.
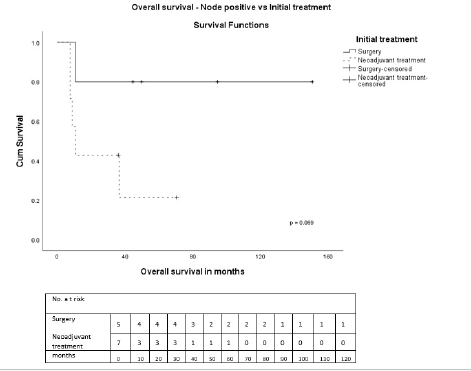
Figure 5. OS—Node-positive versus initial treatment.
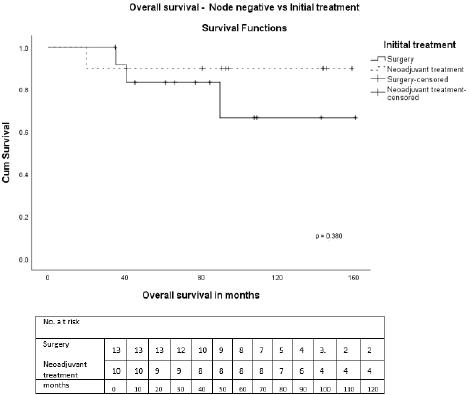
Figure 6. OS—Node-negative versus initial treatment.
The limitation of our study is the retrospective nature and small number. In our study, the tumours were almost equally staged in clinical stages I and II (n = 17) and stage III (n = 18). Also, irrespective of the tumour’s size, it was observed that 60% had negative axillary nodes, and most of them were receptor-negative (75%). An equal number of patients had undergone initial surgery or NAT, followed by surgery. However, as expected in receptor-negative and triple-negative tumours, the response to neoadjuvant chemotherapy did not increase complete pathological rates as expected. Only one patient had a complete pathological response, and 7 (41%) out of 17 who received NAT continued to be node-positive. Also, the lungs was the common site of recurrence within 15 months in those who had completed planned treatments, explaining the aggressive and chemoresistant nature of the disease (Table 2). Another patient also had brain metastasis during NAT and was considered inoperable. Whether upfront surgery in operable patients will improve survival is a subject to be studied in the future.
Conclusion
MPC is a chemoresistant tumour (ORR 31%) with a 5-year survival of 64%. NAT did not provide a survival benefit in node-positive subjects. These tumours have no significant response to chemotherapy, and there is a high chance of local disease progression and metastasis, making future surgical options after chemotherapy difficult. Upfront surgery should be considered for operable diseases (including stage III), followed by a decision on adjuvant therapy. Further studies are warranted to find the optimal treatment, and effective systemic therapy regimens are yet to be defined in MPC.
Conflicts of interest
The authors have no conflicts of interest to report.
Funding statement
The authors received no financial support for the research, authorship, and/or publication of this article.
References
1. Reis-Filho JS, Lakhani SR, and Gobbi H, et al (2012) Metaplastic carcinoma WHO Classification of Tumours of the Breast eds SR Lakhani, SJ Schnitt, and PH Tan, et al (Lyon: IARC Press) pp 48–52 [IARC Publications Website - WHO Classification of Tumours]
2. Lien HC, Lin CW, and Mao TL, et al (2004) p53 overexpression and mutation in metaplastic carcinoma of the breast: genetic evidence for a monoclonal origin of both the carcinomatous and the heterogeneous sarcomatous components J Pathol 204 131–139 https://doi.org/10.1002/path.1624 PMID: 15376261
3. Geyer FC, Weigelt B, and Natrajan R, et al (2010) Molecular analysis reveals a genetic basis for the phenotypic diversity of metaplastic breast carcinomas J Pathol 220 562–573 https://doi.org/10.1002/path.2675 PMID: 20099298
4. Bae SY, Lee SK, and Koo MY, et al (2011) The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients Breast Cancer Res Treat 126 471–478 https://doi.org/10.1007/s10549-011-1359-8 PMID: 21287362
5. Beatty JD, Atwood M, and Tickman R, et al (2006) Metaplastic breast cancer: clinical significance Am J Surg 191 657–664 https://doi.org/10.1016/j.amjsurg.2006.01.038 PMID: 16647355
6. Nelson RA, Guye ML, and Luu T, et al (2015) Survival outcomes of metaplastic breast cancer patients: results from a US population-based analysis Ann Surg Oncol 22(1) 24–31 https://doi.org/10.1245/s10434-014-3890-4
7. Luini A, Aguilar M, and Gatti G, et al (2007) Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature Breast Cancer Res Treat 101 349–353 https://doi.org/10.1007/s10549-006-9301-1
8. Song Y, Liu X, and Zhang G, et al (2013) Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators World J Surg Oncol 11 129 https://doi.org/10.1186/1477-7819-11-129 PMID: 23738706 PMCID: 3679991
