Evaluation of prognostic factors in patients undergoing first-line chemotherapy for advanced biliary tract cancer: a retrospective analysis from a South American cancer centre
Tiago Cordeiro Felismino1a, Felipe Marcio Araujo Oliveira1, Camilla Albina Zanco Fogassa1, Silvia Nardozza Santerini1, Victor Hugo Fonseca de Jesus1, Rachel Simões Pimenta Riechelmann1, Felipe José Fernandez Coimbra2 and Celso Abdon Lopes Mello1
1Clinical Oncology Department, AC Camargo Cancer Center, Rua Professor Antonio Prudente 211, São Paulo, 01509-001, Brazil
2Surgical Oncology Department, AC Camargo Cancer Center, Rua Professor Antonio Prudente 211, São Paulo, 01509-001, Brazil
ahttps://orcid.org/0000-0002-1055-8118
Abstract
Introduction: Biliary tract cancers (BTCs) are rare tumours with regional differences. Prognostic factors are poorly understood. Gemcitabine + platinum (GP) is the standard first-line chemotherapy in metastatic patients. We aimed to search for prognostic factors in patients with advanced disease in a cancer centre in South America.
Methods: We conducted a retrospective analysis of patients with advanced BTC treated with chemotherapy. Variables were age (< or ≥70 years), Eastern Cooperative Oncology Group (ECOG) performance status (0/1 versus 2/3), gender, primary site (intrahepatic (IHC), extrahepatic (EHC), gallbladder (GB)), staging (locally advanced versus metastatic), metastatic sites, albumin (>3.5 g/dL versus <3.5 g/dL), biliary obstruction and first-line chemotherapy (GP, 5FU-based or single-agent). Cox regression method was used to explore factors.
Results: From 2010 to 2017, 104 patients were included. Median age was 62 years (32–86) and 22.1% were older than 70 years. Most patients had ECOG performance status 0/1 (63.4%), were female (51.9%) and were metastatic (82.7%). Bone metastases were found in 19.2%. Primary IHC, EHC and GB were 54.8%, 36.5% and 8.7%, respectively. GP was used by 79.8%. Median follow-up was 32.4 months. Median overall survival (mOS) was 11.4 months. In univariate analysis, male (p = 0.007), albumin < 3.5 g/dL (p = 0.001), biliary obstruction (p = 0.006), 5FU-based (p = 0.006) and single-agent (p < 0.0001) were associated with worse OS. ECOG performance status 2/3 (p = 0.058) and bone metastases (p = 0.051) were marginally related. In multivariate analysis, male (p = 0.003), bone metastases (p = 0.023), biliary obstruction (p = 0.001), 5FU-based (p = 0.016) and single-agent (p = 0.023) were independently associated with inferior OS.
Conclusion: In this retrospective study, we observed that male patients, bone metastases, biliary obstruction and regimens other than GP had worse survival. Larger studies should be conducted to confirm our findings.
Keywords: biliary tract neoplasms, prognosis, chemotherapy
Correspondence to: Tiago Cordeiro Felismino
Email: tiago.felismino@accamargo.org.br
Published: 17/01/2022
Received: 26/02/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Biliary tract cancers (BTCs) are a rare group of malignances [1]. Main subsites are intrahepatic (IHC), extrahepatic cholangiocarcinoma (EHC) and gallbladder (GB) cancer. Incidence is higher in China and southeast Asia [2]. Recently, an increase in the incidence of IHC has been described [3]. However, the incidence of GB cancer has decreased, mainly due to the increase in cholecystectomy. In Latin America, Bolivia has the highest age-adjusted incidence rate for both sex (14.0/100,000) followed by Chile (9.3/100,000) and Peru (4.8/100,000) [4, 5]. The regional differences observed in incidence are mostly due to differences in environmental exposures to various chemicals, genetic predisposition and regional intrinsic risk factors that predispose to carcinogenesis [4]. In the metastatic setting, prognosis is poor. Gemcitabine plus Cisplatin (GC) is considered the standard therapy in the first-line setting based in the ABC-02 trial. Overall survival (OS) reached 11.7 months in the doublet regimen, compared to 8.1 months in gemcitabine monotherapy arm [6]. Recently, ABC-06 showed superiority of FOLFOX over best supportive care in second-line patients [7]. Main prognostic factors are poorly defined due to the rarity and heterogeneity of this disease. Herein we aim to describe main prognostic factors for metastatic BTC in a South American cohort of patients treated at a large cancer centre, who received first-line chemotherapy.
