Treatment pattern and outcomes in de novo T790M-mutated non-small cell lung cancer
Goutam Santosh Panda, Vanita Noronha, Darshit Shah, George John, Anuradha Chougule, Vijay Patil, Rajiv Kumar, Nandini Menon, Ajay Singh, Pratik Chandrani, Abhishek Mahajan and Kumar Prabhash
Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Dr. E Borges Road, Parel, Mumbai 400012, India
Abstract
Introduction: Limited data exists for non-small cell lung cancer (NSCLC) patients harbouring de novo T790M mutation.
Methods: NSCLC patients, with de novo T790M, who registered at our institute between 01/03/2015 and 31/12/2019, were considered for retrospective analysis of treatment pattern and clinical outcomes, i.e., progression-free survival (PFS) and overall survival (OS).
Results: Of 1,542 epidermal growth factor receptor (EGFR)-mutated patients, 40 (2.59%) had de novo T790M. Most were male (27, 67.5%) and smokers (23, 57.5%). The commonest site of metastasis was the lungs (31, 77.5%), while 7 (17.5%) had central nervous system (CNS) involvement. Additional EGFR gene mutations and anaplastic lymphoma kinase (ALK) positivity were observed in 20 (50.0%) and 4 (10.0%) cases, respectively. The first-line systemic therapy and the number of patients receiving it were as follows: osimertinib by 14 (35.0%), first-generation EGFR tyrosine kinase inhibitors (TKIs) by 10 (25.0%), gefitinib + chemotherapy by 3 (7.5%), chemotherapy by 7 (17.5%) and gefitinib + bevacizumab by 2 (5%). One patient defaulted before starting any treatment. Hence, 39 were considered for survival analysis. The median PFS and OS for the entire cohort were 10.4 (95% CI = 7.6–19.7) months and 24.9 (95% CI = 15.7–NA) months, respectively. The median PFS for patients on osimertinib was 19.8 (95% CI = 11.6–28.0) months versus 8.8 (95% CI = 6.6–10.9) months for those on other systemic therapy. No CNS involvement, use of osimertinib or first-generation EGFR TKI plus chemotherapy or ALK inhibitor in ALK-positive cases prognosticated better PFS. When compared to other systemic therapies, osimertinib improved PFS in patients with or without additional EGFR mutations, although it was statistically significant for the former group only (p = 0.002).
Conclusion: The incidence of de novo T790M is low. Osimertinib in frontline therapy provides promising outcomes.
Keywords: outcome, de novo T790M, non-small cell lung cancer, treatment pattern
Correspondence to: Vanita Noronha
Email: vanita.noronha@gmail.com
Published: 06/05/2022
Received: 27/01/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Epidermal growth factor receptor (EGFR)-activating mutations are the most common driver mutations in non-small cell lung cancer (NSCLC). EGFR-activating mutations can be found in exons 18–21 of the EGFR gene. Exon 19 deletions and an exon 21 L858R point mutation are the most common mutations in patients with advanced NSCLC [1–3]. First- and second-generation EGFR tyrosine kinase inhibitors (EGFR-TKIs) outperform platinum-based treatment in NSCLC patients with activating EGFR mutations [4, 5]. Even better outcomes are observed in these patients with the use of third-generation EGFR-TKI or combining chemotherapy with first-generation TKI [6, 7]. Following TKI therapy, the T790M mutation accounts for 50% of the acquired resistance mutations. When receiving TKI treatment, this mutation is thought to arise under selection pressure. De novo T790M mutations occur at a different rate depending on the population examined and the mutation detection technology used. According to direct sequencing, this mutation can be detected in 0.4%–3% of all NSCLCs and 1%–8% of all EGFR-mutant NSCLCs. The T790M mutation causes threonine to be replaced with methionine at position 790. As a result, the T790M mutation’s affinity for ATP increases, outperforming the reversible inhibitors. Osimertinib, a third-generation EGFR-TKI, is approved for patients with T790M, but in second-line therapy. The T790M detection rate can be as high as 79% using more sensitive techniques, e.g., mutant-enriched polymerase chain reaction (PCR) and mass spectrometry [8–10]. However, barring a few trials with a small number of patients, no good data exist for patients with de novo T790M, which has been linked to inferior outcomes [8, 10–12]. Reversible TKIs show limited activity in these cases and usually cytotoxic chemotherapy is used to treat these patients [13]. Given the paucity of data regarding management and outcome of patients with de novo T790M, we undertook a retrospective study to analyse the treatment pattern and clinical outcomes of NSCLC patients with de novo T790 mutation.
