Cutaneous T cell lymphomas (CTCLs) are a group of non-Hodgkin lymphomas that develop from T cells and mainly impact the skin with painful lesions.
The two main subtypes of CTCL are mycosis fungoides and Sézary syndrome.
CTCLs are extremely rare, with approximately six cases per 1 million people each year. It is unclear how CTCL develops, and unfortunately there are limited treatment options and no cure.
Moffitt Cancer Center treats approximately 16% of CTCL patients nationwide. In order to improve their understanding of how CTCL develops in hopes of developing new therapies, a team of Moffitt immunologists and haematologists, including Jose Conejo-Garcia, Ph.D., Javier Pinilla-Ibarz,, M.D., Ph.D. and Lubomir Sokol, M.D., Ph.D., conducted a series of studies.
In an article published in The Journal of Clinical Investigation, they demonstrate that decreased expression of the protein SATB1 contributes to CTCL development and that drugs that cause SATB1 to become re-expressed may be potential treatment options for this disease.
The protein SATB1 plays an important role in cell death, proliferation and invasion, and has also been shown to be involved in the processes that control T cells differentiations.
Recently, it was reported that mycosis fungoides is associated with lower levels of SATB1, suggesting that it may play an important role in the development of this disease.
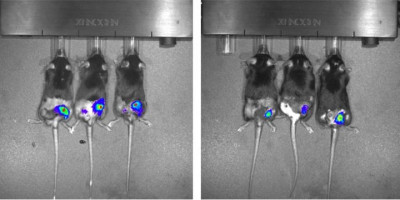
To understand how SATB1 contributes to CTCL, Moffitt researchers created a mouse model that lacked SATB1 in combination with over expression of NOTCH1, which is known to be involved in the development of CTCL.
The mice developed enlarged spleens and livers, swollen lymph nodes, a high level of T cells and lived for a significantly shorter time than control mice.
When the researchers examined the skin of the mice that were missing SATB1, they discovered characteristics that were similar to CTCL, suggesting that loss of SATB1 cooperates with NOTCH1 to promote CTCL development.
The researchers confirmed these observations by showing that T cells from patients with Sézary syndrome have significantly lower levels of SATB1 than cells from healthy donors.
The researchers wanted to determine mechanistically how loss of SATB1 contributes to the development of CTCL in mice by assessing signalling pathways that were activated both upstream and downstream of SATB1.
They performed a series of laboratory experiments to show SATB1 regulated the downstream protein STAT5 and other protein chemical receptors in T cells that cause them to expand in number.
Next, the researchers focused on upstream processes to determine how SATB1 loss occurs in patients' samples.
They discovered that a process called epigenetic regulation plays an important role in this loss.
Specifically, they showed that DNA regulatory proteins called histones become methylated, which results in loss of SATB1 expression.
They also discovered that drugs that prevent this methylation cause SATB1 to become re-expressed.
These methylation inhibitors also prevented the growth of Sézary syndrome cells more effectively than the drug romidepsin, which is commonly used to treat CTCL patients.
These observations suggest that drugs that target these methylation processes may be viable options to treat CTCL patients.
The researchers hope that their preclinical studies will eventually lead to clinical trials of methylation inhibitors in CTCL patients.
"Our results offer new insight into the pathophysiology of CTCL, as well as a mechanistic rationale for targeting histone methyltransferases to abrogate malignant expansion and skin homing in advanced CTCL patients," said Carly Harro, study first author and student in Moffitt's Cancer Biology Ph.D. Program.