Methods
We conducted a retrospective analysis of patients treated at a cancer centre in Brazil, from 2010 to 2017. Patients were included if they were 18 years of age or older, had histologic diagnosis of metastatic, recurrent or locally advanced unresectable BTC (IHC or EHC and gallbladder cancer). All patients were deemed eligible to receive first-line chemotherapy by their treating physician. Exclusion criteria were absence of histologic confirmation, absence of data regarding systemic treatment and mixed histologies (hepatocellular-cholangiocarcinoma). Medical files were used as source of information. This study was approved by the local Ethics Committee (2680/19).
Statistical analysis
Descriptive statistics were used for main demographic characteristics. Survival curves were estimated using Kaplan–Meier method and compared with log-rank test. OS was defined as date of first chemotherapy cycle and death from any cause. Progression-free survival (PFS) was defined as date of first chemotherapy cycle and disease progression or death from any cause. Radiologic assessments of response and clinical benefit were performed according to local guidelines, typically by means of clinical examination every 2 weeks and computed tomography or magnetic resonance imaging every 8 weeks. Progressive disease was identified by the treating physician.
X2 tests were used to analyse categorical variables distributions between genders. To evaluate prognostic factors, univariate and multivariate analysis were performed using the Cox regression method. Age (<70 years versus >70 years), gender, staging (locally advanced versus metastatic), primary site (EHC versus IHC versus GB carcinoma), Eastern Cooperative Oncology Group (ECOG) performance status (0–1 versus 2–3), bone metastasis, baseline Carbohydrate Antigen 19-9 (CA19-9), albumin (<3.5 g/dL versus > 3.5 g/dL), biliary obstruction and first-line treatment (gemcitabine + platinum (GP) versus 5-Fluorouracil (5-FU) based versus monotherapy) were used in the univariate model. All tests were considered statistically significant with a two-sided p value of < 0.05. Statistical analysis was performed with IBM SPSS 20.
Results
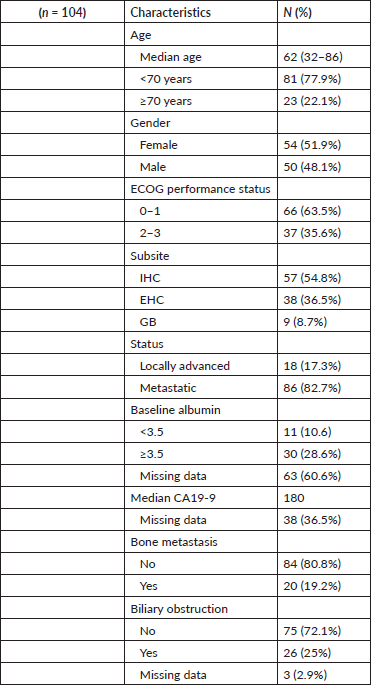
Between July/2009 and March/2017, 104 patients were identified. Main demographics are shown in Table 1. Median age was 62 years (32–86). Patients older than 70 years were 23 (22.1%). Male/female proportion was 50 (48.1%) and 54 (51.9%), respectively. Primary site was IHC in 57 (54.8%), EHC in 38 (36.5%) and GB in 9 (8.7%). Metastatic/locally advanced unresectable were 86 (82.7%) and 18 (17.3%). ECOG performance status 0/1 and 2/3 were present in 66 (63.5%) and 37 (35.6%). Main sites of metastasis were liver in 67 (64.4%), distant lymph nodes in 26 (25%) and bone in 20 (19.2%). Median baseline CA19-9 was 185 U/mL (0.6–34.833). Biliary obstruction at diagnosis of advanced disease was present in 26 (25%).