Methods
De novo T790M-mutated NSCLC patients who registered at our institute between 1/3/2015 and 31/12/2019 were considered for analysis. NSCLC patients harbouring additional driver mutations were also included. Prior history of malignancy was not an exclusion criterion. Approval from the Institutional Ethics Committee was taken and waiver for consent was obtained. No funding was used for this study.
The database maintained in the Department of Medical Oncology and Molecular Pathology was searched for de novo T790M-mutated patients. The World Health Organisation 2010 classification system was followed for making a histological diagnosis of NSCLC.
The primary objective of this study was to determine overall survival (OS). The secondary objectives were to study the pattern of care, determine the progression-free survival (PFS) and prognostic factors.
The QiaAmp formalin-fixed paraffin-embedded (FFPE) Tissue Kit was utilised for DNA extraction from six FFPE tissue sections of 14 mm each. The DNA was amplified for the EGFR exons 18–21 using a nested PCR method, followed by mutation analysis by TaqMan-based real-time PCR technique, as published earlier by our group [14].
The Response Evaluation Criteria in Solid Tumours 1.1 (RECIST 1.1) was employed for documenting response to therapy. The overall response rate (ORR) was calculated as the percentage of patients who achieved complete response (CR) or partial response (PR) on a particular therapy, while for the disease control rate (DCR), patients who attained stable disease, CR and PR were considered.
Statistical analysis
Statistical analysis was carried out using SPSS (IBM SPSS Statistics for Windows, Version 25.0). The Kaplan–Meier method was used for estimating survival and the log-rank test was employed for comparison. Independent prognostic factors were determined by Cox’s multivariate analysis of significant prognostic factors on univariate analysis.
PFS was the period between diagnosis of metastatic carcinoma of the lungs and the first event, which might be progression (clinical and/or radiological), second malignancy or death. Patients who did not have an event or were lost to follow-up for more than 6 months were censored. OS was computed from the date of diagnosis to death from any cause or the last documented follow-up. The cut-off date for data collection was 1 December 2021.
Results
Baseline characteristics
We had 1,542 NSCLC patients who harboured EGFR mutations and were registered at our institute during the above-mentioned time period. De novo T790M mutations were detected in 40 of 1,542 patients (2.59%). There were 27 (67.5%) males and 13 (32.5%) females among the 40 patients, with 23 (57.5%) smokers and the rest were never smokers (Table 1). The median age at presentation was 54.5 years (range = 27–75). In four cases, granular cytoplasmic positivity for anaplastic lymphoma kinase 1 (ALK1) was observed by immunohistochemistry (IHC) and 20 (50%) patients also had other EGFR mutations in exons 18, 19 and 21, such as L858R/L861Q and G719X. Figure 1a and b shows the additional driver mutations found in these patients.
The most common site of metastasis was the lungs, followed by bone. Central nervous system (CNS) involvement at baseline was noted in seven (17.5%) patients. Brain imaging/cerebrospinal fluid (CSF) studies were considered for patients who had symptoms and/or signs of CNS involvement. The exceptions were NSCLC patients, with no evidence of metastasis on computed tomography (CT) thorax, for whom a whole-body fluorodeoxyglucose positron emission tomography and computed tomography (FDG PET/CT) was ordered to rule out metastasis. Prior history of malignancy (carcinoma of breast and ovary in one each) was noted in two patients, while one patient had synchronous non-secretory pituitary macro-adenoma.