9. Park HS, Park S, and Kim JH, et al Clinicopathologic features and outcomes of metaplastic breast carcinoma: comparison with invasive ductal carcinoma of the breast Yonsei Med J 51 864–869 [https://www.eymj.org/DOIx.php?id=10.3349/ymj.2010.51.6.864] PMID: 20879052 PMCID: 2995974
10. Lai HW, Tseng LM, and Chang TW, et al (2013) The prognostic significance of metaplastic carcinoma of the breast (MCB)—a case controlled comparison study with infiltrating ductal carcinoma Breast 22 968–973 https://doi.org/10.1016/j.breast.2013.05.010 PMID: 23787124
11. Cimino-Mathews A, Verma S, Figueroa-Magalhaes MC, et al (2016). A clinicopathologic analysis of 45 patients with metaplastic breast carcinoma Am J Clin Pathol 145 365–372 https://doi.org/10.1093/ajcp/aqv097 PMID: 27124919
12. Choi BB and Shu KS (2012) Metaplastic carcinoma of the breast: multimodality imaging and histopathologic assessment Acta Radiol 53 5–11 https://doi.org/10.1258/ar.2011.110341
13. Jung SY, Kim HY, and Nam BH, et al (2010) Worse prognosis of metaplastic breast cancer patients than other patients with triplenegative breast cancer Breast Cancer Res Treat 120 627–637 https://doi.org/10.1007/s10549-010-0780-8 PMID: 20143153
14. Montagna E, Maisonneuve P, and Rotmensz N, et al (2013) Heterogeneity of triple negative breast cancer: histologic subtyping to inform the outcome Clin Breast Cancer 13 31–39 https://doi.org/10.1016/j.clbc.2012.09.002
15. Fayaz S, Demian GA, and Eissa HE, et al (2017) Metaplastic breast carcinoma: Analysis of 31 cases from a single institute J Egypt Natl Canc Inst 29 141–145 https://doi.org/10.1016/j.jnci.2017.05.002 PMID: 28669452
16. Hasdemir OA, Tokgoz S, and Koybasioglu F, et al (2018) Clinicopathological features of metaplastic carcinoma Adv Clin Exp Med 27(4) 509–513 https://doi.org/10.17219/acem/68293 PMID: 29558027
17. Pezzi MC, Lina PP, and Karin C, et al (2007) Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base Ann Surg Oncol 14 166e173
18. Leibl S and Moinfar F (2005) Metaplastic breast carcinomas are negative for Her-2 but frequently express EGFR (Her-1): potential relevance to adjuvant treatment with EGFR tyrosine kinase inhibitors? J Clin Pathol 58 700–704 https://doi.org/10.1136/jcp.2004.025163
19. Zhang Y, Lv F, and Yang Y, et al (2015) Clinicopathological features and prognosis of metaplastic breast carcinoma: experience of a major Chinese cancer center PLoS One 10(6) e0131409 https://doi.org/10.1371/journal.pone.0131409 PMID: 26115045 PMCID: 4482719
20. Hu Q, Chen WX, and Zhong SL, et al (2013) Current progress in the treatment of metaplastic breast carcinoma Asian Pac J Cancer Prev 14 6221–6225 https://doi.org/10.7314/APJCP.2013.14.11.6221
21. Abouharb S and Moulder S (2015) Metaplastic breast cancer: clinical overview and molecular aberrations for potential targeted therapy Curr Oncol Rep 17(3) 431 https://doi.org/10.1007/s11912-014-0431-z PMID: 25691085
22. Günhan-Bilgen I, Memiş A, and Ustün EE, et al (2002) Metaplastic carcinoma of the breast: clinical, mammographic, and sonographic findings with histopathologic correlation AJR Am J Roentgenol 178 1421–1425 [https://www.ajronline.org/doi/full/10.2214/ajr.178.6.1781421] https://doi.org/10.2214/ajr.178.6.1781421 PMID: 12034610
23. Dave G, Cosmatos H, and Do T, et al (2006) Metaplastic carcinoma of the breast: a retrospective review Int J Radiat Oncol Biol Phys 64 771–775 https://doi.org/10.1016/j.ijrobp.2005.08.024
24. Rakha EA, Tan PH, and Varga Z, et al (2015) Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study Br J Cancer 112(2) 283–289 https://doi.org/10.1038/bjc.2014.592 PMCID: 4453452
25. He X, Ji J, and Dong R, et al (2019) Prognosis in different subtypes of metaplastic breast cancer: a population-based analysis Breast Cancer Res Treat 173 329–341 https://doi.org/10.1007/s10549-018-5005-6
26. Xu D and Hou L (2019) Clinicopathologic characteristics of mixed epithelial/mesenchymal metaplastic breast carcinoma (carcinosarcoma): a meta-analysis of Chinese patients Pol J Pathol 70(3) 174–182 https://doi.org/10.5114/pjp.2019.90393 PMID: 31820860