Table 1. Patients characteristics.
Regarding first-line chemotherapy (Table 2), GP, 5-FU based and monotherapy were used in 83 (79.8%), 12 (11.5%) and 9 (8.7%), respectively. GP combination was 88% Cisplatin and 12% Oxaliplatin. FOLFOX/CAPOX and FOLFIRINOX were used in ten and two patients, respectively. Gemcitabine and Capecitabine monotherapy were used in 6 and 3 patients, respectively. Fifty-nine (56.7%) patients underwent second-line chemotherapy. Main second-line regimens were FOLFIRI (33.9%) and FOLFOX (32.2%).
Numerically, more female patients used GP as first-line (85.2% versus 74%, p = 0.32). Access to second-line was not statistically different among gender (59.3% for female versus 54% for male, p = 0.69).
Table 2. First-line regimens.

Survival
Median follow-up time was 34.2 months (95% CI: 29.9–38.4). At time of analysis, seventy-six (73.1%) patients had died. Median OS (mOS) was 11.4 months (95% CI: 9.0–13.7) (Figure 1). Median progression-free survival in first-line was 5.6 months (95% CI: 4.0–7.2).
In univariate analysis, male patients (hazard ratio (HR): 1.88 (95% CI: 1.19–2.98); p = 0.007), albumin < 3.5 g/dL (HR: 4.73 (95% CI: 1.96–11.4); p = 0.001), biliary obstruction (HR: 2.15 (95% CI: 1.24–3.71); p = 0.006), 5FU-based (HR: 2.64 (95% CI: 1.31–5.30); p = 0.006) and single-agent (HR: 6.27 (95% CI: 2.36–16.6); p < 0.0001) were associated with worse OS. Bone metastases (HR: 1.82 (95% CI: 0.99–3.34); p = 0.051) and ECOG performance status 2–3 (HR: 1.58 (95% CI: 0.98–2.53); p = 0.058) were marginally associated with prognosis.
In multivariate analysis, male patients (HR: 4.18 (95% CI: 1.62–10.8); p = 0.003), bone metastases (HR: 3.53 (95% CI: 1.18–10.5); p = 0.023), biliary obstruction (HR: 4.28 (95% CI: 1.75–10.440; p = 0.001), 5FU-based (HR: 6.24 (95% CI: 1.41–27.6); p = 0.016) and single agent chemotherapy (HR: 7.22 (95% CI: 1.30–39.8); p = 0.023) were significantly and independently associated with inferior OS (Table 3). mOS was 15.9 months and 7.3 months for female and male patients, respectively (Figure 2).
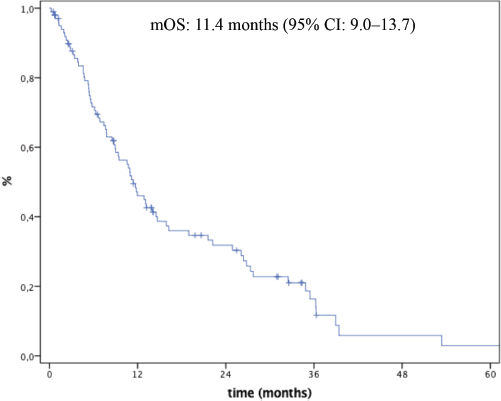
Figure 1. Overall survival.
Table 3. Univariate and multivariate analysis for OS.