First-line (1L) treatment
Of these 40 patients, only one patient was non-metastatic at presentation and was initially treated with curative intent, i.e., surgical resection, followed by adjuvant chemotherapy. Later, this patient developed metastatic disease and was treated accordingly. The other 39 patients had metastatic disease at presentation. One patient defaulted before the start of any treatment at our institute and was not reachable. Hence, 39 patients were considered for survival analysis. Treatment for metastatic disease included first-generation TKI (erlotinib/gefitinib) alone in 10 (25.0%) patients, third-generation TKI (osimertinib) in 14 (35.0%) patients, gefitinib plus bevacizumab in 2 patients (5.0%), gefitinib plus chemotherapy in 3 (7.5%) patients, once-in-3-weeks pemetrexed and carboplatin doublet regimen in 6 out of 40 patients (15%), while etoposide and carboplatin doublet was given to 1 patient (Table 1). ALK inhibitor was advised for three out of four cases which were positive for ALK by IHC. No patient received combined targeted therapy, i.e., EGFR TKI plus ALK inhibitors.
Overall response rate (ORR) and disease control rate (DCR)
ORR for the entire cohort was 48.7% and the DCR was 89.7%. The ORR and DCR for patients receiving osimertinib was 64.3% and 92.9%, respectively. The same for patients on systemic therapy other than osimertinib was 40% and 88%, respectively. This is presented in Table 2. The ORR and DCR with osimertinib for patients harbouring only T790M were 37.5% and 87.5%, respectively. For patients receiving systemic therapies other than osimertinib and having no additional EGFR mutation, the ORR and DCR were 41.7% and 83.3%, respectively.
Treatment post-progression on 1L
Of all 39 patients analysed for survival, 26 patients had events for PFS (death = 2 patients; progression = 24 patients). Of these 26 patients, 18 patients received second-line therapy after progression on first-line systemic therapy, while the remaining 8 patients defaulted. The second-line treatment included osimertinib and chemotherapy in eight patients each, chemotherapy plus first-generation EGFR-TKI and ALK inhibitor in one patient each. Of all the patients experiencing events, repeat biopsy was carried out for only three patients and it could not be carried out for the rest of the patients due to various reasons such as poor ECOG PS post-progression, logistics for molecular testing and patient defaulting.
Survival
The median follow-up in surviving patients was 34.9 (95% CI = 28.1–41.6) months. There were 26 (26/39, 66.7%) progressions and a total of 22 deaths, with no death attributable to the toxicity of systemic therapy.
A) PFS: At the time of analysis, 26 (26/39, 72.2%) patients experienced events for PFS. Only two patients failed in the brain. The median PFS was 10.4 (95% CI = 7.6–19.7) months (Figure 2). The projected 3-year PFS was 13.0%.
B) OS: There were 22 deaths, none due to the toxicity of systemic therapy. The median OS was 24.9 (95% CI = 15.7–NA) months and the 3-year OS estimate was 24.6% (Figure 3).
Table 1. Baseline parameters of patients with de novo T790M mutation.
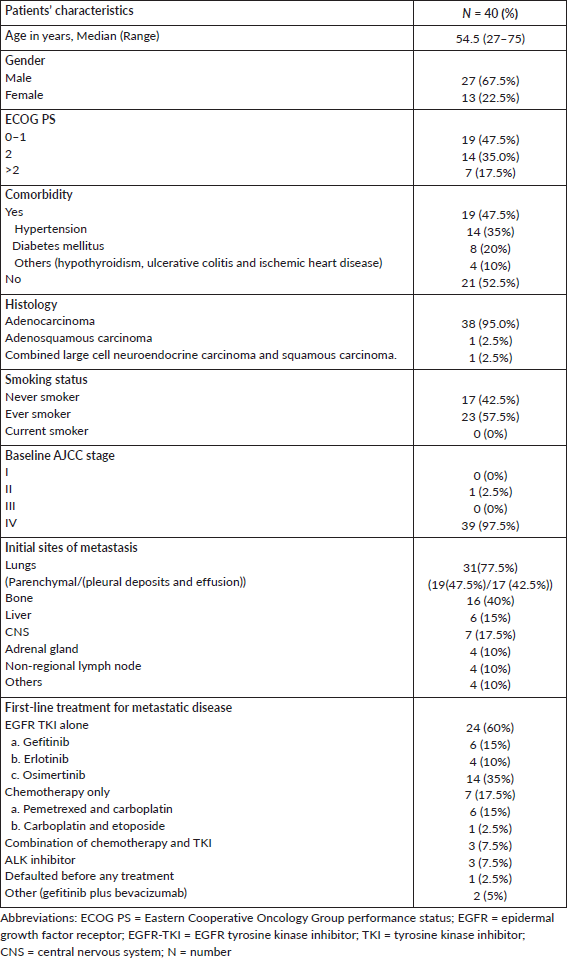
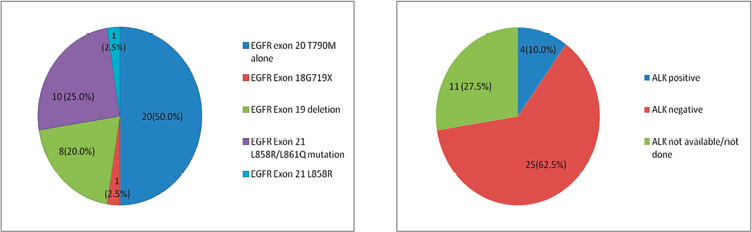
Figure 1. (a): Additional EGFR mutations in patients with de novo T790M. (b): Additional ALK rearrangement with de novo T790M.
Table 2. ORR and DCR with various treatments for patients with de novo T790M.
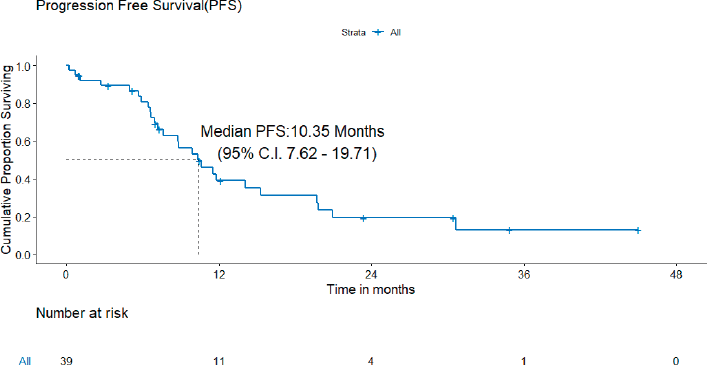
Figure 2. PFS of patients with de novo T790M.
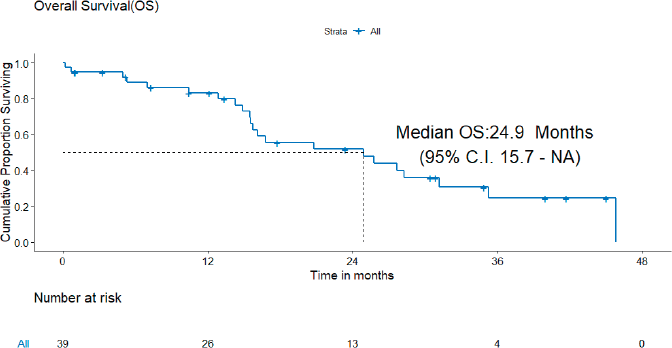
Figure 3. OS of patients with de novo T790M
Patients with de novo T790M only
Osimertinib led to a median PFS and OS of 14.1 (95% CI = 4.6–23.6) months and 27.7 (95% CI = 0–56.7) months, respectively. Systemic therapies other than osimertinib led to median PFS and OS of 8.8 (95% CI = 4.5–13.0) and 14.9 (95% CI = 12.4–17.4) months in patients with de novo T790M-only EGFR mutation.