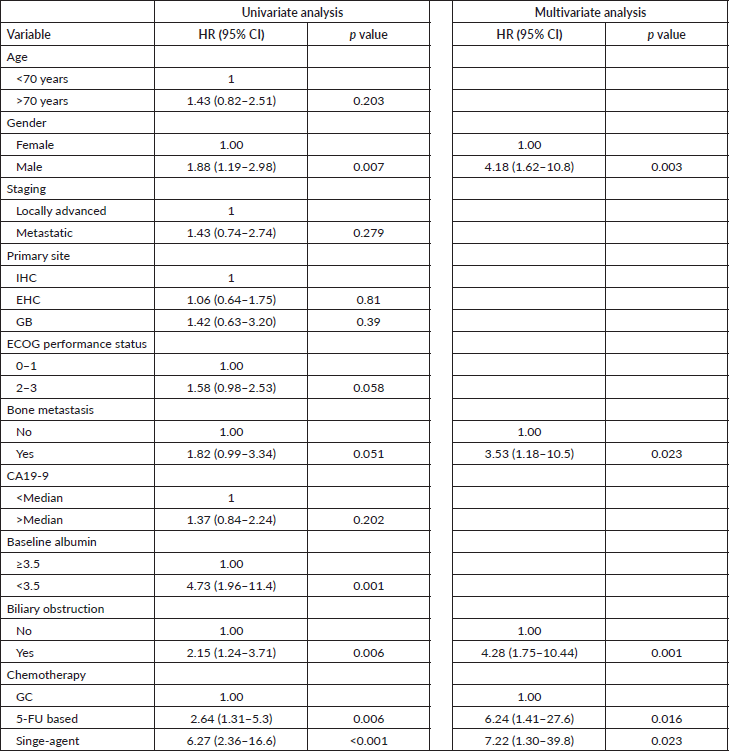
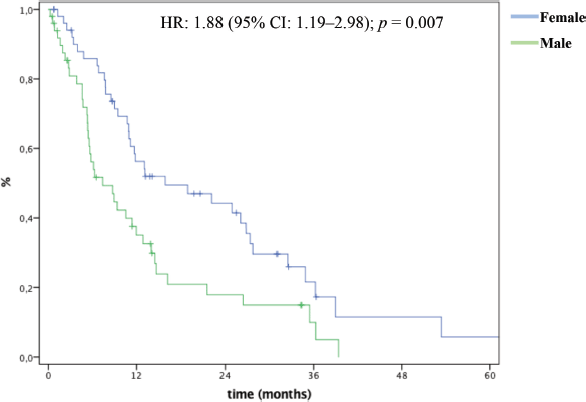
Figure 2. OS by gender.
Discussion
BTC is a rare gastrointestinal neoplasm but with increasing incidence over the past years, especially for IHC [3]. Surgery is the only curative procedure for a minority of patients who present with localised disease [8]. Survival of patients undergoing palliative chemotherapy for metastatic or irresectable disease is still poor [9]. Any effort to better understand this pathology is worth done. Our study confirmed the survival rates observed in main prospective trials in the metastatic scenario. In our multivariate model for OS, gender, presence of bone metastasis, biliary obstruction and first-line chemotherapy regimen other than GC were correlated to inferior OS.
Our data bring relevant information about two aspects of metastatic BTC. The first is regarding the standard first-line treatment. Patients treated with GP regimens presented a more favourable outcome. GP was superior to Gemcitabine in the Phase III ABC-02 study and was established as the standard of treatment first-line therapy for metastatic biliary tract carcinomas [6]. On the other, based on prospective trials, Gemcitabine plus Oxaliplatin (GemOx) is considered an alternative regimen for first-line [10, 11], although direct comparison of GP and GemOx has not been conducted in a randomised trial.