Patients with additional EGFR mutations
On the contrary, patients on osimertinib who harboured additional EGFR mutations had median PFS and OS of 19.8 (95% CI = 19.7–20.0) months and 24.9 (95% CI = 17.6–32.2) months, respectively. The same for patients on other systemic therapies were 7.6 (95% CI = 4.6–10.6) months and 20.8 (95% CI = 13.2–28.4), respectively.
Prognostic factors
Entire cohort: In univariate analysis, no baseline CNS involvement (p = 0.04), using osimertinib (p = 0.01), using osimertinib/first-generation EGFR TKI plus chemotherapy/ALK inhibitor in ALK-positive NSCLC cases (p = 0.005) were predictors of superior PFS, while the only absence of CNS involvement was an indicator of better OS. The median PFS for patients on osimertinib was 19.8 (95% CI = 11.6–28.0) months versus 8.8 (95% CI = 6.6–10.9) months for those on other systemic therapy (Figure 4). The multivariate analysis could not be carried out due to fewer events. Poor PS at presentation tended to predict inferior OS (p = 0.051). Although osimertinib compared to other systemic therapies led to an improvement in PFS for both cohorts, i.e., patients with additional EGFR mutation and de novo T790M alone, it was statistically significant for patients with additional EGFR mutations only (p = 0.002).
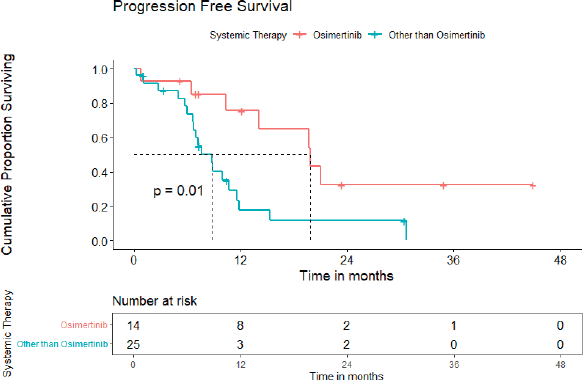
Figure 4. PFS for patients with de novo T790M using osimertinib versus other systemic therapies.
Discussion
The incidence of EGFR (exons 18–21) mutation in Indian ethnicity is 23%. Of these, EGFR exon 19 deletion and exon 21 mutation together constitute >90% [14]. The incidence of de novo T790M NSCLC varies from 1% to 38% and it depends on the detection method applied [2, 9, 12, 15]. We used real-time PCR with an assay sensitivity limit of detection of 1% for detecting EGFR mutations [14]. The incidence of T790M mutation at baseline was low in our cohort (2.59%). Two prior studies by our group also reported a low incidence of de novo T790M. In a study by Kate et al., only 10 (0.8%) of 1,260 patients harbouring an EGFR mutation had the de novo T790M mutation [16]. Similarly, the incidence of de novo T790M mutations reported by Noronha et al [17] was low (4/247; 1.6%) among patients with the EGFR exon 20-mutated lung cancer cases. However, a meta-analysis of 22 studies revealed the de novo T790M mutation rate to be in the range of 22%–80% [18]. Interestingly, we observed EGFR T790M at a 2.6% frequency, more in line with another study from China [19]. We suggest future experiments with highly sensitive NGS or droplet digital PCR (ddPCR) for a better assessment of T790M mutation rate in the Indian cohort. Most of the patients were non-smokers as seen with other EGFR-sensitising mutations [6, 7, 20]. The incidence of baseline CNS involvement in our study was 17.5%. This corroborates with our previous studies [7, 21] in which the incidence was 13.9% and 18%, respectively. The reported incidence of CNS involvement in EGFR-positive NSCLC ranges from 20% to 55% [22–26]. A co-existing EGFR mutation was noted in approximately half of the patients (19/40, 47.5%) in our study, similar to other studies [27, 28].
The T790M is known to be insensitive to first-generation TKIs. Hence, for patients with the T790M mutation, osimertinib is recommended, but only in the second or subsequent lines of treatment. The clinical outcomes for these patients have been studied in few retrospective studies with a limited sample size. ORR for patients on osimertinib in our cohort was 64.3%. The response rate with osimertinib was 85.7% in the AURA trial, in which 6 out of 7 patients with T790M mutation had a response to osimertinib [29]. An objective response rate of 80% in osimertinib arm and 76% in the standard EGFR TKI arm was observed in a phase-3 randomised trial of patients with untreated NSCLC harbouring exon 19 deletion or L858R mutation in EGFR gene [6]. Similarly, osimertinib in second line for NSCLC patients with T790M conferred an objective response rate of 71% [30]. The median PFS of patients with de novo T790M was superior statistically when treated with osimertinib and so was OS, although not statistically significant. However, in our cohort, patients with de novo T790M alone had inferior response rates (ORR and DCR) and survival outcomes (PFS and OS) compared to those having additional EGFR mutations. However, these worse outcomes were not statistically significant. The inferior outcomes in patients with de novo T790M alone could be due to the absence of other EGFR driver mutations, especially sensitising ones, small sample size, retrospective the nature of study and a real-world scenario, which has its own intricacies.
The outcome of patients harbouring de novo T790M was poor with a median PFS of 10 months, while the same for patients with sensitising EGFR mutations was 16 months on gefitinib+chemotherapy [7]. Our median PFS of 10 months in these patients with de novo T790M is likewise comparable to the result reported by other investigators with a limited sample size [9, 12, 31]. The median PFS was slightly higher (23.1 months (95% CI = 14.1–NE)) for the recently published phase-IIa study comprising 22 patients, with all receiving osimertinib [32] compared to the median PFS of 19.8 months in our cohort of patients receiving osimertinib. The possible explanations for higher PFS are small sample size, T790M coexistent in low allelic frequency with exon 19 deletion or L858R mutation and separate ethnicity from our cohort. However, the response rate and disease control rate were similar (77.3% and 86.4%, respectively). In our study, although the outcome with gefitinib+chemotherapy was comparable to osimertinib, a small number of patients (only three) received the former treatment. Hence, a firm conclusion cannot be drawn on the efficacy of gefitinib plus chemotherapy for these patients. Moreover, osimertinib compared to other systemic therapies led to superior PFS in patients with or without additional EGFR mutations, although the improvement in PFS was statistically significant for those with additional EGFR mutations.
Limitation
The limitation of this study includes all the limitations of a retrospective study. Also, we did not analyse the patients’ allelic frequency as a prognostic factor, which may have an impact on the outcome. Although carried out in a limited number of patients, its clinical relevance cannot be overlooked due to the rarity of such cases and the lack of any standard guidelines. We report the outcome of such patients with relatively good follow-up.
Conclusion
The incidence of de novo T790 mutations in NSCLC is low. Osimertinib can be considered as frontline therapy for these patients with a promising outcome. Future prospective studies are needed for better understanding of disease biology, treatment and prognosis of patients with this rare mutation.
Abbreviations
EGFR = Epidermal growth factor receptor
TKI = Tyrosine kinase inhibitor
NSCLC = Non-small cell lung cancer
CNS = Central nervous system
ALK = Anaplastic lymphoma kinase
RECIST 1.1 = Response Evaluation Criteria in Solid Tumours 1.1
PFS = Progression-free survival
OS = Overall survival
FDG PET/CT = Fluorodeoxyglucose positron emission tomography and computed tomography
Conflicts of interest/competing interests
None.