Recently, the ABC-06 trial established FOLFOX as a new standard for second-line treatment of BTC [7]. Based on the good toxicity profile of FOLFOX and the activity of fluoropyrimidine for gastrointestinal carcinomas in general, this regimen is employed in the first-line for patients with metastatic BTC in specific situations. In 2017, Schinzari et al [12] published the results of the phase II trial comparing FOLFOX4 with de Gramont regimen. In this trial, FOLFOX4 had superior PFS (5.2 versus 2.8 months (HR: 0.47 (95% CI: 0.25–0.89); p = 0.0031) and OS (13.0 versus 7.5 months (HR: 0.31 (95% CI: 0.15–0.63); p = 0.0013). However, in our study, 7.6% of patients were treated with FOLFOX in first-line and despite the small sample, outcomes were worse than those of patients treated with Gemcitabine combinations, reinforcing the role of GP. Clinical reasons not to employ GP were not accurately assessed in our analysis.
Second, female gender was significatively associated with better survival. We found that survival was almost doubled among female patients. In analogous tumours like metastatic pancreatic cancer, gender is a controversial prognostic factor [13, 14] . However, one retrospective analysis suggested that gender may influence responses to FOLFIRINOX [15].
Clinical data endorses our findings. Bridgewater et al [16] described main prognostic factors in ABC-02 patients and in an international dataset. Male patients were identified as having worse OS (HR: 1.28 (95% CI: 1.01–1.60); p = 0.037). Baton et al [17] published a series of 59 hilar cholangiocarcinoma patients who underwent resection. Male gender was related to poor OS (HR: 5.4 (95% CI: 2.2–13.5); p = 0.0002). Another retrospective large series from Korea with 740 BTC patients [18] also showed worse survival for male patients (HR: 0.83 (95% CI: 0.69–0.99); p = 0.04).
Some aspects of BTC regarding access to therapeutics may help clarify this difference. Undergoing second and further lines of treatment is probably a strong prognostic factor. In a large study of second-line therapy, out of 378 patients that received first-line chemotherapy, only 96 patients (25%) received second-line. Female/male ratio was 31% and 21%, respectively (p = 0.03) [19]. In our data, second-line therapy was evenly distributed between genders.
Currently, it is clear that there are striking differences in gastrointestinal cancer incidences among male and female patients. These differences are in part explained by exposition to risk factors such as smoking [20]. Nevertheless, host factors probably play an important role in incidence variations. As an example, androgen levels may be related to the higher incidence of oesophageal adenocarcinoma [21]. However, little is known about gender as a prognostic factor in gastrointestinal malignancies. For instance, in gastro-oesophageal cancer, The Cancer Genome Atlas Program (TCGA) revealed a higher rate of microsatellite instability (MSI-H) among women [22]. It is clear now that MSI-H patients have a better prognosis [23]. In colorectal cancer, women have a higher proportion of right-sided tumours which are linked with poor outcomes [24, 25].
Drug effects are also variable between genders. 5-FU based chemotherapy tends to be more toxic in women. A polled analysis of more than 28,000 patients, demonstrated that neutropenia and gastrointestinal toxicity were more likely to affect female patients [26]. Benefit of immunotherapy also seems to vary according to sex. A large meta-analysis with 11,351 patients with different types of advanced cancer concluded that the magnitude of treatment was greater among men. HR was 0.72 and 0.86 in male and female patients, respectively, in the comparison of immune checkpoint inhibitors and control groups [27].
Finally, it is noteworthy that we have a growing body of evidence regarding molecular biology of BTC. Fibroblast Growth Factor Receptor (FGFR) fusions and Isocitrate Dehydrogenase (IDH) mutations are among targetable alterations in this scenario. Clinical trials have shown benefit of targeted therapies for such patients [28, 29]. Describing main characteristics of these patients, including eventual gender differences, will be paramount to enlighten these findings.