Funding
No financial assistance was utilised for this study.
Ethical approval
Approval from institutional ethics committee (IEC) was taken and waiver of consent was obtained from IEC.
References
1. Wu Y-L, Zhou C, and Hu C-P, et al (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial Lancet Oncol [Internet] 15(2) 213–222 https://doi.org/10.1016/S1470-2045(13)70604-1
2. Mok TS, Wu Y-L, and Thongprasert S, et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma N Engl J Med [Internet] 361(10) 947–957 https://doi.org/10.1056/NEJMoa0810699
3. Yoshida K, Yatabe Y, and Park JY, et al (2007) Prospective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer J Thorac Oncol [Internet] 2(1) 22–28 [https://www.ncbi.nlm.nih.gov/pubmed/17410005] https://doi.org/10.1097/01243894-200701000-00006
4. Maemondo M, Inoue A, and Kobayashi K, et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR N Engl J Med [Internet] 362(25) 2380–2388 https://doi.org/10.1056/NEJMoa0909530
5. Sequist LV, Yang JC-H, and Yamamoto N, et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations J Clin Oncol [Internet] 31(27) 3327–3334 https://doi.org/10.1200/JCO.2012.44.2806
6. Soria J-C, Ohe Y, and Vansteenkiste J, et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer N Engl J Med [Internet] 378(2) 113–125 https://doi.org/10.1056/NEJMoa1713137
7. Noronha V, Patil VM, and Joshi A, et al (2020) Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFRmutated lung cancer J Clin Oncol [Internet] 38(2) 124–136 https://doi.org/10.1200/JCO.19.01154
8. Inukai M, Toyooka S, and Ito S, et al (2006) Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer Cancer Res [Internet] 66(16) 7854–7858 https://doi.org/10.1158/0008-5472.CAN-06-1951
9. Rosell R, Molina MA, and Costa C, et al (2011) Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinibtreated advanced non-small-cell lung cancer patients with EGFR mutations Clin Cancer Res [Internet] 17(5) 1160–1168 https://doi.org/10.1158/1078-0432.CCR-10-2158
10. Su K-Y, Chen H-Y, and Li K-C, et al (2012) Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer J Clin Oncol [Internet] 30(4) 433–440 https://doi.org/10.1200/JCO.2011.38.3224
11. Fukuoka M, Wu Y-L, and Thongprasert S, et al (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol [Internet] 29(21) 2866–2874 https://doi.org/10.1200/JCO.2010.33.4235
12. Maheswaran S, Sequist LV, and Nagrath S, et al (2008) Detection of mutations in EGFR in circulating lung-cancer cells N Engl J Med [Internet] 359(4) 366–377 https://doi.org/10.1056/NEJMoa0800668
13. Prabhash K (2019) Treatment of advanced nonsmall cell lung cancer: First line, maintenance and second line – Indian consensus statement update South Asian J Cancer [Internet] 8(1) 1–17 https://doi.org/10.4103/sajc.sajc_227_18
14. Chougule A, Prabhash K, and Noronha V, et al (2013) Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity PLoS One [Internet] 8(10) e76164 https://doi.org/10.1371/journal.pone.0076164
15. Sequist LV, Martins RG, and Spigel D, et al (2008) First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations J Clin Oncol [Internet] 26(15) 2442–2449 https://doi.org/10.1200/JCO.2007.14.8494
16. Kate S, Chougule A, and Joshi A, et al (2019) Outcome of uncommon EGFR mutation positive newly diagnosed advanced non-small cell lung cancer patients: a single center retrospective analysis Lung Cancer [Internet] 10 1–10
17. Noronha V, Choughule A, and Patil VM, et al (2017) Epidermal growth factor receptor exon 20 mutation in lung cancer: types, incidence, clinical features and impact on treatment Onco Targets Ther [Internet] 10 2903–2908 https://doi.org/10.2147/OTT.S133245
18. Liu Y, Sun L, and Xiong Z-C, et al (2017) Meta-analysis of the impact of de novo and acquired EGFR T790M mutations on the prognosis of patients with non-small cell lung cancer receiving EGFR-TKIs Onco Targets Ther [Internet] 10 2267–2279 https://doi.org/10.2147/OTT.S133082
19. Tian P, Wang Y, and Wang W, et al (2018) High-throughput sequencing reveals distinct genetic features and clinical implications of NSCLC with de novo and acquired EGFR T790M mutation Lung Cancer [Internet] 124 205–210 https://doi.org/10.1016/j.lungcan.2018.08.014
20. Shi Y, Au JS-K, and Thongprasert S, et al (2014) A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol [Internet] 9(2) 154–162 https://doi.org/10.1097/JTO.0000000000000033
21. Noronha V, Joshi A, and Gokarn A, et al (2014) The importance of brain metastasis in EGFR mutation positive NSCLC patients Chemother Res Pract [Internet] 2014 856156
22. Rosell R, Moran T, and Queralt C, et al (2009) Screening for epidermal growth factor receptor mutations in lung cancer N Engl J Med [Internet] 361(10) 958–967 https://doi.org/10.1056/NEJMoa0904554
23. Gahr S, Stoehr R, and Geissinger E, et al (2013) EGFR mutational status in a large series of Caucasian European NSCLC patients: data from daily practice Br J Cancer [Internet] 109(7) 1821–1828 https://doi.org/10.1038/bjc.2013.511
24. Remon J and Besse B (2018) Brain Metastases in oncogene-addicted non-small cell lung cancer patients: incidence and treatment Front Oncol [Internet] 8 88 https://doi.org/10.3389/fonc.2018.00088
25. Iuchi T, Shingyoji M, and Itakura M, et al (2015) Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations Int J Clin Oncol [Internet] 20(4) 674–679 https://doi.org/10.1007/s10147-014-0760-9
26. Rangachari D, Yamaguchi N, and VanderLaan PA, et al (2015) Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers Lung Cancer [Internet] 88(1) 108–111 https://doi.org/10.1016/j.lungcan.2015.01.020
27. Lee SH, Kim EY, and Kim A, et al (2020) Clinical implication and usefulness of de novo EGFR T790M mutation in lung adenocarcinoma with EGFR-tyrosine kinase inhibitor sensitizing mutation Cancer Biol Ther [Internet] 21(8) 741–748 https://doi.org/10.1080/15384047.2020.1776579
28. Wang S, Yan B, and Zhang Y, et al (2019) Different characteristics and survival in non-small cell lung cancer patients with primary and acquired EGFR T790M mutation Int J Cancer [Internet] 144(11) 2880–2886 https://doi.org/10.1002/ijc.32015
29. Yang JC-H, Ahn M-J, and Kim D-W, et al (2017) Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA Study Phase II Extension Component J Clin Oncol [Internet] 35(12) 1288–1296 https://doi.org/10.1200/JCO.2016.70.3223
30. Mok TS, Wu Y-L, and Ahn M-J, et al (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer N Engl J Med [Internet] 376(7) 629–640 https://doi.org/10.1056/NEJMoa1612674
31. Costa C, Molina MA, and Drozdowskyj A, et al (2014) The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial Clin Cancer Res [Internet] 20(7) 2001–2010 https://doi.org/10.1158/1078-0432.CCR-13-2233
32. Majem M, Sullivan I, and Viteri S, et al (2021) First-line osimertinib in patients with epidermal growth factor receptor-mutant non-small-cell lung cancer and with a coexisting low allelic fraction of Thr790Met Eur J Cancer [Internet] 159 174–181 https://doi.org/10.1016/j.ejca.2021.09.039