Conclusion
In summary, our data identified prognostic factors related to outcome in metastatic BTC. Although FOLFOX has become the standard on the second-line, in our series patients receiving first-line treatment other than gemcitabine combination had worse prognosis. We also showed that bone metastasis, biliary obstruction and male gender were correlated with worse prognosis. These data could be useful for future trials in selecting patients with higher risk to more intensive and precise therapy. Further studies and multicentric collaborations are fundamental to validate our findings and also to understand the biology of this rare neoplasm.
Funding
None.
Conflicts of interest/competing interests
The authors declare that they have no conflicts of interest.
Ethics approval
Approved by local ethics committee (2680/19).
Consent to participate
Not applicable.
Authors' contributions
All authors contributed to study concept and design; data acquisition; data analysis and interpretation; drafting of the manuscript; critical revision of the manuscript intellectual content and statistical analysis.
References
1. Bridgewater JA, Goodman KA, and Kalyan A, et al (2016) Biliary tract cancer: epidemiology, radiotherapy and molecular profiling Am Soc Clin Oncol Educ Book 35 194–203 https://doi.org/10.1200/EDBK_160831
2. Sripa B, Kaewkes S, and Sithithaworn P, et al (2007) Liver fluke induces cholangiocarcinoma PLoS Med 4 201 https://doi.org/10.1371/journal.pmed.0040201
3. Patel T (2001) Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States Hepatology 33(6) 1353–1357 https://doi.org/10.1053/jhep.2001.25087 PMID: 11391522
4. Ferlay J, Ervik M, and Lam F, et al (2018) Global Cancer Observatory: Cancer Today (Lyon: International Agency for Research on Cancer) https://gco.iarc.fr/today
5. Rawla P, Sunkara T, and Thandra KC, et al (2019) Epidemiology of gallbladder cancer Clin Exp Hepatol 5(2) 93–102 https://doi.org/10.5114/ceh.2019.85166 PMID: 31501784 PMCID: 6728871
6. Valle J, Wasan H, and Palmer DH, et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer N Engl J Med 362(14) 1273–1281 https://doi.org/10.1056/NEJMoa0908721 PMID: 20375404
7. Lamarca A, Palmer DH, and Wasan HS, et al (2019) ABC-06 a randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC + mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy J Clin Oncol 37(15_suppl) 4003 https://doi.org/10.1200/JCO.2019.37.15_suppl.4003
8. Nakeeb A, Pitt HA, and Sohn TA, et al (1996) Cholangiocarcinoma A spectrum of intrahepatic, perihilar, and distal tumors Ann Surg 224(4) 463–475 https://doi.org/10.1097/00000658-199610000-00005 PMID: 8857851 PMCID: 1235406
9. Tariq NU, McNamara MG, and Valle JW (2019) Biliary tract cancers: current knowledge, clinical candidates and future challenges Cancer Manag Res 11 2623–2642 https://doi.org/10.2147/CMAR.S157092 PMID: 31015767 PMCID: 6446989
10. André T, Tournigand C, and Rosmorduc O, et al (2004) Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study Ann Oncol 15(9) 1339–1343 https://doi.org/10.1093/annonc/mdh351 PMID: 15319238
11. Kim ST, Kang JH, and Lee J, et al (2019) Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: a multicenter, open-label, randomized, phase III, noninferiority trial Ann Oncol 30(5) 788–795 https://doi.org/10.1093/annonc/mdz058 PMID: 30785198
12. Schinzari G, Rossi E, and Mambella G, et al (2017) First-line treatment of advanced biliary ducts carcinoma: a randomized phase II study evaluating 5-FU/LV plus oxaliplatin (Folfox 4) versus 5-FU/LV (de Gramont Regimen) Anticancer Res 37(9) 5193–5197 PMID: 28870954
13. Lambert A, Jarlier M, and Gourgou Bourgade S, et al (2017) Response to FOLFIRINOX by gender in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ ACCORD 11 randomized trial PLoS One 12(9) e0183288 https://doi.org/10.1371/journal.pone.0183288 PMID: 28931010 PMCID: 5606928
14. Katz MH, Hu CY, and Fleming JB, et al (2012) Clinical calculator of conditional survival estimates for resected and unresected survivors of pancreatic cancer Arch Surg 147(6) 513–519 https://doi.org/10.1001/archsurg.2011.2281 PMID: 22351874 PMCID: 3740148
15. Hohla F, Hopfinger G, and Romeder F, et al (2014) Female gender may predict response to FOLFIRINOX in patients with unresectable pancreatic cancer: a single institution retrospective review Int J Oncol 44(1) 319–326 https://doi.org/10.3892/ijo.2013.2176
16. Bridgewater J, Lopes A, and Wasan H, et al (2016) Prognostic factors for progression-free and overall survival in advanced biliary tract cancer Ann Oncol 27(1) 134–140 https://doi.org/10.1093/annonc/mdv483
17. Baton O, Azoulay D, and Adam DV, et al (2007) Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes J Am Coll Surg 204(2) 250–260 https://doi.org/10.1016/j.jamcollsurg.2006.10.028 PMID: 17254929
18. Kim BJ, Hyung J, and Yoo C, et al (2017) Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients Cancer Chemother Pharmacol 80(1) 209–215 https://doi.org/10.1007/s00280-017-3353-2 PMID: 28597043
19. Walter T, Horgan AM, and McNamara M, et al (2013) Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study Eur J Cancer 49(2) 329–335 https://doi.org/10.1016/j.ejca.2012.08.003
20. Wagner AD, Oertelt-Prigione S, and Adjei A, et al (2019) Gender medicine and oncology: report and consensus of an ESMO workshop Ann Oncol 30(12) 1914–1924 https://doi.org/10.1093/annonc/mdz414 PMID: 31613312
21. Petrick JL, Falk RT, and Hyland PL, et al (2018) Association between circulating levels of sex steroid hormones and esophageal adenocarcinoma in the FINBAR Study PLoS One 13(1) e0190325 https://doi.org/10.1371/journal.pone.0190325 PMID: 29342161 PMCID: 5771564
22. Polom K, Marano L, and Marrelli D, et al (2018) Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer Br J Surg 105(3) 159–167 https://doi.org/10.1002/bjs.10663
23. Pietrantonio F, Miceli R, and Raimondi A, et al (2019) Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer J Clin Oncol 37(35) 3392–3400 https://doi.org/10.1200/JCO.19.01124 PMID: 31513484
24. White A, Ironmonger L, and Steele RJC, et al (2018) A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK BMC Cancer 18(1) 906 https://doi.org/10.1186/s12885-018-4786-7 PMID: 30236083 PMCID: 6149054
25. Lemmens VE, Klaver YL, and Verwaal VJ, et al (2011) Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study Int J Cancer 128(11) 2717–2725 https://doi.org/10.1002/ijc.25596
26. Wagner AD, Grothey A, and Andre T, et al (2018) Association of sex and adverse events (AEs) of adjuvant chemotherapy (ACT) in early stage colon cancer (CC): a pooled analysis of 28, 636 patients (pts) in the ACCENT database J Clin Oncol 36(15_suppl) 3603 https://doi.org/10.1200/JCO.2018.36.15_suppl.3603
27. McQuade JL, Daniel CR, and Hess KR, et al (2018) Sex as a predictor of response to cancer immunotherapy Lancet Oncol 19(8) e376 https://doi.org/10.1016/S1470-2045(18)30483-2 PMID: 30102226
28. Abou-Alfa GK, Sahai V, and Hollebecque A, et al (2020) Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study Lancet Oncol 21(5) 671–684 https://doi.org/10.1016/S1470-2045(20)30109-1 PMID: 32203698 PMCID: 8461541
29. Abou-Alfa GK, Macarulla Mercade T, and Javle M, et al (2019) LBA10_PR - ClarIDHy: a global, phase 3, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation Ann Oncol 2019(suppl_5) v851–v934






